Abstract
AIM: To evaluate the effect of mitochondrial tumor necrosis factor receptor-associated protein-1 (TRAP-1) on the lymph node metastasis (LNM) in Chinese colorectal cancer (CRC) patients, and develop potential LNM-associated biomarkers for CRC using quantitative real-time polymerase chain reaction (RT-PCR) analysis.
METHODS: Differences in mitochondrial TRAP-1 gene expression between primary CRC with LNM (LNM CRC) and without LNM (non-LNM CRC) were assessed in 96 Chinese colorectal carcinoma samples using quantitative RT-PCR analysis, Western blotting, and confirmed with immunohistochemical assay. The relationship between clinicopathological parameters and potential diagnostic biomarkers was also examined.
RESULTS: TRAP-1 was significantly upregulated in LNM CRC compared with non-LNM CRC, which was confirmed by RT-PCR, Western blotting and immunohistochemical assay. The expression of TRAP-1 in two different metastatic potential human colorectal cancer cell lines, LoVo and HT29, was analyzed with Western blotting. The expression level of TRAP-1 was dramatically higher in LoVo than in HT29. Overexpression of TRAP-1 was significantly associated with LNM (90.2% in LNM group vs 22% in non-LNM group, P < 0.001), the advanced tumor node metastasis stage (89.1% in LNM group vs 26.9% in non-LNM group, P < 0.001), the increased 5-year recurrence rate (82.7% in LNM group vs 22.6% in non-LNM group, P < 0.001) and the decreased 5-year overall survival rate (48.4% in LNM vs 83.2% in non-LNM group, P < 0.001). Univariate and multivariate analyses indicated that TRAP-1 expression was an independent prognostic factor for recurrence and survival of CRC patients (Hazard ratio of 2.445 in recurrence, P = 0.017; 2.867 in survival, P = 0.028).
CONCLUSION: Mitochondria TRAP-1 affects the lymph node metastasis in CRC, and may be a potential biomarker for LNM and a prognostic factor in CRC. Over-expression of TRAP-1 is a predictive factor for the poor outcome of colorectal cancer patients.
Keywords: Colorectal cancer, Lymph node metastasis, Prognosis, Quantitative real-time polymerase chain reaction analysis, Hsp90 family, Mitochondria tumor necrosis factor receptor-associated protein-1
INTRODUCTION
Invasion into adjacent tissues and metastasis to distant sites are major features of malignant cancer cells, which are complex processes that require a coordinated action of a large assortment of growth factors and their receptors, as well as downstream signaling intermediates[1]. Colorectal cancer (CRC) is the third most common cancer in both men and women and the second most common cause of cancer-related death[2]. CRC frequently migrates through the lymphatic route, depositing tumor cells into local lymph nodes, namely lymph node metastasis (LNM). The status of the local lymph nodes delivers crucial information concerning cancer staging, prognosis, and clinical decision making. The existence of LNM notably reduces the chance of CRC survivals[3].
Unfortunately, the mechanisms related to LNM remain poorly understood at present because LNM is a complicated process that involves cancer cell detachment from the primary tumor, migration, invasion, adhesion and implantation in the new environment. A variety of dysregulated molecules play a significant role in this highly sophisticated process[4,5]. Therefore, LNM has become a focus of cancer studies.
Clinicopathological features such as poor differentiation, depth of wall penetration, lymphovascular invasion, and tumor size are considered to be associated with CRC with LNM (LNM CRC)[6,7].
Proper staging is important for choosing the right treatment for a patient: the most useful staging system is the tumor node metastasis (TNM) system established collaboratively by the American Joint Committee on Cancer and the International Union for Cancer Control[8].
However, these characteristics are still insufficient to predict the existence of LNM. In order to improve the diagnosis and prognosis of CRC, there is an urgent need to identify specific tumor molecular markers to recognize patients with LNM, which can define a subset of CRC patients who could benefit from rational management.
Heat shock protein 90 (Hsp90) is an abundant molecular chaperone that is further overexpressed or activated in cancer cells, suggesting that it could be a crucial regulator of growth and/or survival of tumor cells[9,10]. Recent data have shown that Hsp90 family may function as novel regulators of mitochondrial permeability transition, especially in tumor cells[11-13]. Inhibitors of Hsp90 have been studied for the treatment of cancer, and a small molecule Hsp90 antagonist derived from geldanamycin (GA), i.e., 17-allylamino-demethoxygeldanamycin (17-AAG), has entered clinical testing in cancer patients, but progress has been questionable, and the response to this agent proved difficult to interpret[14,15]. This reflected a modest anticancer activity that was inconsistent with a predicted essential role of Hsp90 in tumor maintenance, paradoxical activation of oncogenic kinases, induction of anti-apoptotic mechanisms, and increased metastatic dissemination[16].
Accordingly, Hsp90 and its ortholog, and tumor necrosis factor receptor-associated protein-1 (TRAP-1) are abundantly localized to the mitochondria of tumor, but not present in most of the normal cells. Mitochondria play a critical role in cell survival and cell death[17]. Consistent with a general role of Hsp90 as a drug target in colorectal cancer, the mitochondria-compartmentalized cytoprotective pathway could provide a novel therapeutic target to enhance tumor cell apoptosis[13].
TRAP-1 associated with cancer has been reported recently in several studies, especially in metastasis of cancer cells[18-20], but there were a limited number of studies on the association of TRAP-1 with metastasis in Chinese CRC patients. In this study, we employed quantitative real-time polymerase chain reaction (RT-PCR) to analyze the expression of mitochondrial TRAP-1 in the groups of LNM CRC and non-LNM CRC. We studied the correlation between expression of TRAP-1 in LNM CRC and non-LNM CRC with quantitative RT-PCR and Western blotting. Confirmed with immunohistochemical (IHC) study, we also investigated the relationship between TRAP-1 expression and lymph node metastatic phenotype of CRC, and determined the prognostic value on the metastasis in Chinese CRC cases.
MATERIALS AND METHODS
Patient population and characteristics of tissue samples
A total of 96 Chinese colorectal carcinoma samples were collected in our hospital (Fudan University Shanghai Cancer Center, Shanghai, China) after informed consent was obtained from the patients. None of the patients received chemotherapy or radiotherapy before surgery. Resected specimens were reviewed by two senior pathologists according to the criteria described in the American Joint Committee on Cancer: Cancer Staging Manual (7th edition, 2009)[8]. The number of lymph nodes retrieved was not less than 12 in the non-LNM CRC. None of them had distant metastasis. The fresh colorectal tumor tissues were obtained immediately after surgery, washed twice with chilled phosphate buffered saline, immediately stored in liquid nitrogen at -80 °C in our tissue bank until further use. This study was approved by the Cancer Center Research Ethics Committee of Fudan University.
Cell lines and culture
For detection of the expression of TRAP-1 in different metastatic potential human colorectal cancer cells, we chose two human colorectal cancer cell lines, LoVo and HT29 in the experiment, to make sure if the TRAP-1 expressed differently in different metastatic potential human colorectal cancer cell lines. The cell line HT29 was cultured in McCoy 5a medium containing 5% fetal bovine serum (FBS). LoVo cells were cultured in F-12K medium containing 10% FBS. All the cell lines were maintained at 37˚C in 95% air and 5% CO2.
RNA isolation and reverse transcription
Total RNA was isolated from the human tissue or cultured colon cancer cell lines using the TRizol according to manufacturer’s instructions (Invitrogen). After the RNA concentration measurement and the integrity of the isolated RNA analysis, 1 μg of RNA was reverse-transcribed into cDNA according to the manufacturer’s protocol (Promega).
Quantitative RT-PCR
Quantitative RT-PCR was analyzed using SYBR Green Supermix (Promega). For PCR, 10 ng of the RT reaction was used for a 25-μL reaction using the ABI Prism 7700 sequence detector system (Applied Biosystems, Branchburg, NJ, United States). Target genes were normalized to β-actin and quantified using the comparative Ct method[21]. TRAP-1 expression levels were measured in triplicate, with a good reproducibility, and the average was calculated.
β-actin was applied as an internal control. The primers for β-actin (205 bp) were 5’-TGACGTGGACATCCGCAAAG-3’ (sense) and 5’-CTGGAAGGTGGAC AGCGAGG-3’ (antisense). The primers for TRAP-1 (185 bp) were 5’-ATGGTGG CTGACAGAGTGGAGG-3’ (sense) and 5’-GCAGTCGGATTTCAGGTGGA TG-3’ (antisense).
Western blotting
Briefly, 30-μg protein samples from each case were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequently transferred to polyvinylidene fluoride membranes. The membranes were incubated with rabbit polyclonal antibody against TRAP-1 (1:1000 dilution; Abcam, Cambridge, United Kingdom) and then incubated with a horseradish-peroxidase-conjugated secondary antibody (1:100 dilution; Proteintech, Chicago, IL, United States). β-actin was detected simultaneously as a loading control (anti-β-actin, 1:1000 dilution; Kangchen, Beijing, China). All blots were visualized using an ECL detection system (Amersham, Arlington Heights, IL, United States) and quantitated by densitometry using an LAS-3000 imager.
Immunohistochemistry
TRAP-1 expression was examined immunohistochemically using paraffin-embedded tissues. In brief, 4-μm-thick tissue sections were heated in 6.5 mmol/L citrate buffer (pH 6.0) at 100 °C for 28 min, and incubated with antibody against TRAP-1 (1:200 dilution). Immunostaining was performed using the DAKO EnVision System (Dako Diagnostics, Zug, Switzerland). In the negative control group, PBS was used instead of primary antibody. TRAP-1 expression was scored by two independent experienced pathologists. Each sample was graded according to the intensity and extent of staining. The intensity of staining was scored as 0 (no staining), 1 (weak staining), and 2 (strong staining). The extent of staining was based on the percentage of positive tumor cells: 0 (no staining), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%). The final score was assessed by summarizing the results of intensity and extent of staining. The case was considered negative if the final score was 0 or 1 (-) or 2 or 3 (±), and positive if the score was 4 or 5 (+) or 6 or 7 (++). In most cases, the two reviewers provided consistent results. Any inconsistencies were resolved through discussion to achieve a consensus score.
Statistical analysis
The Student t test was used to evaluate the differences in TRAP-1 expression between LNM CRC and non-LNM CRC. The χ2 test was used to assess the relationship between TRAP-1 expression and clinicopathological factors. The cumulative recurrence and survival probability were estimated using the Kaplan-Meier method, and differences were calculated by log-rank test. Prognostic factors were determined using Cox regression analysis. The recurrence-free and overall survival duration were calculated from the first resection of the primary tumor to first evidence of recurrence or to death from any cause, respectively. The diagnosis of recurrence was based on the typical features presented on computed tomography/magnetic resonance imaging and elevated serum carcinoembryonic antigen. All P values were two-sided, and P < 0.05 was considered to be significant. Statistical analyses were performed using SPSS 13.0 software.
RESULTS
TRAP-1 expression in CRC specimens and different metastatic potentials of two human colorectal cancer cell lines, LoVo and HT29, were determined by Western blotting and quantitative RT-PCR. The expression of TRAP-1 in LoVo cells and HT29 cells was analyzed with Western blotting. The expression level of TRAP-1 was dramatically higher in LoVo than in HT29 cells. Representative Western blotting results are presented in Figure 1A.
Figure 1.
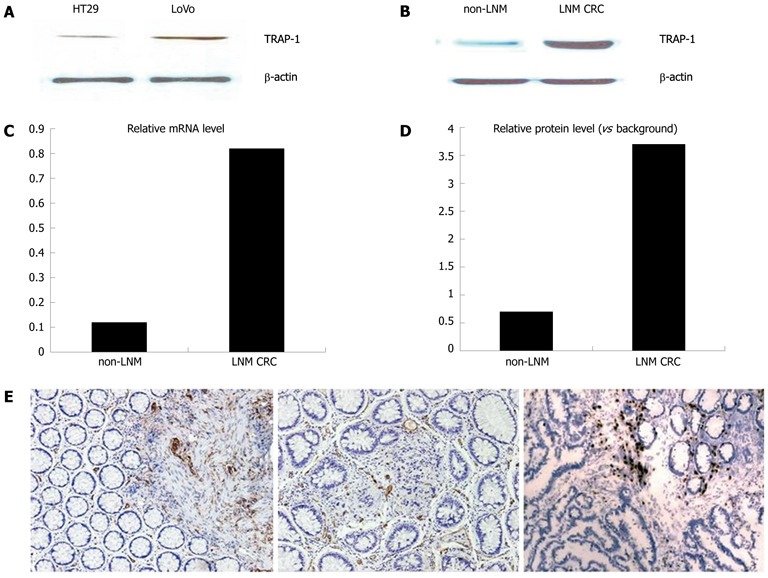
Confirmation of the overexpression of tumor necrosis factor receptor-associated protein-1 in colorectal cancer. A: Western blotting analysis for tumor necrosis factor receptor-associated protein-1 (TRAP-1) expression in different metastatic potential LoVo cell and HT29 cell. β-actin was used as the internal loading control. The histogram shows the relative expression levels of TRAP-1 in LoVo and HT29 cell; B: Western blotting analysis for TRAP-1 expression in non-lymph node metastasis (LNM) and LNM groups. Data represent the mean ± SE (P < 0.001, Student t test); C, D: mRNA level of TRAP-1 via quantitative real-time polymerase chain reaction. TRAP-1 was consistently increased in the LNM group compared with non-LNM group. The mRNA level was normalized to that of β-actin. Data represent the mean ± SE (P < 0.001, Student t test); E: Immunohistochemical labeling for the TRAP-1 in the CRC sample. TRAP-1 was identified in non-LNM cancer tissues (weak in middle) and strong staining in LNM cancer group (right), but was rare in normal mucosa (left).
Quantitative RT-PCR and Western blotting were used to analyze the TRAP-1 expression in different groups of CRC. Relative gene and protein expression quantifications were calculated by the comparative Ct method using β-actin as an endogenous control. The results revealed that TRAP-1 mRNA and protein levels were higher in LNM CRC than in non-LNM CRC (P < 0.001, Figure 1B and C, D), which is consistent with the results by the Western blotting.
Immunohistochemistry was applied to confirm the upregulation of TRAP-1 in different groups (Figure 1E). In non-LNM CRC, there was weak staining in cancer cells compared to the strong staining in both primary and matched metastatic lymph node cancer cells in LNM CRC samples.
Association between TRAP-1 expression and clinicopathological features and postoperative prognosis of CRC patients
To detect the relationship between TRAP-1 expression and clinicopathological features and whether TRAP-1 could be a prognostic factor in predicting clinical outcomes of CRC patients, we evaluated TRAP-1 expression with the same samples. In the LNM CRC samples, 87% were positive for TRAP-1 expression, whereas 10.2% of the non-LNM CRC samples had positive expression.
Statistical analysis revealed that positive expression of TRAP-1 was significantly associated with LNM, and advanced TNM stage (P < 0.001). However, no significant correlations were observed between TRAP-1 expression and other clinicopathological parameters of sex, age, tumor size, tumor differentiation and tumor location (Table 1).
Table 1.
Relationship between tumor necrosis factor receptor-associated protein-1 expression and clinicopathological factors in colorectal cancer
| Clinicopathological factors | n |
TRAP-1 expression |
P value1 | |
| Negative | Positive | |||
| Sex | ||||
| Male | 46 | 16 | 30 | 0.225 |
| Female | 50 | 23 | 30 | |
| Age (yr) | ||||
| ≤ 60 | 66 | 30 | 36 | 0.486 |
| > 60 | 30 | 10 | 20 | |
| Tumor size (cm) | ||||
| ≤ 5 | 70 | 30 | 40 | 0.065 |
| > 5 | 26 | 11 | 15 | |
| Tumor location | ||||
| Colon | 31 | 11 | 20 | 0.915 |
| Rectum | 65 | 32 | 33 | |
| Tumor differentiation2 | ||||
| I-II | 80 | 45 | 35 | 0.212 |
| III-IV | 16 | 6 | 10 | |
| Tumor status2 | ||||
| T1-2 | 34 | 14 | 20 | 0.512 |
| T3-4 | 62 | 31 | 31 | |
| Lymph node metastasis2 | ||||
| N0 | 45 | 35 | 10 | < 0.001 |
| N1-2 | 51 | 5 | 46 | |
| TNM stage2 | ||||
| I-II | 41 | 30 | 11 | < 0.001 |
| III-IV | 55 | 6 | 49 | |
Statistical analysis was performed with χ2 test;
Grading of differentiation status and tumor node metastasis (TNM) classification for colorectal cancer were based on the American Joint Committee on Cancer: Cancer Staging Manual (7th edition, 2009). The tumors were classified into two groups: Well differentiated (grades I and II) and poorly differentiated (grades III and IV).
Furthermore, we found that patients with TRAP-1-positive CRC had significantly poorer prognosis than those with TRAP-1-negative CRC. The 5-year cumulative recurrence rate was significantly higher in patients with TRAP-1-positive CRC than in the TRAP-1-negative group (P < 0.001, Figure 2A). The 5-year cumulative survival rate in patients with TRAP-1-positive CRC was much lower than in those with TRAP-1-negative CRC (P < 0.001, Figure 2B). Univariate analyses revealed that LNM, TNM stage and TRAP-1 expression were associated with recurrence and overall survival. In multivariate analysis, LNM, TNM stage and TRAP-1 expression were also independent prognostic factors for recurrence and overall survival (P < 0.05, Table 2).
Figure 2.
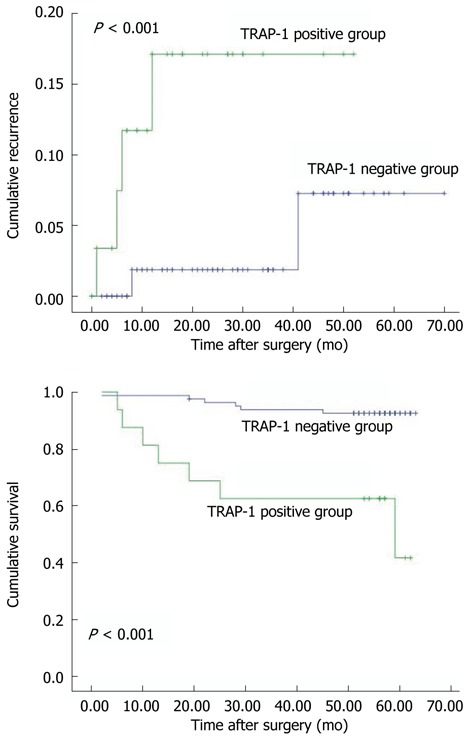
Overexpression of tumor necrosis factor receptor-associated protein-1 correlated with poor prognosis in 96 colorectal cancer patients. A: Cumulative recurrence; B: Cumulative survival. The tumor necrosis factor receptor-associated protein-1 (TRAP-1)-positive vs TRAP-1-negative groups (P < 0.001, log-rank test).
Table 2.
Univariate and multivariate analyses of recurrence and survival (Cox regression)
| Variables |
Recurrence |
Survival |
||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Univariate analysis | ||||
| Sex | ||||
| Male/female | 0.813 (0.479-1.518) | 0.604 | 0.829 (0.424-1.618) | 0.662 |
| Age (yr) | ||||
| ≤ 60/> 60 | 1.506 (0.804-2.712) | 0.163 | 1.822 (0.947-3.528) | 0.065 |
| Tumor size (cm) | ||||
| ≤ 5/> 5 | 0.876 (0.163-0.665) | 0.687 | 0.880 (0.438-1.723) | 0.704 |
| Tumor location | ||||
| Colon/rectum | 0.812 (0.445-1.423) | 0.518 | 0.904 (0.476-1.734) | 0.778 |
| Tumor differentiation | ||||
| I-II/III-IV | 1.212 (0.654-2.308) | 0.501 | 1.151 (0.576-2.358) | 0.65 |
| Tumor status | ||||
| T1-2/T3-4 | 0.866 (0.475-1.618) | 0.687 | 1.020 (0.504-2.028) | 0.904 |
| Lymph node metastasis | ||||
| N0/N1-2 | 2.707 (1.502-4.912) | 0.001 | 2.812 (1.413-5.509) | 0.002 |
| TNM stage | ||||
| I-II/III-IV | 3.554 (1.932-6.526) | < 0.001 | 3.385 (1.677-6.843) | < 0.001 |
| TRAP-1 expression | ||||
| Negative/positive | 3.657 (1.919-6.957) | < 0.001 | 4.145 (1.913-8.712) | < 0.001 |
| Multivariate analysis | ||||
| LNM | ||||
| N0/N1-2 | 0.210 (0.051-0.758) | 0.018 | 0.196 (0.041-0.852) | 0.028 |
| TNM stage | ||||
| I-II/III-IV | 8.905 (2.072-38.190) | 0.003 | 9.037 (1.703-48.105) | 0.010 |
| TRAP-1 expression | ||||
| Negative/positive | 2.445 (1.065-5.712) | 0.017 | 2.867 (1.113-7.36) | 0.028 |
HR: Hazard ratio; CI: Confidence interval; LNM: Lymph node metastasis; TNM: Tumor node metastasis; TRAP-1: Tumor necrosis factor receptor-associated protein-1.
DISCUSSION
Metastasis remains one of the major challenges in management of CRC patients. LNM is the most common form of metastasis in CRC. To study the correlation between expression of TRAP-1 and LNM metastasis in East Asian CRC patients, develop potential LNM-associated biomarkers for CRC, we used the quantitative RT-PCR to determine the expression of TRAP-1 in clinical LNM CRC and non-LNM CRC patients, and employed immunohistochemical assay in the same samples to confirm the outcome of PCR and Western blotting.
Recently, several studies have shown that TRAP-1 is an important factor relevant to progression and prognosis in various human cancers, such as glioblastoma[22], ovarian[23], prostate[24], colorectal[25], and bladder[26] cancer. In particular, some studies have revealed that overexpression of TRAP-1 strongly indicates the presence of LNM[22-24], which accords with the objectives of our study. However, similar investigations have been limited to the relationship between TRAP-1 expression and LNM in CRC. Therefore, it is of interest to notice that TRAP-1, one of the significantly upregulated proteins identified in LNM CRC compared with non-LNM CRC, has been confirmed at the protein and mRNA levels.
TRAP-1 is abundantly and differentially expressed in metastatic CRC in humans, but in normal colon cells, TRAP-1 was undetectable or minimally expressed. The different distribution of TRAP-1 in colorectal cancer cells vs normal cells is in agreement with another survey of different tumor types, where TRAP-1 was also differentially expressed in tumors of breast, lung, prostate and pancreas as compared with normal matched tissues[13].The up-regulation of TRAP-1 in LNM CRC played a major role in crucial biological functions that influence various aspects of cell physiology, including proliferation and apoptosis, and differentiation and morphogenesis. It is also significantly involved in cell adhesion and motility, and cancer invasion and metastasis[27,28].
The relationship between TRAP-1 expression and the LNM phenotype of CRC was also studied in the experiment with the same CRC samples. We found that the increase in TRAP-1 expression level was significantly correlated with LNM and advanced TNM stage, which suggests that TRAP-1 plays an important part in the progression of CRC from a localized to lymph node metastatic disease. In addition, patients with TRAP-1-positive CRC had an increasing risk of recurrence and significantly reduced overall survival. Univariate and multivariate analyses indicated that TRAP-1 expression is an independent prognostic factor for recurrence and overall survival in CRC, which indicates the considerable prognostic value of TRAP-1 expression.
In conclusion, the present study provided evidences in the association between differently expressed TRAP-1 and LNM in CRC based on a quantitative mRNA expression analysis. TRAP-1 was identified and confirmed to be significantly overexpressed in LNM CRC. Further evaluation by Western blotting and IHC assay using the same sample sets suggested that TRAP-1 acts as a potential biomarker for LNM and prognosis in CRC.
Most epithelial malignancies, including colorectal cancer exhibit a higher anti-apoptotic threshold, which contributes to disease progression[29-31]. However, many questions remain to be answered[32-34] with respect to the cellular function of TRAP-1 and how it exerts its influence on metastatic progression, and the molecular basis for the different localization of Hsp90 and TRAP-1 to the tumor vs normal mitochondria awaits further studies.
COMMENTS
Background
Colorectal cancer (CRC) is the third most common cancer in both men and women and the second most common cause of cancer-related death. CRC frequently migrates through lymph node metastasis (LNM), but the variables used to predict the existence of LNM are not available. In order to improve the diagnosis and prognosis of CRC, there is an urgent need to identify specific tumor molecular markers to recognize patients with LNM, which can define a subset of CRC patients who could benefit from rational management.
Research frontiers
Accordingly, Hsp90, and its ortholog, tumor necrosis factor receptor-associated protein-1 (TRAP-1) are abundantly localized to mitochondria of tumor, but not to the normal cells. Mitochondria play a critical role in cell survival and cell death. Consistent with a general role of Hsp90 as a drug target in colorectal cancer, the mitochondria-compartmentalized cytoprotective pathway could provide a novel therapeutic target to enhance tumor cell apoptosis.
Innovations and breakthroughs
The association between TRAP-1 and cancer has been reported in several studies recently, especially the relationship of TRAP-1 with metastasis of cancer cells. There was a limited number of studies on the association of TRAP-1 with metastasis in Chinese CRC patients. In this study, the authors analyzed the expression of mitochondrial in different groups of LNM CRC and non-LNM CRC using quantitative real-time polymerase chain reaction (RT-PCR), and investigated the correlation between expression of TRAP-1 in LNM CRC and non-LNM CRC with quantitative RT-PCR and Western blotting. Confirmed with immunohistochemical assay in the same samples, the authors also investigated the relationship between TRAP-1 expression and lymph node metastatic phenotype of CRC, and determined the prognostic value on the metastasis in Chinese CRC cases.
Applications
This study suggests that the mitochondria TRAP-1 affect the lymph node metastasis in CRC, and it may be a potential biomarker for LNM and a prognostic factor in CRC. Over-expression of TRAP-1 is a predictive factor for the poor outcome of CRC patients.
Terminology
Hsp90 ortholog, TRAP-1 are abundantly localized to mitochondria of tumor, but not to normal cells. TRAP-1 is a 75 kDa heat shock protein that is encoded in humans by the TRAP-1 gene.
Peer review
The study is well-designed, although there are some technical points that need rethinking during the interpretation of the results. The topic is of great clinical importance as colorectal carcinoma represents a worldwide health problem, and finding possible biomarkers that can be prognostic factors may help the clinicians in their daily routine.
Footnotes
Supported by The Grants from Shanghai Health Bureau, No. JG1103
Peer reviewer: Dr. Ferenc Sipos, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi u. 46., 1088 Budapest, Hungary
S- Editor Gou SX L- Editor Ma JY E- Editor Zhang DN
References
- 1.Fidler IJ. Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metastasis Rev. 1991;10:229–243. doi: 10.1007/BF00050794. [DOI] [PubMed] [Google Scholar]
- 2.Strimpakos AS, Cunningham D, Mikropoulos C, Petkar I, Barbachano Y, Chau I. The impact of carcinoembryonic antigen flare in patients with advanced colorectal cancer receiving first-line chemotherapy. Ann Oncol. 2010;21:1013–1019. doi: 10.1093/annonc/mdp449. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 4.Royston D, Jackson DG. Mechanisms of lymphatic metastasis in human colorectal adenocarcinoma. J Pathol. 2009;217:608–619. doi: 10.1002/path.2517. [DOI] [PubMed] [Google Scholar]
- 5.Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol. 2007;25:4298–4307. doi: 10.1200/JCO.2006.07.1092. [DOI] [PubMed] [Google Scholar]
- 6.Ricciardi R, Madoff RD, Rothenberger DA, Baxter NN. Population-based analyses of lymph node metastases in colorectal cancer. Clin Gastroenterol Hepatol. 2006;4:1522–1527. doi: 10.1016/j.cgh.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Fang W, Fan B, Xiong B. Analysis of pathological risk factors for lymph node metastasis in colorectal cancer. Hepatogastroenterology. 2009;56:663–666. [PubMed] [Google Scholar]
- 8.Carolyn C. Cancer staging handbook seventh edition. Available from: http: //www.cancerstaging.org/staging/index.html.
- 9.Romanucci M, Marinelli A, Sarli G, Della Salda L. Heat shock protein expression in canine malignant mammary tumours. BMC Cancer. 2006;6:171. doi: 10.1186/1471-2407-6-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 11.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 12.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 13.Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Neckers L, Ivy SP. Heat shock protein 90. Curr Opin Oncol. 2003;15:419–424. doi: 10.1097/00001622-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Collins I, Workman P. New approaches to molecular cancer therapeutics. Nat Chem Biol. 2006;2:689–700. doi: 10.1038/nchembio840. [DOI] [PubMed] [Google Scholar]
- 16.Drysdale MJ, Brough PA, Massey A, Jensen MR, Schoepfer J. Targeting Hsp90 for the treatment of cancer. Curr Opin Drug Discov Devel. 2006;9:483–495. [PubMed] [Google Scholar]
- 17.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 18.Leav I, Plescia J, Goel HL, Li J, Jiang Z, Cohen RJ, Languino LR, Altieri DC. Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer. Am J Pathol. 2010;176:393–401. doi: 10.2353/ajpath.2010.090521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. 6th ed. New York: Springer-Verlag; 2002. AJCC Cancer Staging Manual; pp. 113–124. [Google Scholar]
- 20.Montesano Gesualdi N, Chirico G, Pirozzi G, Costantino E, Landriscina M, Esposito F. Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis. Stress. 2007;10:342–350. doi: 10.1080/10253890701314863. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Siegelin MD, Plescia J, Raskett CM, Gilbert CA, Ross AH, Altieri DC. Global targeting of subcellular heat shock protein-90 networks for therapy of glioblastoma. Mol Cancer Ther. 2010;9:1638–1646. doi: 10.1158/1535-7163.MCT-10-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landriscina M, Amoroso MR, Piscazzi A, Esposito F. Heat shock proteins, cell survival and drug resistance: the mitochondrial chaperone TRAP1, a potential novel target for ovarian cancer therapy. Gynecol Oncol. 2010;117:177–182. doi: 10.1016/j.ygyno.2009.10.078. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen MC, Tu GH, Koprivnikar KE, Gonzalez-Edick M, Jooss KU, Harding TC. Antibody responses to galectin-8, TARP and TRAP1 in prostate cancer patients treated with a GM-CSF-secreting cellular immunotherapy. Cancer Immunol Immunother. 2010;59:1313–1323. doi: 10.1007/s00262-010-0858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costantino E, Maddalena F, Calise S, Piscazzi A, Tirino V, Fersini A, Ambrosi A, Neri V, Esposito F, Landriscina M. TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptotis in human colorectal carcinoma cells. Cancer Lett. 2009;279:39–46. doi: 10.1016/j.canlet.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Jin Y, Murata H, Sakaguchi M, Kataoka K, Watanabe M, Nasu Y, Kumon H, Huh NH. Partial sensitization of human bladder cancer cells to a gene-therapeutic adenovirus carrying REIC/Dkk-3 by downregulation of BRPK/PINK1. Oncol Rep. 2012;27:695–699. doi: 10.3892/or.2011.1543. [DOI] [PubMed] [Google Scholar]
- 27.Kubota K, Inoue K, Hashimoto R, Kumamoto N, Kosuga A, Tatsumi M, Kamijima K, Kunugi H, Iwata N, Ozaki N, et al. Tumor necrosis factor receptor-associated protein 1 regulates cell adhesion and synaptic morphology via modulation of N-cadherin expression. J Neurochem. 2009;110:496–508. doi: 10.1111/j.1471-4159.2009.06099.x. [DOI] [PubMed] [Google Scholar]
- 28.Neckers L, Kern A, Tsutsumi S. Hsp90 inhibitors disrupt mitochondrial homeostasis in cancer cells. Chem Biol. 2007;14:1204–1206. doi: 10.1016/j.chembiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Huang X, Zhang X, Farahvash B, Olumi AF. Novel targeted pro-apoptotic agents for the treatment of prostate cancer. J Urol. 2007;178:1846–1854. doi: 10.1016/j.juro.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 30.Altieri DC, Stein GS, Lian JB, Languino LR. TRAP-1, the mitochondrial Hsp90. Biochim Biophys Acta. 2012;1823:767–773. doi: 10.1016/j.bbamcr.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua G, Zhang Q, Fan Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J Biol Chem. 2007;282:20553–20560. doi: 10.1074/jbc.M703196200. [DOI] [PubMed] [Google Scholar]
- 32.Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275:3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- 34.Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers. 2010;93:211–217. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]


