Abstract
Liposarcoma is one of the most common soft tissue sarcomas found in adults, and it usually occurs in the retroperitoneum and the extremities. Here, we describe a case of dedifferentiated liposarcoma originating from a well-differentiated liposarcoma of the mesorectum that presented as a protruding mass in the rectal lumen. Hartmann’s operation with total mesorectal excision was performed and the tumor was removed radically. No management guidelines are currently available for liposarcoma of the rectum. We propose that complete surgical resection be required for the treatment of rectal liposarcoma and that a long-term detailed follow up is necessary.
Keywords: Dedifferentiated liposarcoma, Soft tissue sarcoma, Rectum, Management, Surgery
INTRODUCTION
Liposarcoma is one of the most common malignant soft tissue tumors found in adults. Most liposarcoma patients are between 40 and 60 years of age, and the incidence in men and women with this disease is approximately equal. Currently, classification of liposarcoma is divided into five subtypes: myxoid, pleomorphic, dedifferentiated, round cell, and well-differentiated liposarcoma (WDLPS)[1]. WDLPS is the most common histological subgroup, whereas dedifferentiated liposarcoma (DDLPS) has a comparatively worse prognosis. In this report we describe a WDLPS arising from the mesorectum and presenting as an endoluminal mass. Additionally, this tumor had histological characteristics consistent with DDLPS and had transmurally invaded into the rectum.
CASE REPORT
A 77-year-old Japanese male patient complaining of constipation and urinary retention, was admitted to Kurashiki Central Hospital and examined by a gastroenterologist. The patient had diabetes mellitus that was under control with anti-diabetic drugs. Physical examination revealed a large mass in the lower abdomen, and an abdominal computed tomography (CT) scan showed a large tumor mass in the rectum accompanied by prostatomegaly. Pelvic magnetic resonance imaging revealed a 9 cm × 7 cm × 5 cm sized polypoid tumor in the rectal cavity, with the dorsal side of the mesorectum appearing to protrude into the tumor (Figure 1). A colonoscopy revealed a smooth-surfaced, oval-shaped, large tumor in the rectum, with the lower end located about 10 cm from the anal verge. The first preoperative colonoscopic biopsy retrieved some necrotic but no malignant tissue. The immunohistochemical analysis of the second biopsied specimen demonstrated positive staining for CD34, CD31 and Ki67, and negative staining for c-kit, AE1/AE3 and epithelial membrane antigen, with the MIB-1 index at 30%. Preoperative diagnosis was not definitive but indicated a possible high-grade sarcoma. The tumor marker carbohydrate antigen 19-9 was detected at an abnormally high level, while other tumor markers were within normal ranges. The gastroenterologist suspected that the preoperative diagnosis was angiosarcoma, malignant solitary fibrous tumor, or c-kit negative gastrointestinal stromal tumor and introduced the patient to the surgical department for surgical resection. A preoperative digital examination confirmed that the distance between the lower end of the tumor and the anal verge was 10 cm and that the tumor was mobile.
Figure 1.
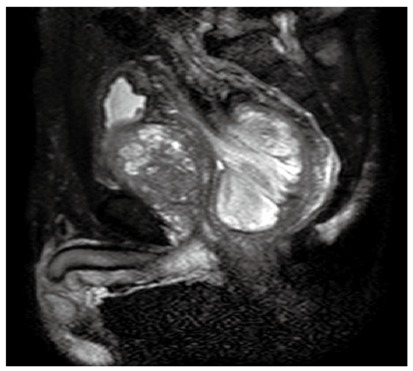
T2-weighted magnetic resonance image demonstrating a high-intensity mass in rectum with prostatomegaly.
The patient underwent a laparotomy, which revealed a large palpable tumor in the rectum. Since the preoperative diagnosis indicated a possible high-grade sarcoma, the local recurrence rate after the operation was not expected to be low, even if the tumor was resected radically. Therefore, total mesorectal excision was performed in addition to Hartmann’s operation to help decrease the risk of pelvic recurrence. A part of the mesorectum was slightly hard and the tumor was resected radically. Gross examination revealed a large submucosal tumor with invagination of the surrounding large intestine. Following surgery, the patient was discharged without any postoperative complications.
The resected solid tumor was 85 mm × 67 mm × 32 mm in size and pedunculated (Figure 2A). Histological examination revealed a high-grade sarcoma consisting of spindle-shaped tumor cells with hyperchromatic oval nuclei and eosinophilic cytoplasm (Figure 2B, C). The differentiation status of the tumor was determined morphologically as well as immunohistochemically. The tumor was positive for Bcl-2, vimentin, desmin, CD34, CD56, CD10, and CD99. However, the mass was found to be present transmurally in the rectum, and was continuous to WDLPS, consisting of mature adipose tissue, intervening fibrous tissue, and scattered atypical cells with large, unusual nuclei in the mesorectum. Thus, a diagnosis of DDLPS arising from WDLPS was rendered.
Figure 2.

Dedifferentiated liposarcoma in rectum. A: Gross picture showing a huge dedifferentiated liposarcoma in rectum; B: Dedifferentiated component identified in polypoid lesion at rectum [hematoxylin and eosin (HE), × 400]; C: Well-differentiated liposarcoma at mesorectum (HE, × 400).
Three months postoperatively, no recurrence or metastasis was identified via CT scan.
DISCUSSION
Retroperitoneal malignant tumors are rare; however, liposarcoma is the most common type[2-6]. Liposarcoma tends to occur in the fourth to sixth decades of life, with no difference in frequency among the sexes. Liposarcomas have been divided into five subtypes by the World Health Organization (well differentiated, dedifferentiated, myxoid, pleomorphic, and mixed type)[1]. In 1979, Evans[7] was the first to characterize a liposarcoma. He described a combination of WDLPS and a non-lipogenic dedifferentiated sarcoma-like component. In 1971 Dahlin et al[8] described the dedifferentiated chondrosarcoma as a morphologically biomorphic neoplasm showing areas of well-differentiated, low-grade tumors juxtaposed with high-grade non-chondroblastic tumors without obvious areas of gradual transition. Dedifferentiation can take place through a de novo mechanism or through the recurrence of WDLPS in which additional changes have occurred[9]. The original definition of DDLPS has been modified over time. Dedifferentiation into exclusively low-grade areas or into a combination of low and high-grade areas has been included in this subtype. DDLPS has a less aggressive clinical course than other types of high grade sarcoma, although the underlying mechanism remains unclear. Approximately 40% of DDLPSs will recur locally and 17% will metastasize and 28% of patients will ultimately die as a result of the tumor[1].
In our case, the mesorectum spindle cells in the pleomorphic lipoma were positive for CD34, indicating a well-differentiated liposarcoma. Preoperative immunohistochemical staining with CD117 was negative, suggesting that the tumor was not a typical GIST. Postoperative histological findings revealed a transition from WDLPS to a non-lipogenic sarcoma with a variable grade at the polypoid lesion indicating a DDLPS.
The most common sites of DDLPS are the retroperitoneum and extremities, with other anatomic locations occasionally reported. Excluding case of DDLPS in the retroperitoneum, we found nine cases of localized DDLPS. Six of the DDLPS cases occurred in the small bowel mesentery[10,11] and two cases of primary advanced DDLPS occurred in the colon[12,13]. The final case of DDLPS occurred in the sigmoid mesocolon[14]. The case we present here is an example of primary retroperitoneal WDLPS with secondary involvement of the rectum. An unusual feature of this case was that the DDLPS was detected in a polypoid lesion in the rectum.
The appropriate diagnostic and therapeutic approach to treat DDLPS has not yet been determined; although, it is generally accepted that complete surgical resection of the tumor should be performed to increase the cure rate of this disease. In our case, total mesorectal excision of the rectum was performed and was expected to have removed the tumor radically. The patient has been followed-up and no signs have been detected to suggest further need for therapy. However, prognosis of DDLPS mainly depends on local recurrence and almost all retroperitoneal cases recur locally in 10-20 years following treatment[15]. Therefore, we recommend that complete surgical resection and long-term follow up after surgery be required for the treatment of DDLPS due to the risk of recurrence.
ACKNOWLEDGMENTS
We thank Hitoshi Nakahori, MD for his contribution to this manuscript.
Footnotes
Peer reviewer: Dr. Marek Bebenek, MD, PhD, Department of Surgical Oncology, Regional Comprehensive Cancer Center, Hirszfelda 12, 53-413 Wroclaw, Poland
S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of tumors. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. pp. 227–232. [Google Scholar]
- 2.Armstrong JR, Cohn I. Primary malignant retroperitoneal tumors. Am J Surg. 1965;110:937–943. doi: 10.1016/0002-9610(65)90181-9. [DOI] [PubMed] [Google Scholar]
- 3.Binder SC, Katz B, Sheridan B. Retroperitoneal liposarcoma. Ann Surg. 1978;187:257–261. doi: 10.1097/00000658-197803000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yiu-Chiu V, Chiu L. Ultrasonography and computed tomography of retroperitoneal liposarcoma. J Comput Tomogr. 1981;5:98–109. doi: 10.1016/0149-936x(81)90022-9. [DOI] [PubMed] [Google Scholar]
- 5.Cody HS, Turnbull AD, Fortner JG, Hajdu SI. The continuing challenge of retroperitoneal sarcomas. Cancer. 1981;47:2147–2152. doi: 10.1002/1097-0142(19810501)47:9<2147::aid-cncr2820470907>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Potter DA, Glenn J, Kinsella T, Glatstein E, Lack EE, Restrepo C, White DE, Seipp CA, Wesley R, Rosenberg SA. Patterns of recurrence in patients with high-grade soft-tissue sarcomas. J Clin Oncol. 1985;3:353–366. doi: 10.1200/JCO.1985.3.3.353. [DOI] [PubMed] [Google Scholar]
- 7.Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3:507–523. doi: 10.1097/00000478-197912000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Dahlin DC, Beabout JW. Dedifferentiation of low-grade chondrosarcomas. Cancer. 1971;28:461–466. doi: 10.1002/1097-0142(197108)28:2<461::aid-cncr2820280227>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Enzinger FM, Weiss SW. Soft Tissue Tumors. 3rd ed. St. Louis: CV Mosby; 1995. pp. 432–466. [Google Scholar]
- 10.Hasegawa T, Seki K, Hasegawa F, Matsuno Y, Shimodo T, Hirose T, Sano T, Hirohashi S. Dedifferentiated liposarcoma of retroperitoneum and mesentery: varied growth patterns and histological grades--a clinicopathologic study of 32 cases. Hum Pathol. 2000;31:717–727. doi: 10.1053/hupa.2000.8222. [DOI] [PubMed] [Google Scholar]
- 11.Cha EJ. Dedifferentiated liposarcoma of the small bowel mesentery presenting as a submucosal mass. World J Gastrointest Oncol. 2011;3:116–118. doi: 10.4251/wjgo.v3.i7.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Annibale M, Cosimelli M, Covello R, Stasi E. Liposarcoma of the colon presenting as an endoluminal mass. World J Surg Oncol. 2009;7:78. doi: 10.1186/1477-7819-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarboui S, Moussi A, Jarraya H, Ben Mna K, Abdesselem MM, Kourda A, Ben Jilani S, Guettier C, Zaouche A. Primary dedifferentiated liposarcoma of the colon: a case report. Gastroenterol Clin Biol. 2009;33:1016–1018. doi: 10.1016/j.gcb.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Winn B, Gao J, Akbari H, Bhattacharya B. Dedifferentiated liposarcoma arising from the sigmoid mesocolon: a case report. World J Gastroenterol. 2007;13:4147–4148. doi: 10.3748/wjg.v13.i30.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coindre JM, Pédeutour F, Aurias A. Well-differentiated and dedifferentiated liposarcomas. Virchows Arch. 2010;456:167–179. doi: 10.1007/s00428-009-0815-x. [DOI] [PubMed] [Google Scholar]


