Abstract
We report an extremely rare case of synchronous double cancers of the common bile duct without pancreaticobiliary maljunction. Only two similar cases have been reported in the English literature. Endoscopic re-trograde cholangiopancreatography showed a tuberous filling defect in the middle and superior parts of the common bile duct, and mild stenosis in the inferior duct. Computed tomography (CT) showed a well enhanced mass in the middle and superior parts of the common bile duct. A single cancer of the middle and superior bile duct was suspected and extra-hepatic bile duct resection was performed. CT eleven months after surgery revealed enhanced inferior bile duct wall and a slightly enhanced tumor within it. Retrospective review of the CT images taken before first surgery showed enhanced inferior bile duct wall without intrabiliary tumor only on the delayed phase. The inferior bile duct tumor was suspected to have originally co-existed with the middle and superior bile duct tumor. Pancreaticoduodenectomy was performed subsequently. Histopathological examination revealed that the middle and superior bile duct tumor was a moderately differentiated tubular adenocarcinoma while the inferior bile duct tumor was a papillary adenocarcinoma. The two tumors were separated and had different histological findings and growth patterns, further suggesting that they were two primary cancers.
Keywords: Bile duct cancer, Synchronous double cancer, Common bile duct, Pancreaticobiliary maljunction, Two primary cancers
INTRODUCTION
Synchronous double cancers in the biliary system are rare[1]. Most such tumors are double cancers in the common bile duct (CBD) and the gallbladder associated with pancreaticobiliary maljunction (PBM)[1-3]. We report here an extremely rare surgical case of synchronous double cancers of the CBD without PBM. To our knowledge, only two similar cases have been reported in the English literature[4,5].
CASE REPORT
A 78-year-old male presented with abdominal pain and jaundice. Computed tomography (CT) showed dilatation of the intrahepatic bile duct and a well-enhanced mass in the middle and upper parts of the CBD (Figure 1A). No mass was detected in the inferior part of the CBD (Figure 1B). The results of laboratory tests showed elevated serum levels of several liver enzymes (total bilirubin 19.6 mg/dL, glutamic oxaloacetic transaminase 54 mg/dL, glutamic pyruvic transaminase 103 mg/dL), and high levels of carcinoembryonic antigen (5.4 ng/dL) and carbohydrate antigen 19-9 (375 U/mL). Endoscopic retrograde cholangiopancreatography (ERCP) showed the absence of PBM and the presence of a tuberous filling defect in the middle and superior parts of the CBD and mild stenosis in the inferior bile duct (Figure 1C). Endoscopy showed no abnormal finding in the papilla of Vater. Although a mild stenosis of the inferior part of the CBD was detected on ERCP, no tumor could be detected in that part of the duct on CT, suggesting that the stenosis was not caused by a malignant tumor. The provisional diagnosis was a solitary tumor in the middle and superior parts of the bile duct. Extra-hepatic bile duct resection with regional lymph node dissection was performed. Histopathological examination showed a moderately differentiated tubular adenocarcinoma with invasive growth (Figure 2). Both the proximal and distal ductal margins were negative. The postoperative course was uneventful. A repeat CT taken 11 mo after surgery showed enhanced inferior bile duct wall and a slightly enhanced tumor measuring 1.6 cm in diameter within the duct (Figure 3A). Cholangioscopy revealed a papillary tumor in the remaining inferior bile duct (Figure 3B). Retrospective review of the CT images before the first surgery confirmed the lack of abnormal findings in the inferior bile duct on the arterial and portal venous phases. However, the enhanced inferior bile duct wall was detected only on the delayed phase (Figure 3C). Based on the clinical course and CT findings, the inferior bile duct tumor was suspected to have originally co-existed with the middle and superior bile duct tumor although it was not detected before the first surgery. Pancreaticoduodenectomy was performed as the second surgery. Histopathological examination showed a papillary adenocarcinoma with expansive growth (Figure 4), and a negative distal ductal margin. Postoperatively, the patient received adjuvant chemotherapy with S-1. However, multiple liver metastases were detected 10 mo after the second surgery. The patient received additional chemotherapy with gemcitabine for the recurrent metastases, but the metastatic foci showed aggressive growth, resulting in death at 31 mo after the first surgery (18 mo after the second surgery).
Figure 1.
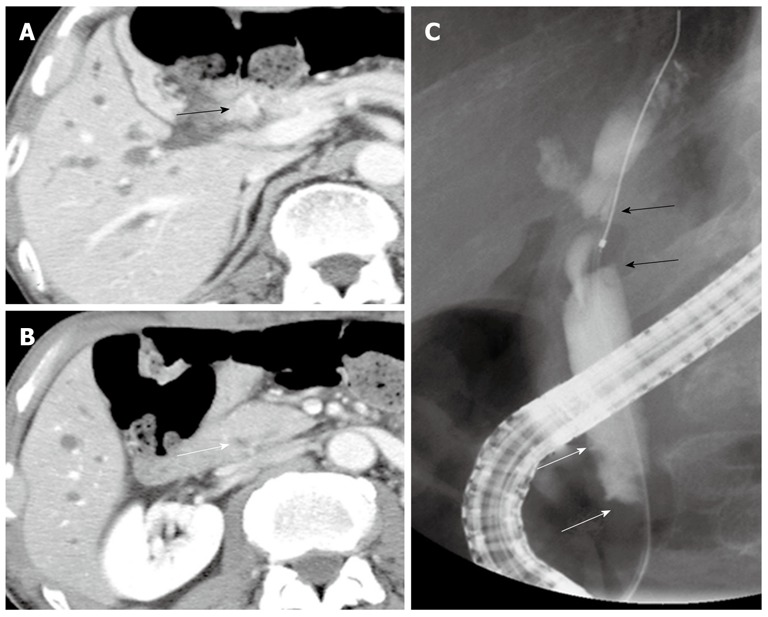
Imaging findings before the first surgery. A: Computed tomography showed a well enhanced mass in the middle and superior parts of the bile duct (black arrow); B: No mass was detected in the inferior part of the bile duct (white arrow); C: Endoscopic retrograde cholangiopancreatography revealed a tuberous filling defect in the middle and superior parts of the bile duct (black arrows) and mild stenosis in the inferior part (white arrows).
Figure 2.
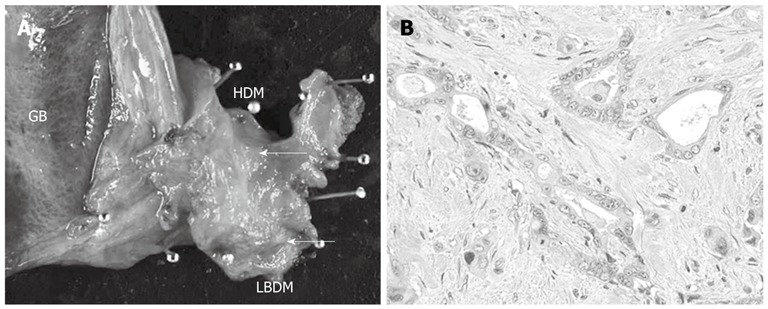
Macroscopic and microscopic findings of resected specimen at the first surgery. A: Resected specimen of the extra-hepatic bile duct showed whitish tuberous tumor in the middle and inferior parts of the bile duct (cancer marked by the white arrows); B: Microscopically, the tumor was moderately differentiated tubular adenocarcinoma with invasive growth (hematoxylin-eosin; magnification; × 100). GB: Gallbladder; HDM: Hepatic duct margin; LBDM: Lower bile duct margin.
Figure 3.
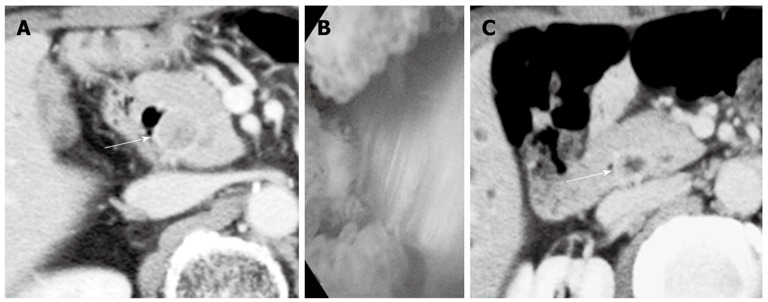
Imaging findings before the second surgery and retrospectively reviewed computed tomography finding before the first surgery. A: Computed tomography (CT) taken 11 mo after the first surgery showed enhanced inferior bile duct wall (white arrow) and slightly enhanced tumor within the duct; B: Cholangioscopy revealed a papillary tumor in the remaining inferior bile duct; C: Retrospective review of the CT images before the first surgery revealed enhanced inferior bile duct wall (white arrow) only on the delayed phase.
Figure 4.
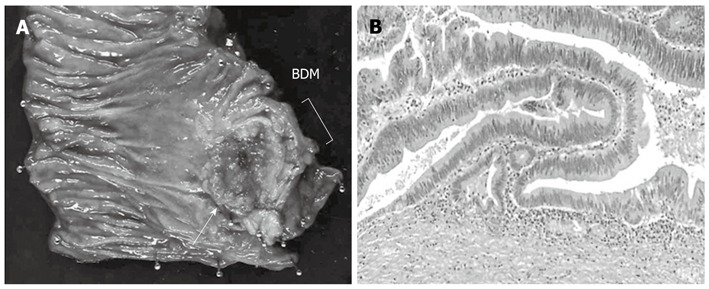
Macroscopic and microscopic findings of resected specimen at the second surgery. A: Specimen resected during pancreaticoduodenectomy. Note the papillary tumor in the inferior bile duct (white arrow); B: Microscopic examination showed papillary adenocarcinoma with expansive growth (hematoxylin-eosin, magnification; × 100). Tumor cells were confined within the fibromuscular coat. BDM: Bile duct margin.
DISCUSSION
Synchronous double cancers in the biliary system are rare. Most of the reported cases are double cancers of the CBD and the gallbladder associated with PBM[1-3]. Fujii et al[6] reported that 62.5% of synchronous double cancers and 100% of metachronous double cancers of the biliary tract were associated with PBM. Biliary cancers with PBM are thought to develop multicentrally, due to the effect of pancreatic juice reflux on the mucosa of the biliary tract[7]. Our patient had synchronous double cancers of the middle and superior bile ducts and the inferior bile duct. Although ERCP before the first surgery revealed a mild stenosis in the inferior part of the bile duct, no mass was detected on CT, and the diagnosis was a single bile duct cancer. However, retrospective review of the first CT images identified enhanced inferior bile duct wall only on the delayed phase, and the site of the enhanced wall was identical to the site of the inferior bile duct tumor detected on the CT eleven months after the first surgery. These imaging findings and the clinical course indicate that the inferior bile duct tumor originally co-existed with the tumor identified in the middle and superior bile duct. We believe that other tests such as endoscopic ultrasonography or cholangioscopy should have been conducted before the first surgery, considering the finding of ERCP. As an extremely rare entity, synchronous double cancers of the CBD without PBM can exist. When a tumor is suspected in the biliary tract, careful and meticulous preoperative assessment is necessary.
A search of the PubMed database identified only two reports of synchronous double cancers of the CBD without PBM in the English-language literature[4,5]. With regard to cases of biliary cancer without PBM, the presence of synchronous double tumors poses the question of whether they are independent primary tumors or one tumor that have metastasized from a single tumor. Differentiation between these events is important since these two origins imply different stage of the disease, as well as different subsequent treatment and prognosis. In their case report, Ogawa et al[6] described synchronous double cancers of the superior and middle bile duct and inferior bile duct. The upper cancer was pathologically diagnosed as poorly differentiated adenocarcinoma, while the lower one was moderately differentiated adenocarcinoma. In their case, the upper cancer was considered a metastasis from the lower one, based on genetic analysis of loss of heterozygosity[8,9]. Bedoui et al[5] reported another case of synchronous double cancers of the middle bile duct and inferior bile duct, and both cancers were pathologically diagnosed as adenocarcinomas. That case was preoperatively diagnosed as a single bile duct cancer, similar to the present case. The diagnosis of another inferior bile duct tumor was made during surgery. In their case, there was no communication in either the mucosal layer or the subepithelial layer between the two cancers, and the two tumors were thought to be primary. In our case, the two cancers were separate entities with different histopathological diagnosis, suggesting that they were two primary tumors.
Footnotes
Peer reviewer: Kyu Taek Lee, MD, PhD, Professor, Department of Medicine Samsung Medical Center, Sungkyunkwan, University School of Medicine, No.50 Irwon-dong, Gangnam-gu, Seoul 135-710, South Korea
S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Takayashiki T, Miyazaki M, Kato A, Ito H, Nakagawa K, Ambiru S, Shimizu H, Furuya S, Nakajima N. Double cancer of gallbladder and bile duct associated with anomalous junction of the pancreaticobiliary ductal system. Hepatogastroenterology. 2002;49:109–112. [PubMed] [Google Scholar]
- 2.Kamisawa T, Takuma K, Itokawa F, Itoi T. Endoscopic diagnosis of pancreaticobiliary maljunction. World J Gastrointest Endosc. 2011;3:1–5. doi: 10.4253/wjge.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasuya K, Nagakawa Y, Matsudo T, Ozawa T, Tsuchida A, Aoki T, Itoi T, Itokawa F. p53 gene mutation and p53 protein overexpression in a patient with simultaneous double cancer of the gallbladder and bile duct associated with pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 2009;16:376–381. doi: 10.1007/s00534-008-0030-1. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa A, Sugo H, Takamori S, Kojima K, Fukasawa M, Beppu T, Futagawa S, Fujii H. Double cancers in the common bile duct: molecular genetic findings with an analysis of LOH. J Hepatobiliary Pancreat Surg. 2001;8:374–378. doi: 10.1007/s005340170011. [DOI] [PubMed] [Google Scholar]
- 5.Bedoui R, Ajmi M, Nouira R, Dziri C. Synchronous double cancer of the common bile duct. Am J Surg. 2011;201:e1–e2. doi: 10.1016/j.amjsurg.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Fujii T, Kaneko T, Sugimoto H, Okochi O, Inoue S, Takeda S, Nagasaka T, Nakao A. Metachronous double cancer of the gallbladder and common bile duct. J Hepatobiliary Pancreat Surg. 2004;11:280–285. doi: 10.1007/s00534-003-0880-5. [DOI] [PubMed] [Google Scholar]
- 7.Hori H, Ajiki T, Fujita T, Okazaki T, Suzuki Y, Kuroda Y, Fujimori T. Double cancer of gall bladder and bile duct not associated with anomalous junction of the pancreaticobiliary duct system. Jpn J Clin Oncol. 2006;36:638–642. doi: 10.1093/jjco/hyl077. [DOI] [PubMed] [Google Scholar]
- 8.Wee A, Teh M, Raju GC. Clinical importance of p53 protein in gall bladder carcinoma and its precursor lesions. J Clin Pathol. 1994;47:453–456. doi: 10.1136/jcp.47.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe M, Asaka M, Tanaka J, Kurosawa M, Kasai M, Miyazaki T. Point mutation of K-ras gene codon 12 in biliary tract tumors. Gastroenterology. 1994;107:1147–1153. doi: 10.1016/0016-5085(94)90240-2. [DOI] [PubMed] [Google Scholar]


