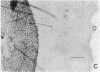
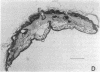
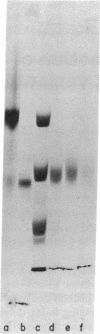
Abstract
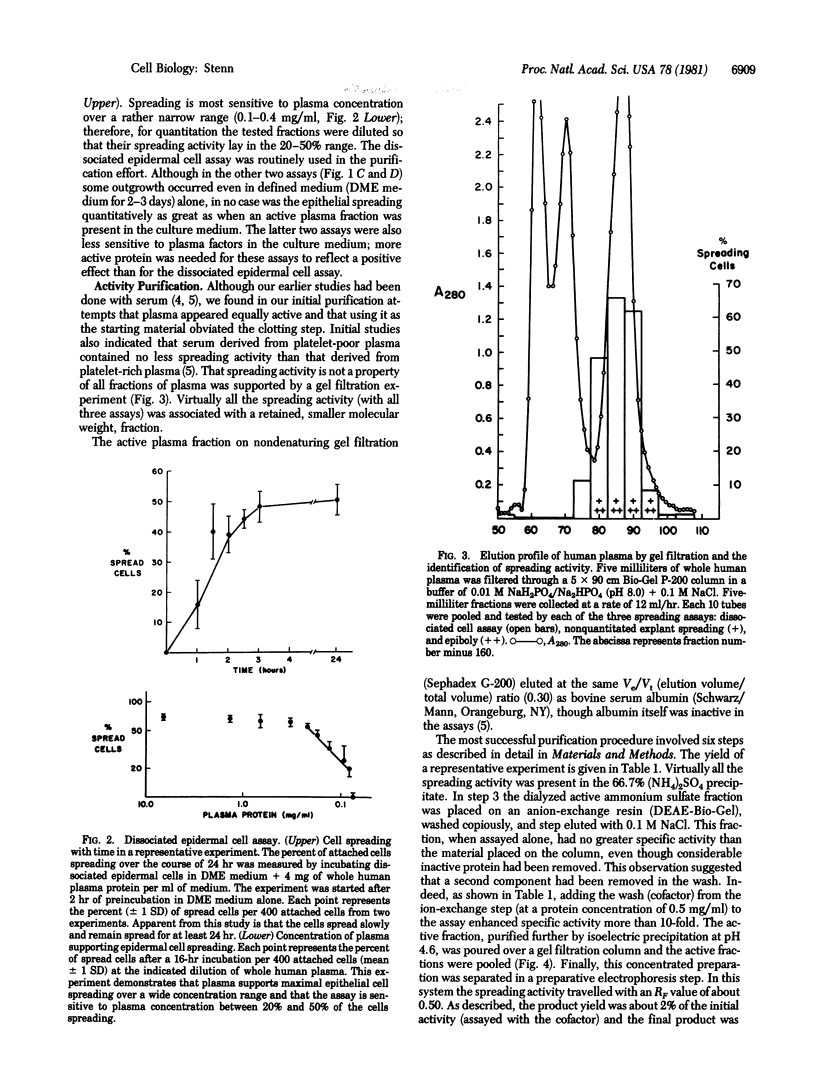
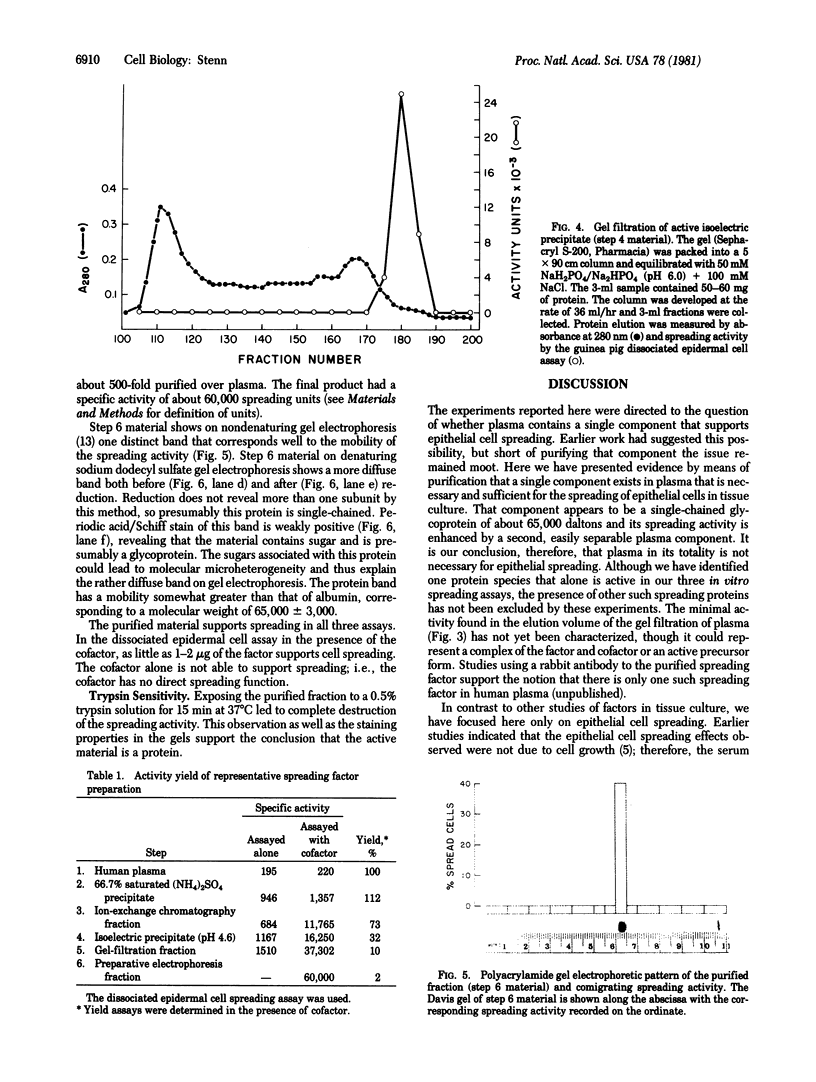
Earlier studies suggested that there is a specific activity in mammalian serum and plasma that supports epidermal (epithelial) cell movement. This activity was shown to be nondialyzable and heat labile. In the studies reported here, using standard biochemical procedures--i.e., ammonium sulfate fractionation, ion-exchange and gel filtration chromatography, isoelectric precipitation, and preparative polyacrylamide gel electrophoresis--we have purified a factor from human plasma that supports epidermal cell movement. The factor travels as an apparent single band on disc gel electrophoresis and corresponds to a glycosylated single-chain protein of approximately 65,000 +/- 3,000 daltons. The purified fraction is necessary and sufficient for dissociated epidermal cells, (ii) outgrowth of epithelial sheets from skin explants, and (iii) epiboly, epithelial sheet movement over a floating skin explant. The purified fraction is active at a concentration of 1-2 micrograms/ml of growth medium. It is destroyed by trypsin and its activity is augmented more than 10-fold by a second, as yet unpurified, fraction of plasma. These studies support the notion that a single protein of plasma supports epidermal cell movement and that this protein may play an important role in wound closure. Because it supports epiboly, the most biologically relevant of the assays, it has been named epibolin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bürk R. R. A factor from a transformed cell line that affects cell migration. Proc Natl Acad Sci U S A. 1973 Feb;70(2):369–372. doi: 10.1073/pnas.70.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürk R. R. Induction of cell proliferation by a migration factor released from a transformed cell line. Exp Cell Res. 1976 Sep;101(2):293–298. doi: 10.1016/0014-4827(76)90380-3. [DOI] [PubMed] [Google Scholar]
- Coombs V., Nissen B. K., Marks R. The epidermal cell migration promoting activity of serum in guinea pig skin in vitro. Arch Dermatol Forsch. 1974 Jun 11;249(4):367–372. doi: 10.1007/BF00557897. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Doly J., Petek F. Polyacrylamide gradient electrophoresis for protein purification on the milligram scale. J Chromatogr. 1977 Jul 11;137(1):69–81. doi: 10.1016/s0021-9673(00)89242-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Federgreen W., Stenn K. S. Fibronectin (LETS) does not support epithelial cell spreading. J Invest Dermatol. 1980 Sep;75(3):261–263. doi: 10.1111/1523-1747.ep12523292. [DOI] [PubMed] [Google Scholar]
- Gibbins J. R. Epithelial migration in organ culture. Effect of incubation in unsupplemented balanced salt solution. Exp Cell Res. 1976 Jul;100(2):374–382. doi: 10.1016/0014-4827(76)90161-0. [DOI] [PubMed] [Google Scholar]
- Hewitt A. T., Kleinman H. K., Pennypacker J. P., Martin G. R. Identification of an adhesion factor for chondrocytes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):385–388. doi: 10.1073/pnas.77.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox P., Griffiths S. A cell spreading factor in human serum that is not cold-insoluble globulin. Exp Cell Res. 1979 Oct 15;123(2):421–424. doi: 10.1016/0014-4827(79)90492-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Skeel A. A serum protein that stimulates macrophage movement, chemotaxis and spreading. Exp Cell Res. 1976 Oct 15;102(2):434–438. doi: 10.1016/0014-4827(76)90065-3. [DOI] [PubMed] [Google Scholar]
- Lipton A., Klinger I., Paul D., Holley R. W. Migration of mouse 3T3 fibroblasts in response to a serum factor. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2799–2801. doi: 10.1073/pnas.68.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrani E., Marks R. Towards characterization of epidermal cell migration promotion activity in serum. Br J Dermatol. 1978 Nov;99(5):513–518. doi: 10.1111/j.1365-2133.1978.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Ooka H., Yamamoto K., Okuma Y., Suga S., Wakasugi M. The migratory activity of rat epidermal cells in vitro--age-related changes and the effect of serum. Exp Gerontol. 1975 Feb;10(1):79–83. doi: 10.1016/0531-5565(75)90017-0. [DOI] [PubMed] [Google Scholar]
- Stenn K. S., Blout E. R. Mechanism of bovine prothrombin activation by an insoluble preparation of bovine factor X a (thrombokinase). Biochemistry. 1972 Nov 21;11(24):4502–4515. doi: 10.1021/bi00774a012. [DOI] [PubMed] [Google Scholar]
- Stenn K. S., Dvoretzky I. Human serum and epithelial spread in tissue culture. Arch Dermatol Res. 1979 Feb 23;264(1):3–15. doi: 10.1007/BF00417274. [DOI] [PubMed] [Google Scholar]
- Stenn K. S., Madri J. A., Roll F. J. Migrating epidermis produces AB2 collagen and requires continual collagen synthesis for movement. Nature. 1979 Jan 18;277(5693):229–232. doi: 10.1038/277229a0. [DOI] [PubMed] [Google Scholar]
- Stenn K. S. The role of serum in the epithelial outgrowth of mouse skin explants. Br J Dermatol. 1978 Apr;98(4):411–416. doi: 10.1111/j.1365-2133.1978.tb06534.x. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M. Mechanisms of morphogenesis in cell cultures. Int Rev Cytol. 1977;50:159–274. doi: 10.1016/s0074-7696(08)60099-6. [DOI] [PubMed] [Google Scholar]
- Wall R. T., Harker L. A., Striker G. E. Human endothelial cell migration: stimulation by a released platelet factor. Lab Invest. 1978 Nov;39(5):523–529. [PubMed] [Google Scholar]
- Whateley J. G., Knox P. Isolation of a serum component that stimulates the spreading of cells in culture. Biochem J. 1980 Feb 1;185(2):349–354. doi: 10.1042/bj1850349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf L., Lipton A. Studies on serum stimulation of mouse fibroblast migration. Exp Cell Res. 1973 Aug;80(2):499–502. doi: 10.1016/0014-4827(73)90334-0. [DOI] [PubMed] [Google Scholar]
- Zetter B. R. Migration of capillary endothelial cells is stimulated by tumour-derived factors. Nature. 1980 May 1;285(5759):41–43. doi: 10.1038/285041a0. [DOI] [PubMed] [Google Scholar]