Abstract
The functional end products of the extant biosynthesis of tetrapyrrole macrocycles in photosynthetic organisms are hydrophobic: chlorophylls and bacteriochlorophylls. A model for the possible prebiogenesis of hydrophobic analogues of nature's photosynthetic pigments was investigated by reaction of acyclic reactants in five media: aqueous solution (pH 7, 60°C, 24 h); aqueous solution containing 0.1 M decanoic acid (which forms a turbid suspension of vesicles); or aqueous solution accompanied by dodecane, mesitylene, or a five-component organic mixture (each of which forms a phase-separated organic layer). The organic mixture was composed of equimolar quantities of decanoic acid, dodecane, mesitylene, naphthalene, and pentyl acetate. The reaction of 1,5-dimethoxy-3-methylpentan-2,4-dione and 1-aminobutan-2-one to give etioporphyrinogens was enhanced in the presence of decanoic acid, affording (following chemical oxidation) etioporphyrins (tetraethyltetramethylporphyrins) in yields of 1.4–10.8% across the concentration range of 3.75–120 mM. The yield of etioporphyrins was greater in the presence of the five-component organic mixture (6.6% at 120 mM) versus that with dodecane or mesitylene (2.1% or 2.9%, respectively). The reaction in aqueous solution with no added oil-slick constituents resulted in phase separation—where the organic reactants themselves form an upper organic layer—and the yield of etioporphyrins was 0.5–2.6%. Analogous reactions leading to uroporphyrins (hydrophilic, eight carboxylic acids) or coproporphyrins (four carboxylic acids) were unaffected by the presence of decanoic acid or dodecane, and all yields were at most ∼2% or ∼8%, respectively. Taken together, the results indicate a facile means for the formation of highly hydrophobic constituents of potential value for prebiotic photosynthesis. Key Words: Origin of life—Prebiotic—Oil slick—Porphyrinogen—Porphyrin—Pyrrole—Partition. Astrobiology 12, 1055–1068.
1. Introduction
The chemical steps that led to the origin of life remain obscure in part due to uncertain conditions and composition of early Earth. It has been suggested that the primitive ocean could have been covered with an oil slick (Lasaga et al., 1971; Cleaves and Miller, 1998; Nilson, 2002). Such an oil slick could have acted as a host for prebiotic compounds, as well as provided strong protection to underlying constituents from solar ultraviolet light (Lasaga et al., 1971; Cleaves and Miller, 1998; Nilson, 2002). The presence of a layer of surfactant at the ocean surface also has been regarded as essential for the formation of aerosol particles containing inverted micellar assemblies (Dobson et al., 2000; Tuck, 2002). Such assemblies could contain diverse functional molecules of importance in prebiotic chemistry. Thus, a primordial oil slick might have played a major role in the origin and development of life on Earth.
A variety of mechanisms have been proffered for formation of a primordial oil slick. In a predominantly methane-nitrogen atmosphere, solar ultraviolet radiation would yield methyl and methylene radicals which upon combination form heavier hydrocarbons. The latter could accumulate to give an oil slick from 1 to 10 m thick (Lasaga et al., 1971; Cleaves and Miller, 1998; Nilson, 2002). An oil slick also could be produced from carbon-rich meteorites containing a variety of extraterrestrial organic molecules, including low-molecular-weight hydrocarbons (Cleaves and Miller, 1998; Sephton, 2002). In this regard, short-chain monocarboxylic acids (Lawless and Yuen, 1979; Huang et al., 2005) and aromatic molecules such as alkylbenzenes and naphthalenes (Komiya and Shimoyama, 1996; Sephton, 2002) have been found in organic material extracted from the Murchison carbonaceous meteorite.
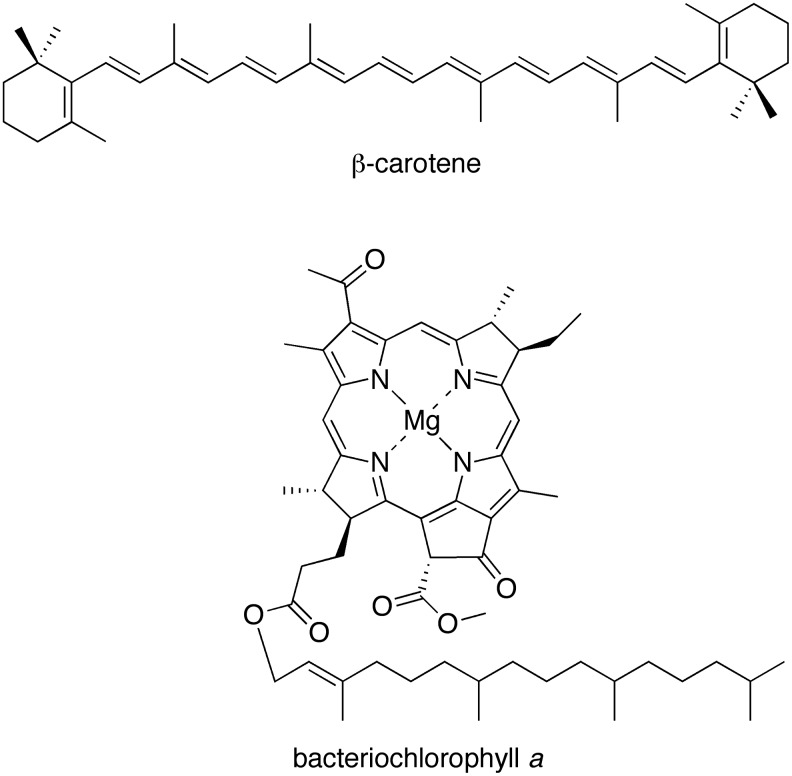
The presence of a primordial oil slick could not only result in accumulation of hydrophobic molecules but also provide a cloistered environment for their formation and subsequent transformation. Such hydrophobic compounds also would be expected to localize in surfactant assemblies including lipid bilayers. The lipid bilayer is characterized by a profound change in static dielectric constant in traversing from the bulk aqueous medium (∼78) to the core of the membrane (∼2.1) (Tien, 1974; Coster, 2003). Indeed, the pigments of modern photosynthesis, which are embedded in membrane-spanning proteins, typically are quite hydrophobic. Examples from photosynthetic bacteria include bacteriochlorophylls and carotenes (Scheer, 2003, 2006). The structures of bacteriochlorophyll a and β-carotene are shown in Scheme 1.
SCHEME 1.
Hydrophobic, functional constituents of extant photosynthesis.
Proposals for tetrapyrrole pigments in early photosynthesis have chiefly focused on macrocycles that resemble those produced via the modern biosynthetic pathway or are derivatives thereof. Examples include the following:
Uroporphyrins, of which uroporphyrin III is derived from the biosynthetic intermediate uroporphyrinogen III. Each uroporphyrin is water-soluble due to the presence of the eight carboxylic acid substituents. Indeed, uroporphyrins and uroporphyrinogens are envisaged as functioning as photocatalysts in aqueous solution (Mercer-Smith and Mauzerall, 1984; Mercer-Smith et al., 1985).
Coproporphyrins, of which coproporphyrin III is the unsaturated analogue of the biosynthetic intermediate coproporphyrinogen III. Coproporphyrins contain only four carboxylic acid groups and are envisaged as functioning as photocatalysts upon complexation with other entities (Mercer-Smith et al., 1985).
Protoporphyrin IX, which is the last common precursor to heme and chlorophylls. Protoporphyrin IX is amphiphilic due to the presence of two carboxylic acid substituents on one edge of the molecule, and is envisaged as functioning in membrane assemblies (Olson and Pierson, 1987; Sivash et al., 1991).
Octaethylporphyrin, an abiotic compound that is quite hydrophobic. Octaethylporphyrin has been investigated in a variety of membrane-based photochemical processes, and the magnesium chelate thereof has been shown to enable light-driven transmembrane proton pumping (Sun and Mauzerall, 1996; Mauzerall and Sun, 2003).
While the photofunctional properties of tetrapyrrole macrocycles would be manifested in a photic world, the intrinsic structural features of tetrapyrroles might prove beneficial in other ways. Indeed, hydrophobic aromatic macrocycles have been proposed to have played a variety of roles in the origin of life, including as scaffolding for organization of nucleic acids (Hud and Anet, 2000) and for assembly of functional containers (Ehrenfreund et al., 2006).
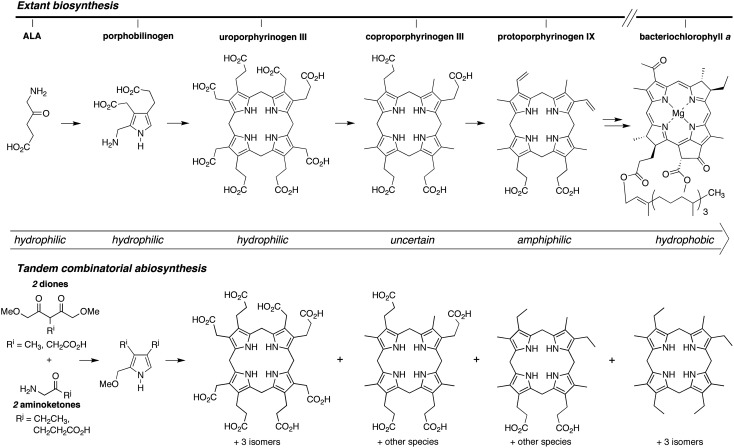
A key feature of the extant biosynthesis of tetrapyrrole macrocycles is the increasing hydrophobicity in proceeding along the pathway from the starting material δ-aminolevulinic acid (ALA) toward functional end products (Mauzerall, 1976, 1998). This progression is illustrated in Scheme 2. Uroporphyrinogen III contains eight carboxylic acids. Decarboxylation of each acetic acid moiety affords coproporphyrinogen III. Decarboxylation of two propionic acid moieties accompanied by dehydrogenation affords the divinyl-diacid protoporphyrinogen IX. The chlorophylls and bacteriochlorophylls are quite hydrophobic. While the porphyrinogen intermediates of the extant pathway may have provided important functional roles in prebiotic chemistry, only the quite advanced biosynthetic products are employed in extant biochemistry.
SCHEME 2.
The stepwise extant biosynthetic formation of tetrapyrrole macrocycles is accompanied by a steady increase in hydrophobicity (top). An all-at-once tandem combinatorial (non-enzymic) process affords macrocycles encompassing the entire range of polarity (bottom). Each porphyrinogen can be oxidized to give the corresponding porphyrin (not shown).
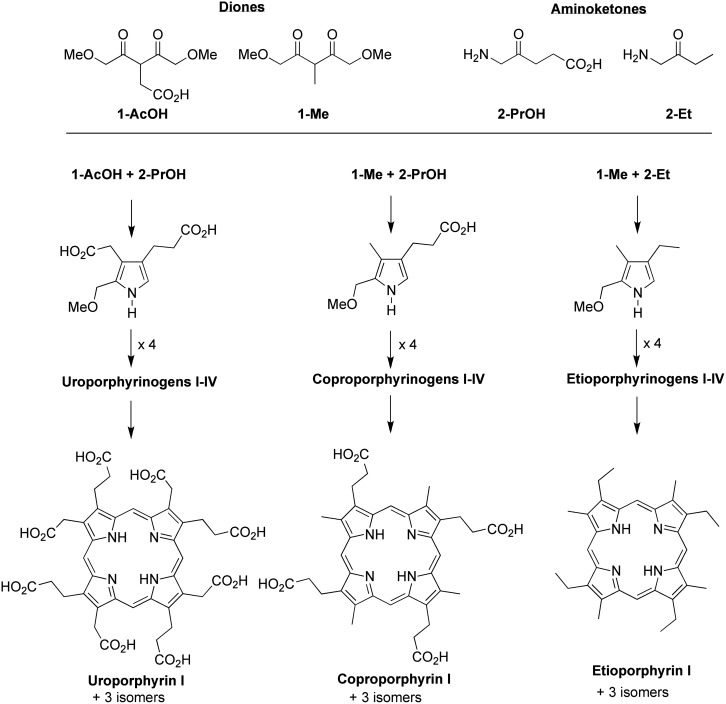
As part of a program to develop a chemical model for the possible prebiogenesis of tetrapyrrole macrocycles, we have been investigating reactions of relatively simple acyclic compounds that proceed via pyrroles to give porphyrinogens (Lindsey et al., 2009, 2011). Recently, we examined the three pairwise reactions of 1-AcOH + 2-PrOH, 1-Me + 2-PrOH, and 1-Me + 2-Et (one dione and one aminoketone each), as are shown in Scheme 3. Each reaction was performed in aqueous solution in the presence or absence of the surfactant sodium dodecylsulfate (Taniguchi et al., 2012). The pairwise reactions (upon chemical oxidation) give the well-known uroporphyrin, coproporphyrin, and etioporphyrin macrocycles, respectively, which represent three different classes of polarity. Uroporphyrins bear eight carboxylic acid substituents and are hydrophilic. Coproporphyrins, with four hydrophilic and four hydrophobic groups, can either aggregate in water or associate with a surfactant. Etioporphyrins have no carboxylic acid substituents, are hydrophobic, and are not expected to form in water in significant yields due to aggregation/precipitation of oligopyrromethane intermediates (Taniguchi et al., 2012).
SCHEME 3.
Three pairwise reactions of one dione and one aminoketone (1-AcOH + 2-PrOH, 1-Me + 2-PrOH, and 1-Me + 2-Et). Each reaction forms a pyrrole, which self-condenses to give porphyrinogens. Oxidation of the porphyrinogens affords the corresponding uroporphyrins, coproporphyrins, or etioporphyrins. One representative porphyrin (type-I isomer) from each pairwise reaction is shown.
A combinatorial process afforded a repertoire of molecules spanning the range of polarity, from uroporphyrinogens to etioporphyrinogens (Scheme 2). The compounds formed predominantly contained 2–6 carboxylic acid units, with relatively little of the porphyrins with extreme hydrophilicity (i.e., uroporphyrinogens) or hydrophobicity (i.e., etioporphyrinogens). Indeed, virtual library analysis (Taniguchi and Lindsey, 2012a, 2012b) indicated the fractional amount of such polar extremes was expected to be 0.0039 (of the total macrocycles) for each (Taniguchi et al., 2011). Thus, although the combinatorial process provided a route to hydrophobic molecules such as etioporphyrins, the expected quantity of such porphyrins would be minute. Otherwise, plausibly prebiotic pathways have heretofore not been demonstrated that directly afford tetrapyrrole analogues of the modern functional constituents of photosynthesis.
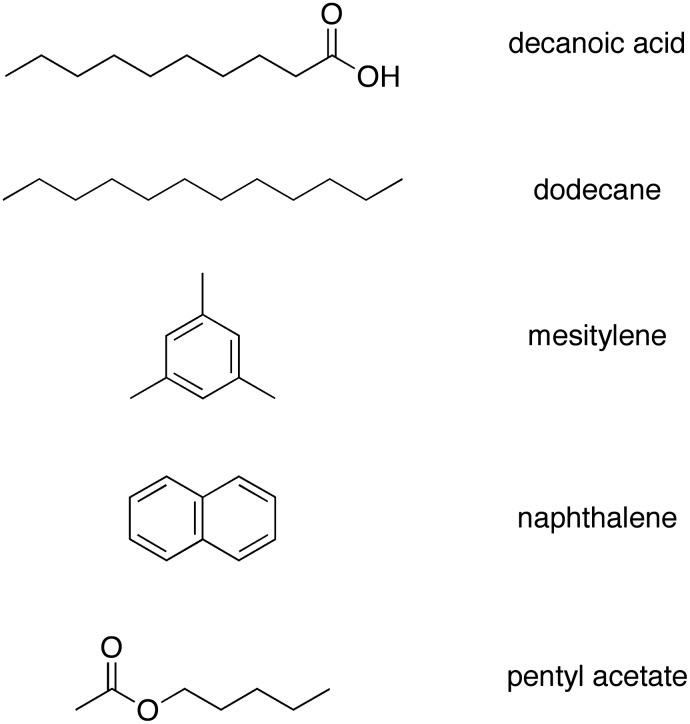
In this paper, the three pairwise reactions were carried out in aqueous solution at pH 7 in the presence or absence of vesicles composed of decanoic acid, and the presence or absence of the aliphatic hydrocarbon dodecane (which gave phase separation). In addition, the reaction forming etioporphyrinogens was examined in a biphasic medium composed of an aqueous solution at pH 7 and the aromatic hydrocarbon mesitylene (1,3,5-trimethylbenzene), as well as in a biphasic medium composed of an aqueous solution at pH 7 and a mixture of hydrocarbons (dodecane, decanoic acid, mesitylene, naphthalene, and pentyl acetate; in equimolar quantities). Our interest herein is to study the ability of an oil slick, formed by low-molecular-weight hydrocarbons, to accumulate and/or facilitate the formation of prebiotic compounds, and thereby provide suitable conditions for the development and origin of life.
2. Methods
2.1. Samples and solutions
δ-Aminolevulinic acid (ALA=2-PrOH; Sigma-Aldrich), etioporphyrin I (Frontier Scientific), and etioporphyrin III (Fisher Scientific) were used as received. 1-Aminobutan-2-one (2-Et) was prepared as described in the literature (Adam et al., 2004). The syntheses of 1,5-dimethoxy-3-methylpentan-2,4-dione (1-Me) and 5-methoxy-3-(methoxyacetyl)levulinic acid (1-AcOH) will be described elsewhere. The organic constituents (dodecane, decanoic acid, mesitylene, naphthalene, and pentyl acetate) were used as received from commercial suppliers.
Stock solutions of 1-AcOH (0.5 M), 1-Me (0.5 M), 2-PrOH (0.5 M), and 2-Et (0.5 M) were prepared in deionized water and stored in frozen form at −15°C. The phosphate buffer stock solution (2 M) was prepared at pH 7 by using potassium hydrogen phosphate (2 M) and potassium dihydrogen phosphate (2 M). The pH values were measured with a pH meter at room temperature (21°C) and are typically within±0.1 pH unit.
2.2. Preparation of decanoic acid vesicles
Decanoic acid (431 mg) was added to 4.5 mL of deionized water. Aqueous NaOH (280 μL, 10 M) was added to the solution in small aliquots, vortexing between additions, until the acid was completely dissolved (pH ∼11). Aqueous HCl (140 μL, 10 M) was then added in small aliquots to the transparent suspension, vortexing between additions, until pH 7 was reached. The volume of the resulting opalescent suspension was adjusted to 5 mL for a final decanoic acid concentration of 0.5 M.
2.3. Reactions
The reactions were carried out at 0.5 mL scale for 24 h at 60°C and pH 7, following experimental procedures described previously (Lindsey et al., 2011; Taniguchi et al., 2012). The 0.5 mL reaction mixtures were prepared by addition of aqueous-based constituents to give a final volume of 0.4 mL, and the volume of the organic “oil slick” constituent was 0.1 mL. The aqueous-based constituents comprised aliquots of 1-AcOH or 1-Me stock solutions, and 125 μL of 2 M potassium phosphate buffer (pH 7) stock solution to give a final buffer concentration of 0.5 M. (Thus, each instance herein where “aqueous solution” is stated refers to the 0.5 M buffered solution at pH 7.) The liquid reaction mixtures were deaerated by three alternating cycles of low vacuum/argon purges and subsequently were frozen. Aliquots of 2-PrOH or 2-Et stock solutions were then added on the open bench top to the frozen solutions containing all other components, thereby affording the final 0.4 mL of aqueous-based constituents. The reaction performed without any organic “oil slick” constituent employed 0.5 mL of the aqueous-based constituents.
The organic “oil slick” constituent comprised 100 μL of one of the following: decanoic acid (0.5 M), dodecane, mesitylene, or a five-component mixture of organic constituents (decanoic acid, dodecane, mesitylene, naphthalene, and pentyl acetate; each in equimolar quantities).
The samples were placed in an enclosed reaction chamber for investigating multiple reactions in parallel, which was evacuated under reduced pressure and then flushed with argon. The cycle was repeated three times. A slow flow of argon was passed continuously through the reaction chamber. None of the reactions was stirred. After 24 h, the crude reaction mixtures were sampled immediately (to determine the porphyrin yield) and subsequently were frozen.
2.4. Porphyrin yields
Porphyrin yield determinations were performed by oxidation of crude reaction mixtures with I2 directly in a cuvette, as described previously (Lindsey et al., 2011; Taniguchi et al., 2012).
(a) The procedure for uroporphyrin and coproporphyrin was as follows. A 50 μL aliquot of a reaction sample (30 mM of each reactant) was transferred to a dilution vial containing 150 μL of water (1/4 dilution). The solution was mixed briefly, and then 50 μL of this diluted sample was transferred to a cuvette containing 2.6 mL of 0.1 M aqueous HCl. Then the cuvette was treated with 56 μL of I2 (50 mM) in ethanol, followed by 56 μL of Na2S2O3 (0.2 M) in water. The absorption spectrum was acquired. The Soret band was very sharp, as expected in aqueous acid, with a full width at half-maximum (fwhm) of 11 nm (see Figs. S1 and S2 in the Supplementary Material, available online at www.liebertonline.com/ast). The yield was determined by using a molar absorption coefficient of 505,000 M−1 cm−1 at the Soret band maximum (Mauzerall, 1960; Taniguchi et al., 2012). For the reactions in the presence of dodecane (which afforded a colorless upper layer), the underlying aqueous layer was sampled, in which case the porphyrin yields were determined assuming a total volume of 0.4 mL.
(b) The procedure for etioporphyrin was as follows. The crude reaction mixture was diluted with water (1 mL), extracted with cyclohexanone (1 mL), and the organic layer was separated. For reaction at 30, 60, or 120 mM, an aliquot of 50, 25, or 12.5 μL, respectively, of the organic layer was transferred to a cuvette containing 2.6 mL of dimethylsulfoxide (DMSO). Then the cuvette was treated with 56 μL of 50 mM I2 in ethanol, followed by 56 μL of Na2S2O3 (0.2 M) in water. The absorption spectrum was acquired. The Soret band was quite broad (fwhm=41 nm), characteristic of octaalkylporphyrins in neutral aqueous solution or organic media (see Figs. S1 and S2 in the Supplementary Material). Accordingly, the yield was determined by using a molar absorption coefficient of 160,000 M−1 cm−1 at the Soret band maximum (Rimington, 1960). For reaction at 3.75, 7.5, or 15 mM, a 50 μL aliquot of the organic phase was transferred to a cuvette containing 2.0 mL of DMSO. The remainder of the procedure was identical.
2.5. Fluorescence emission analyses
For analysis of etioporphyrin by fluorescence spectroscopy, 50 μL of the reaction mixture (120 mM, pH 7, 60°C, 24 h, and 0.1 M decanoic acid) was treated with 56 μL of 50 mM I2 in ethanol followed by 56 μL of 0.2 M Na2S2O3 (in water). The resulting oxidized reaction mixture was treated with brine and extracted with cyclohexanone (Rimington and Benson, 1967). The organic phase was concentrated upon application of a stream of argon. The resulting crude porphyrin was dissolved in 1-propanol. Fluorescence measurements were performed as described previously (Soares et al., 2012).
2.6. Identification of porphyrin isomers by 1H NMR spectroscopy
For analysis of etioporphyrin isomers, the crude porphyrin extract and authentic samples of etioporphyrin I and etioporphyrin III were examined by proton nuclear magnetic resonance (1H NMR) spectroscopy. The crude porphyrin extract was obtained by treatment of the oxidized reaction mixture (120 mM, pH 7, 60°C, 24 h, and 0.1 M decanoic acid) with brine, extraction with cyclohexanone (Rimington and Benson, 1967), and concentration of the organic phase. The 1H NMR spectra were acquired in CS2 containing tetramethylsilane and benzene-d6 as reference and lock. Carbon disulfide is a very attractive solvent for 1H NMR spectroscopy in this case due to the high solubility afforded hydrophobic tetrapyrrole macrocycles, absence of protons, low viscosity, and low boiling point (Lee et al., 1995; Bothner-By et al., 1996).
2.7. Mass spectrometry measurements
For analysis of etioporphyrin by mass spectrometry, an aliquot (100 μL) of the crude mixture obtained from reaction of 1-Me + 2-Et (120 mM, pH 7, 60°C, 24 h, and 0.1 M decanoic acid) was treated with 300 μL of 50 mM I2 in ethanol followed by 300 μL of Na2S2O3 (200 mM) in water. The electrospray ionization mass spectrometry (ESI-MS) spectrum showed a molecular ion peak [m/z=479.3157, (M + H)+] consistent with expectations (calculated 479.3169 for the protonated molecule, where M=C32H38N4).
3. Results
3.1. Three porphyrins of distinct polarity
Each of the reactions (1-AcOH + 2-PrOH; 1-Me + 2-PrOH; 1-Me + 2-Et) was carried out under three conditions that differ only in the composition of the reaction medium. The reaction media include the following: (i) aqueous solution; (ii) aqueous solution in the presence of 0.1 M decanoic acid, a concentration greater than the minimum (40 mM) required for vesicle formation (Apel et al., 2002); and (iii) aqueous solution in the presence of a layer of dodecane. In addition, the reaction of 1-Me + 2-Et was carried out in (iv) aqueous solution in the presence of a layer of mesitylene, and (v) aqueous solution in the presence of the five-component mixture of hydrocarbons.
The chemical structures of all the hydrocarbons are depicted in Scheme 4. In each case, the aqueous solution contained potassium phosphate buffer (0.5 M, pH 7), and the reactions were allowed to proceed under anaerobic conditions for 24 h at 60°C. In each case, the aminoketone and the dione were employed in equimolar quantities. The static dielectric constant of each neat compound (in liquid form) is as follows: dodecane (2.002 at 30°C), mesitylene (2.279 at 20°C), naphthalene (2.54 at 80°C), pentyl acetate (4.75 at 20°C) (Riddick and Bunger, 1970). A value for decanoic acid was not identified. The polarity of these bulk solvents thus is comparable to that of the inner, hydrocarbon region of a lipid bilayer. The solubility of water in each is as follows: dodecane (65 ppm at 25°C), mesitylene (0.0291 wt % at 20°C), and pentyl acetate (1.15 wt % at 20°C) (Riddick and Bunger, 1970). Values for decanoic acid and naphthalene were not identified.
SCHEME 4.
Organic constituents employed in prototypical oil slicks.
To determine the yield of porphyrin, aliquots from the reaction mixtures affording uroporphyrinogens or coproporphyrinogens were diluted in 0.1 M HCl and treated with an ethanolic solution of I2. The I2 treatment converts the porphyrinogens to the corresponding porphyrins (Mauzerall and Granick, 1958). The yield of porphyrins was then determined by absorption spectroscopy. For the reactions to give etioporphyrinogens, the hydrophobicity of the macrocycles (i.e., lack of solubility in aqueous HCl) mandated an alternative analysis procedure. The crude reaction mixture was first extracted with cyclohexanone, then an aliquot of the cyclohexanone extract was dissolved in DMSO, and finally the DMSO solution was treated with an ethanolic solution of I2 followed by absorption spectroscopy.
The results for reactions at 30 mM are shown in Table 1. The yields for 1-Me + 2-Et (etioporphyrins) were modest in aqueous solution alone or in aqueous solution in the presence of dodecane or mesitylene (∼1%), but 6.0% in the presence of decanoic acid (entry 1). The reaction in aqueous solution in the presence of the 5-component organic mixture was 3.6%. A control reaction of 1-Me + 2-PrOH (coproporphyrins) afforded yields that were roughly invariant (7.1–7.6%, entry 2) regardless of the presence or absence of decanoic acid or dodecane. The reaction of 1-AcOH + 2-PrOH (uroporphyrins) in aqueous solution gave a yield nearly comparable to those in the presence of decanoic acid or dodecane (1.2–1.5%, entry 3).
Table 1.
Data for Pairwise Reactions of Diones (1-AcOH or 1-Me) and Aminoketones (2-PrOH or 2-Et)a
| |
|
Yield (%)e |
||||
|---|---|---|---|---|---|---|
| Entry | Porphyrins | Aqueous solution | Decanoic acidf | Dodecaneg | Mesityleneh | 5-comp. mixturei |
| 1 | etiob | 1.4 | 6.0 | 1.1 | 1.0 | 3.6 |
| 2 | coproc | 7.1 | 7.6 | 7.6 | naj | na |
| 3 | urod | 1.5 | 1.2 | 1.4 | na | na |
The pairwise reactions (30 mM for each reactant) were carried out for 24 h at 60°C in aqueous solution (pH 7) in the presence or absence of organic constituents (“oil slick”).
Etio denotes etioporphyrins from the reaction of 1-Me + 2-Et.
Copro denotes coproporphyrins from the reaction of 1-Me + 2-PrOH.
Uro denotes uroporphyrins from the reaction of 1-AcOH + 2-PrOH.
Porphyrin yields upon chemical oxidation (with I2).
Aqueous solution containing 0.1 M decanoic acid.
Aqueous solution (0.4 mL) and dodecane (0.1 mL).
Aqueous solution (0.4 mL) and mesitylene (0.1 mL).
Aqueous solution (0.4 mL) and the five-component organic mixture (0.1 mL).
Not available.
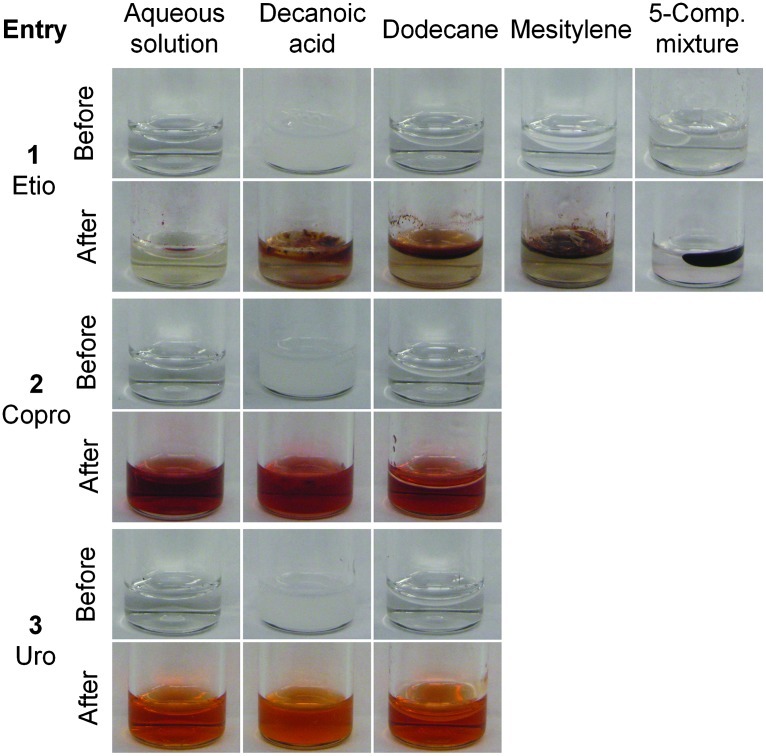
Photographs of the reaction mixtures are shown in Fig. 1. In each case prior to reaction, the aqueous solution was homogeneous, the decanoic acid–containing mixture was turbid, and the dodecane- or mesitylene-containing mixture was biphasic, with a lower aqueous phase and an upper organic layer. In the case of the five-component organic mixture, several phase-separated organic globules were observed at the top of the aqueous solution.
FIG. 1.
Photographs of 30 mM reactions of diones and aminoketones before and after 24 h of reaction in various media prior to chemical oxidation with I2. Entry 1: reactions of 1-Me + 2-Et (to give etioporphyrinogens). Entry 2: reactions of 1-Me + 2-PrOH (to give coproporphyrinogens). Entry 3: reactions of 1-AcOH + 2-PrOH (to give uroporphyrinogens). Etio, Copro, and Uro denote etioporphyrinogens, coproporphyrinogens, and uroporphyrinogens, respectively. The entry numbers in the figure correspond to those in Table 1.
The reaction to form etioporphyrinogens proceeded as follows: after 24 h at 60°C, the reaction in aqueous solution resulted in a red droplet on top of the aqueous solution. The reaction in the presence of decanoic acid afforded phase separation, with red color localized predominantly in the upper layer. The reaction in the presence of dodecane or mesitylene afforded a relatively colorless aqueous solution beneath a red organic layer. The reaction in the presence of the five-component organic mixture afforded a single red globule on top of the colorless aqueous solution.
The reactions to form uroporphyrinogens or coproporphyrinogens were quite different. The two reactions were identical in the composition of the mixture upon visual inspection: the reaction in aqueous solution afforded a red homogeneous solution; the reaction in the presence of decanoic acid afforded a red turbid suspension; and the reaction in the presence of dodecane afforded a red aqueous solution beneath a colorless layer of dodecane.
Each reaction begins with colorless acyclic starting materials, and if the corresponding pyrrole, oligopyrromethanes, and porphyrinogen are the sole species formed, the final reaction mixture should be colorless given that each such species absorbs only in the ultraviolet region (Lindsey et al., 1987; Lindsey and Wagner, 1989). Indeed, porphyrinogens (206 nm) (Mauzerall, 1962), ketones (∼280 nm) (Jaffé and Orchin, 1962), and diketones (275 nm) (Morton and Rosney, 1926) are not colored. The broad experience in tetrapyrrole chemistry is that reactions of pyrroles leading to tetrapyrrole macrocycles typically afford colored reaction mixtures; the color stems from diverse non-porphyrin substances and can include some amount of porphyrin (Lindsey et al., 1987, 1994; Lindsey and Wagner, 1989; Li et al., 1997; Lindsey, 2000; Geier and Lindsey, 2001). The non-porphyrin substances include molecules that contain dipyrrin chromophores, which can form via oxidation or tautomerization processes (Lindsey and Wagner, 1989), as well as tripyrrins and bilins (Moss, 1987). The 2e−/2H+ dehydrogenation of a dipyrromethane unit gives the corresponding dipyrrin, whereas the 6e−/6H+ dehydrogenation of a porphyrinogen or bilane gives the corresponding porphyrin or bilin, respectively. The facile dehydrogenation under ostensibly anaerobic conditions reflects the electron-richness of the trialkyl- or tetraalkyl-pyrrole constituents, the deep thermodynamic well presented by the porphyrin relative to the porphyrinogen, and the presence in the reaction mixture of entities that function as mild oxidants, such as ketones and azafulvene moieties. In summary, the color of the reaction mixtures observed herein signals the presence of diverse reaction products, which can include intermediates along the path to tetrapyrrole macrocycles, by-products, and porphyrins.
3.2. Etioporphyrin formation across a wide concentration range
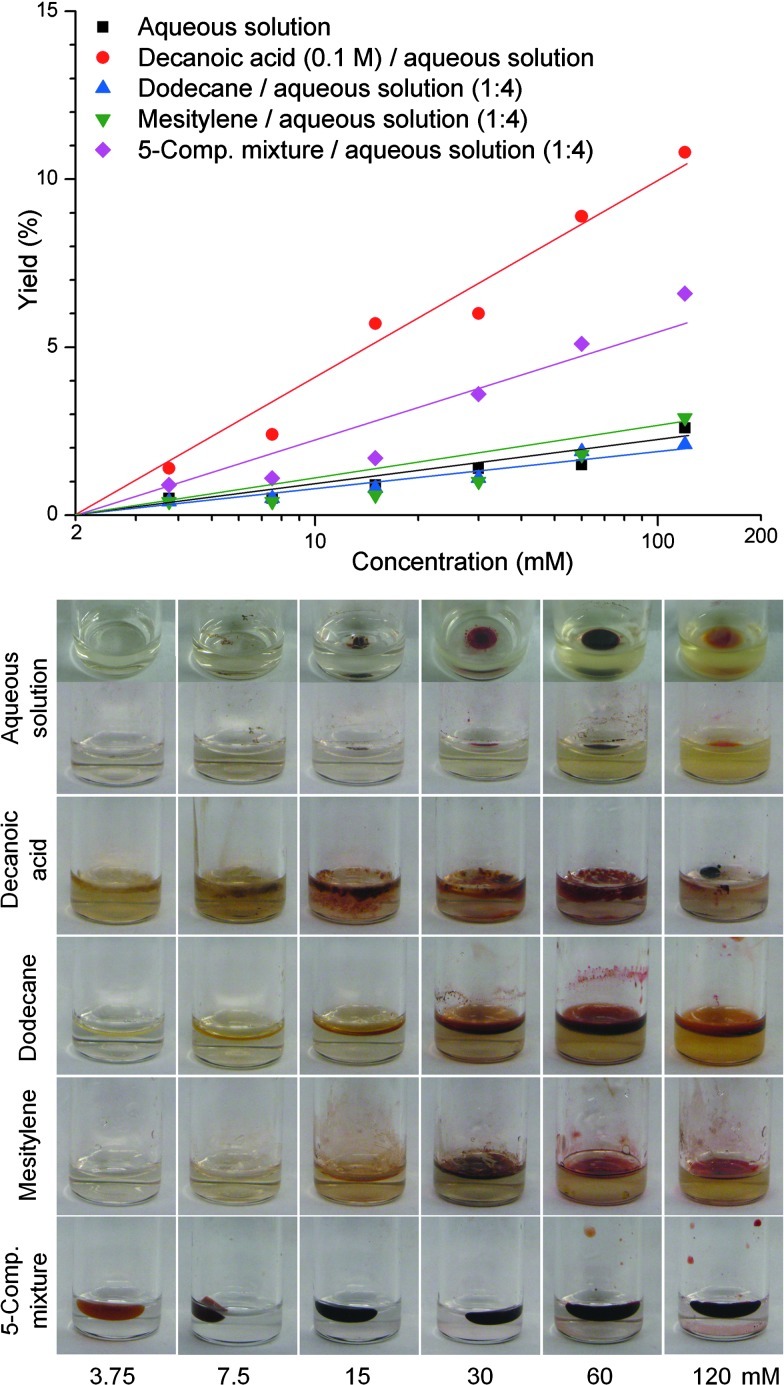
The reaction of 1-Me + 2-Et also was examined at lower and higher concentrations (from 3.75 to 120 mM) than the 30 mM concentration employed above. The results are displayed in Fig. 2. The numerical data corresponding to the figure are provided in the Supplementary Material (Table S1). Five distinct media were employed:
In aqueous solution, the yield increased from 0.5% to 2.6% over this concentration range. As the concentration increased, the size of a phase-separated red droplet increased, suggesting that the reaction largely occurred in the phase-separated layer (see photos).
In the presence of dodecane or mesitylene, the yield increased from 0.4% to 2.9% over this concentration range. The extent of coloration of the dodecane or mesitylene layer increased with increasing reaction concentration.
In the presence of decanoic acid, however, the yield increased from 1.4% to 10.8% over the 3.75–120 mM concentration range. A droplet was formed at the highest concentration of reactants (120 mM reaction), whereas a precipitate was observed for the 3.75–60 mM reactions.
In the presence of the five-component organic mixture, the phase-separated organic globules coalesced to give one globule, which became increasingly colored as the concentration increased. The yield of the reaction increased from 0.9% to 6.6% over the concentration range examined.
FIG. 2.
Top: Yields as a function of reactant concentration for the reaction of 1-Me + 2-Et (to give etioporphyrinogens) carried out for 24 h at 60°C in aqueous solution (pH 7) in the presence or absence of organic constituents (“oil slick”), followed by workup and oxidation with I2. The reaction media included aqueous solution (black squares), aqueous solution containing 0.1 M decanoic acid (red circles), dodecane/aqueous solution (1:4 v/v; blue upright triangles), mesitylene/aqueous solution (1:4 v/v; green inverted triangles), and the five-component organic mixture/aqueous solution (1:4 v/v; magenta diamonds). The lines are provided to guide the eye. Bottom: Photographs of the reaction mixtures (3.75, 7.5, 15, 30, 60, and 120 mM) immediately prior to workup and oxidation with I2. Photos of the reactions in aqueous solution (upper row) are provided in both side and top views for clarity.
3.3. Characterization of etioporphyrin
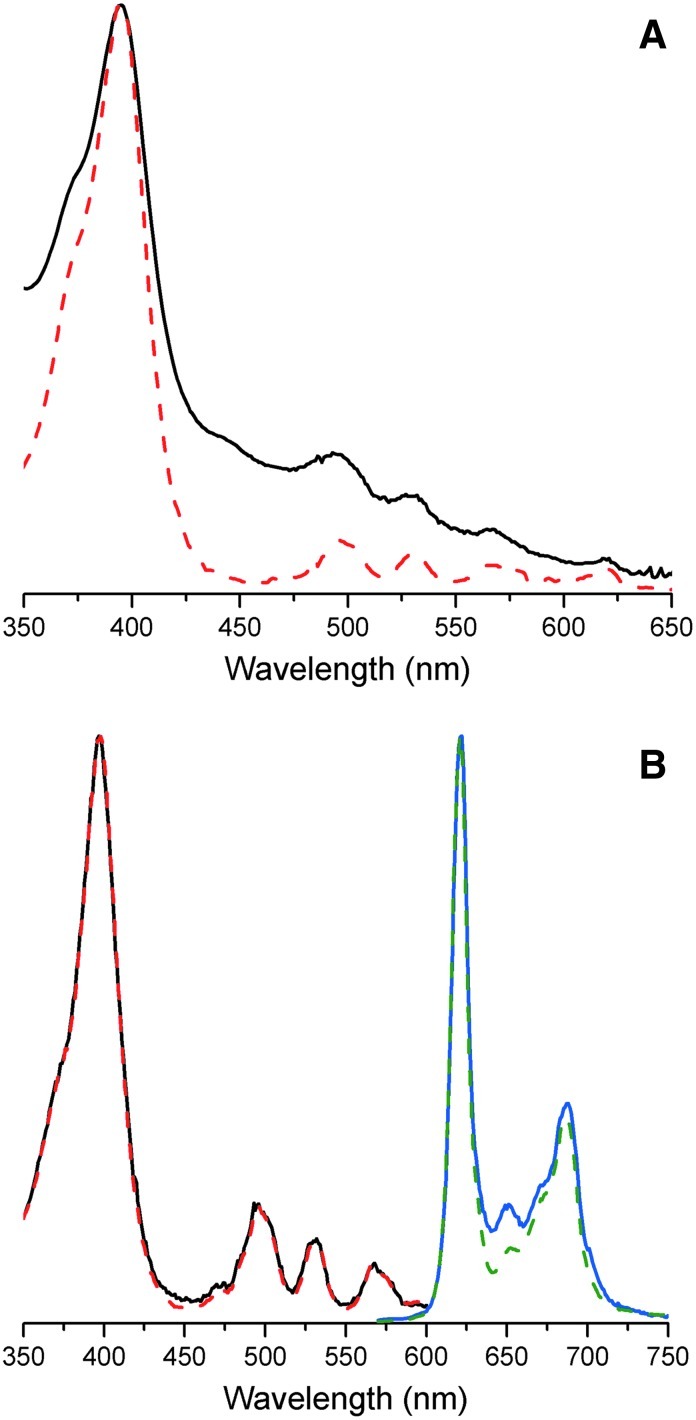
The pairwise reactions of 1-AcOH + 2-PrOH (to form uroporphyrinogens) and of 1-Me + 2-PrOH (to form coproporphyrinogens) carried out in aqueous solution have been analyzed previously (Lindsey et al., 2011), as has the reaction of 1-Me + 2-Et (to form etioporphyrinogens) in the presence of surfactants (Taniguchi et al., 2012). Prior evidence in support of the porphyrinogens and, upon oxidation, the porphyrins was obtained by high-resolution ESI-MS, absorption spectroscopy, and fluorescence spectroscopy. Here, a crude etioporphyrin sample was obtained upon oxidation of a sample from the reaction of 1-Me and 2-Et (120 mM each reactant, pH 7, 60°C, 24 h) in the presence of 0.1 M decanoic acid. The crude etioporphyrin sample was dissolved in 1-propanol and examined by absorption spectroscopy (panel A, Fig. 3). The spectrum exhibited characteristic peaks consistent with an authentic sample of etioporphyrin I; additional nonspecific absorption observed across the visible region of the crude sample is characteristic of bilin and related chromophores. The fluorescence emission spectrum and fluorescence excitation spectrum also were obtained of the crude etioporphyrin sample. The fluorescence spectra of the crude sample were almost identical to those of an authentic sample of etioporphyrin I (panel B, Fig. 3).
FIG. 3.
Characterization data for etioporphyrin obtained from the reaction of 1-Me and 2-Et (120 mM each reactant, pH 7, 60°C, 24 h) in the presence of 0.1 M decanoic acid. (A) Absorption spectra in 1-propanol of the crude etioporphyrin sample (solid black line) and authentic sample of etioporphyrin I (dashed red line). (B) Fluorescence excitation (λem 690 nm) and emission (λex 395 nm) spectra in 1-propanol of the crude etioporphyrin sample (solid lines) compared with those of an authentic sample of etioporphyrin I (dashed lines).
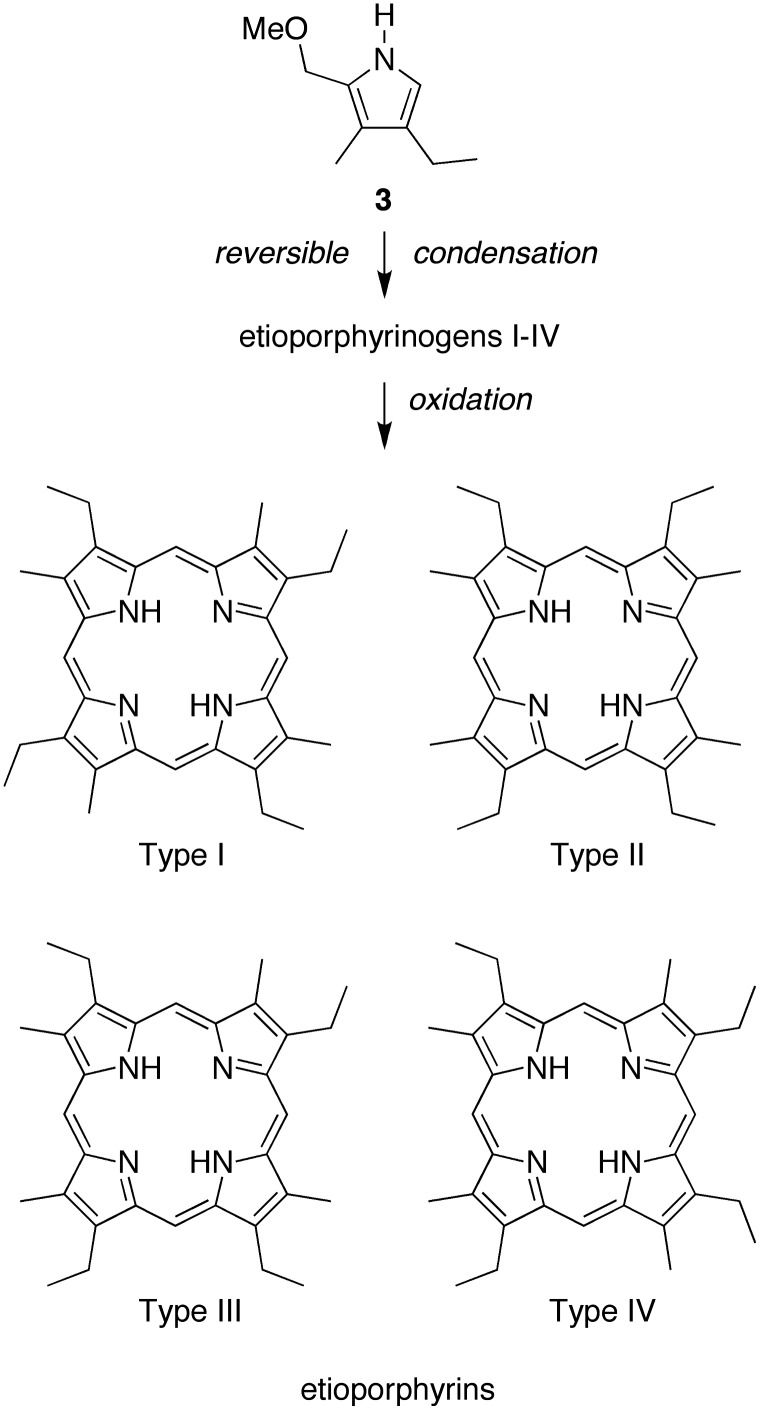
A key issue in porphyrin-forming reactions concerns whether the pyrrole self-condensation affords a unique porphyrinogen, or if exchange processes (i.e., scrambling) among pyrromethane intermediates alter the relative orientation of the pyrrole units (Taniguchi and Lindsey, 2012b). The reaction of dione 1-Me and aminoketone 2-Et is expected to afford the corresponding pyrrole 3 (Scheme 5). If four molecules of pyrrole 3 are joined without exchange, the etioporphyrin type-I species results. If exchange occurs, a mixture of the four etioporphyrin isomers (types I–IV) can be formed. The four isomers of etioporphyrin are shown in Scheme 5.
SCHEME 5.
Route to form etioporphyrin isomers I–IV.
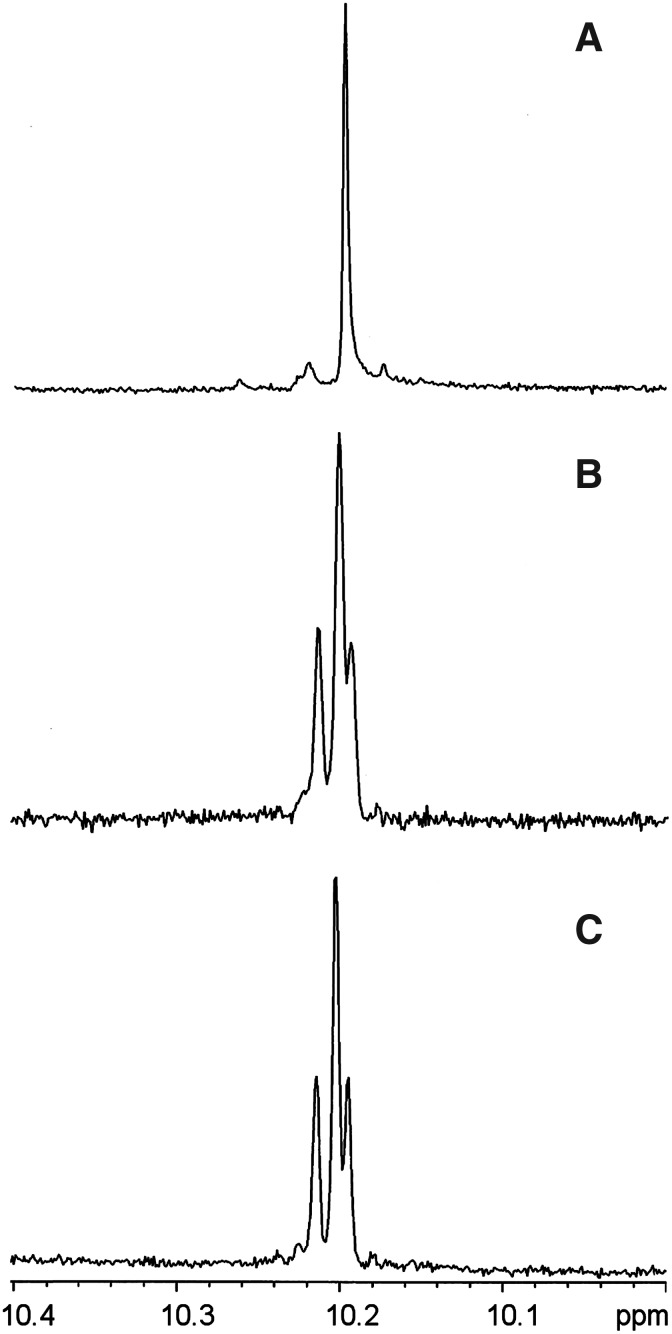
A clear distinction between etioporphyrin I and etioporphyrin isomers II–IV is observed upon examination of the resonance of the meso-protons in the 1H NMR spectrum. Etioporphyrin I affords a singlet (10.20 ppm) given that each meso-proton is flanked by one β-methyl group and one β-ethyl group; the molecule exhibits C4h symmetry (Taniguchi and Lindsey, 2012b). The spectrum of an authentic sample of etioporphyrin I is shown in Fig. 4A. By contrast, the spectrum of an authentic sample of etioporphyrin III exhibits multiple resonances from the meso-protons (Fig. 4B). The multiple resonances stem from the fact that each meso-proton is unique; the molecule has Cs symmetry (Taniguchi and Lindsey, 2012b). Samples of etioporphyrin isomers II and IV are not commercially available. The crude etioporphyrin sample obtained upon reaction of 1-Me and 2-Et was examined by 1H NMR spectroscopy. The spectrum showed multiple peaks in the region attributed to the meso-protons (Fig. 4C). A minimum interpretation is that the sample contains at least one isomer in addition to etioporphyrin I, such as isomer III, which can be derived from isomer I upon rearrangement of a single pyrrole (Taniguchi and Lindsey, 2012b). A reasonable interpretation is that all four isomers of etioporphyrin are present.
FIG. 4.
1H NMR spectroscopic data (at room temperature in CS2 containing benzene-d6 and tetramethylsilane) showing the signal(s) from the meso-protons. (A) Etioporphyrin I. (B) Etioporphyrin III. (C) Crude etioporphyrin sample obtained from reaction of 1-Me and 2-Et.
The physicochemical properties of the four etioporphyrin isomers are expected to be essentially identical. Such properties include partitioning into lipid media, absorption spectra, and excited-state characteristics (lifetime, energetics, intersystem crossing yield), all of which together determine photochemical properties in membrane assemblies. The identification of the existence of the etioporphyrin isomers is not important from a physicochemical perspective for etioporphyrin performance in a prebiotic milieu; however, the existence of such isomers reflects the reversibility of latter stages of the reaction, which is profoundly important in determining the number of tetrapyrrole macrocycles formed upon combinatorial reactions (Taniguchi and Lindsey, 2012b; Taniguchi et al., 2012). The mixture of etioporphyrin isomers observed herein indicates that the pyrrole precursor (3) affords oligopyrromethanes that undergo exchange processes, and would be expected to do so in conjunction with β-alkyl substituted pyrroles that exhibit similar reactivity.
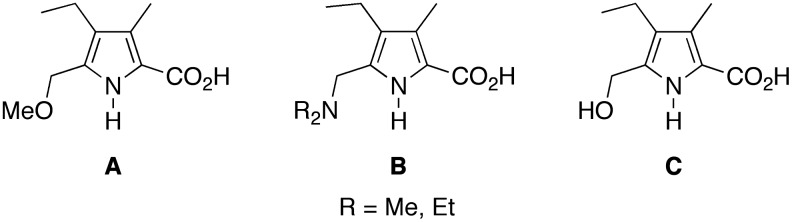
A number of reactions have been carried out to prepare etioporphyrins from substituted pyrroles. Such pyrroles (A–C) are shown in Scheme 6. In each case, the pyrrole was equipped with ethyl and methyl groups at the 3- and 4-positions (to give the characteristic substituents of etioporphyrins), a substituted 2-methyl group (for oligomerization and formation of the porphyrinogen meso-carbon), and a 5-carboxy group (which undergoes decarboxylation in solution to unveil the open α-pyrrolic position and enable self-condensation). The substituent at the 2-methyl site includes methoxy (A) (Jeandon and Callot, 1993), dialkylamino (B) (Nguyen and Smith, 1996), and hydroxy (C) (Pichon-Santander and Scott, 2002), although the latter may well be identical to A due to the presence of methanol upon reaction and workup (Jeandon and Callot, 1993). Other than the 5-carboxy group and the relative position of the ethyl and methyl groups, pyrroles A–C closely resemble that formed upon reaction of 1-Me + 2-Et. Regardless of conditions, even those designed to avoid exchange processes, each prior study with each pyrrole A–C afforded a mixture of etioporphyrin isomers rather than a pure sample of etioporphyrin I.
SCHEME 6.
Pyrroles examined in routes to etioporphyrin I and other isomers.
3.4. Examination of the pyrrole-forming step leading to etioporphyrinogen
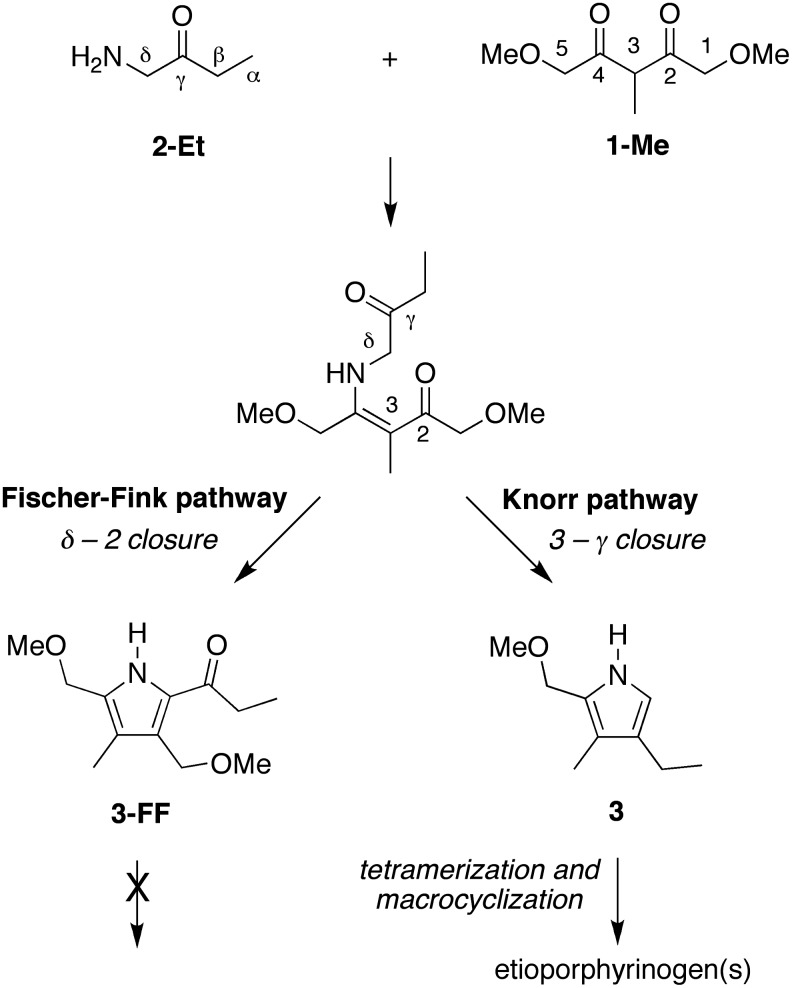
A key issue for structure-directed chemical processes—where molecular transformations occur due to intrinsic reactivity rather than external action by enzymes or other catalysts—concerns the integrity and yields obtained. In the prior studies of pairwise reactions leading to uroporphyrinogen and to coproporphyrinogen, a pyrrole by-product was formed in each case in addition to the pyrrole leading to the macrocycles (Lindsey et al., 2011). The pyrrole by-product is known as the Fischer-Fink pyrrole. Here, a sample of the reaction of 1-Me + 2-Et (120 mM, pH 7, 60°C, 24 h) in the presence of 0.1 M decanoic acid (and prior to oxidation) was analyzed by ESI-MS. A peak consistent with the Fischer-Fink pyrrole was observed at m/z=248.1253 (calculated 248.1257), which stems from the sodium-cationized species [(M + Na)+ where M=C12H19NO3]. The Fischer-Fink pyrrole (3-FF) arises by unwanted reaction of the dione and aminoketone versus the desired route to the Knorr pyrrole (3), as is shown in Scheme 7. The Fischer-Fink pyrrole bears a full complement of substituents at the four carbon positions. One of the α-substituents is an acyl group, which deactivates the pyrrole and blocks reactions required to lead to the porphyrinogen. Thus, the reaction processes observed here for reactants with hydrophobic substituents and in the presence of a surfactant are fully consistent with those observed previously for the reactants bearing polar substituents in aqueous solution (Lindsey et al., 2011).
SCHEME 7.
Competing Knorr and Fischer-Fink pathways to pyrroles.
4. Discussion
The inventory of constituents required for the origin of life remains uncertain. Much emphasis has been placed on the importance of amino acids, nucleotides, and sugars, all of which are soluble in water. More recent attention has been devoted to surfactants such as decanoic acid, which could form primitive vesicles and other assemblies (Apel et al., 2002). By contrast, the origin of hydrophobic compounds has received little attention. The importance of hydrophobic molecules stems in part from their potential function in lipid assemblies, including as photoactive pigments of photosynthesis (Mauzerall and Sun, 2003). The magnesium chelate of octaethylporphyrin, for example, has been shown to give light-driven transmembrane charge transport, which has been proposed as a model for a prebiotic photoreaction system (Sun and Mauzerall, 1996). Octaethylporphyrin is a very close analogue of etioporphyrin. Other structural roles of hydrophobic aromatic hydrocarbons have been proposed (Hud and Anet, 2000; Ehrenfreund et al., 2006).
4.1. Oil slicks and the origin of hydrophobic tetrapyrroles
To investigate reactions in the presence of a model oil slick, several biphasic media were employed here as models for primordial oil slicks on an underlying aqueous solution: a saturated aliphatic hydrocarbon (dodecane); an aromatic hydrocarbon (mesitylene); and a five-component mixture arbitrarily composed of decanoic acid, dodecane, mesitylene, naphthalene, and pentyl acetate. In addition, a monocarboxylic acid (decanoic acid) was employed that forms vesicles. All the reactions studied herein employed an aqueous phase that was buffered with 0.5 M potassium phosphate. Prior studies of analogous reactants in a non-phosphate buffer have also afforded porphyrinogens in yields of as much as 10% (Lindsey et al., 2011).
The reaction of 1-AcOH + 2-PrOH (uroporphyrinogens, eight carboxylic acids) occurs in the aqueous phase, given the near-constant yields obtained for the reactions in aqueous media in the presence or absence of dodecanoic acid or a separate dodecane layer (1.2–1.5%, entry 3 in Table 1). Similarly, the reaction of 1-Me + 2-PrOH (coproporphyrinogens, four carboxylic acids) also occurs in aqueous solution given the comparable yields (7.1–7.6%) regardless of media (entry 2 in Table 1). Indeed, in these reactions the dodecane layer remains colorless (Fig. 1, entries 2 and 3).
The reaction of 1-Me + 2-Et (etioporphyrinogens, zero carboxylic acids) proceeded smoothly in the presence of decanoic acid. The yield increased from 1.4% to 10.8% over the concentration range of 3.75–120 mM. The 120 mM concentration of reactants exceeded the concentration of decanoic acid (100 mM). A surprising feature of this chemistry was that the medium underwent a phase separation, from a turbid suspension to a biphasic system with a lower aqueous layer and an upper organic layer (Fig. 2, bottom). Equally surprising was the reaction in aqueous solution: here, an upper layer of organic material accumulated, the extent of which increased with increasing reaction concentration. The hydrophobic nature of the reactants themselves afforded a phase-separated organic layer. This result suggests that a preexisting oil slick is not mandatory given that an oil slick can form from the hydrophobic reactants, intermediates, by-products, or products themselves.
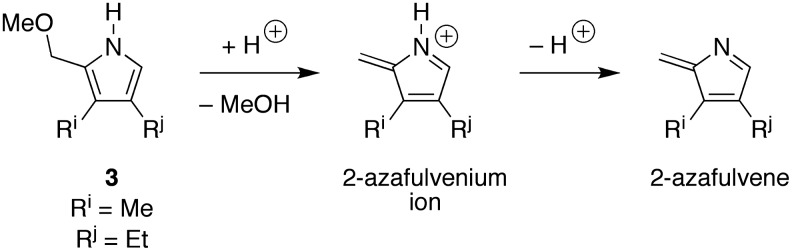
The reaction to give etioporphyrinogens in the presence of dodecane or mesitylene was not as expected: the yield was no higher than that in aqueous solution alone. The reaction in the five-component organic mixture, however, afforded an increase in yield, reaching 6.6% at the highest concentration (120 mM) examined. It may well be that the phase separation of reactants into dodecane or mesitylene creates a reaction environment that is too hydrophobic. Said differently, the reaction at certain steps is expected to benefit from a polar environment, which can stabilize transition states or intermediates that entail the formation of charged or polar species. Examples of such intermediates include an iminium ion and tertiary alcohol during pyrrole formation (Lindsey et al., 2011), and the 2-azafulvenium ion (Scheme 8) formed by displacement of the pyrrolic α-methoxy group. Reaction of a pyrrolic species with the neutral 2-azafulvene, derived by deprotonation of the 2-azafulvenium ion, may still entail formation of polar species. If so, such steps in the absence of specific catalysts would proceed very slowly in a hydrophobic medium such as dodecane or mesitylene, which have low dielectric constants. Thus, pyrrole formation may proceed in aqueous solution and then partition into the organic layer for subsequent oligomerization, or partitioning may only occur following oligomerization and cyclization. Accordingly, the necessity to stabilize transient polar species may result in reaction occurring largely at the aqueous-organic interface, in which case a thin oil slick containing surfactants may better support reaction chemistry of this type than a thick hydrophobic layer. In this context, it warrants emphasis that the role of organic constituents of an oil slick may be multifold, affording solubility, supporting interfacial reactions, and contributing to phase-separated nanoscale or microscale assemblies.
SCHEME 8.
Putative polar or nonpolar reactive species derived from pyrrole 3.
The yields of porphyrins remain <10% in all cases examined. The colored reaction mixture prior to oxidation with I2 in part consists of non-porphyrin species. Moreover, regardless of the ultimate yield of porphyrin, the reactions afford colored products. Oxidation (by chemical or photochemical means) of the crude mixture formed upon condensation of acyclic reactants affords further colored (non-porphyrin) species that absorb across the visible and into the ultraviolet spectral region. The facile formation of colored, non-porphyrin species suggests that such compounds could function as a shield of ultraviolet light (Cleaves and Miller, 1998), thereby protecting biorelevant molecules (e.g., nucleobases) from photodestruction. The distinction between a photoprotective layer and a layer that could impede photosynthesis depends on the wavelength and intensity of absorption; the latter of course depends on concentration. It would appear extremely unlikely that the flux of acyclic precursors (e.g., 1-Me + 2-Et) would suffice to create derived by-products at a concentration commensurate with a “black” photoinsulating layer blanketing the Earth. Whether such non-porphyrin species might have valuable functional properties, on the other hand, remains to be examined.
4.2. Origin of precursors
The route described in the chemical model proposed herein relies on a β-diketone (1-Me) and an α-aminoketone (2-Et) for formation of the corresponding pyrrole, porphyrinogens, and porphyrins. Compounds 1-Me and 2-Et are the respective (formally decarboxylated) hydrophobic analogues of 1-AcOH and 2-PrOH (the biosynthetic reactant ALA), which afford water-soluble products. Prebiotic routes have not yet been described for β-diketones and α-aminoketones regardless of solubility; such building blocks are relatively simple yet are obviously more elaborate than single-carbon or single-nitrogen reactants (e.g., H2CO, HCN). The emerging view of “the generational simplicity of the molecular building blocks of life” (Eschenmoser, 2007a) is juxtaposed against the relevance requirements for a credible prebiotic route (Lahav, 1999): the route should proceed in the early Earth environment, definitive knowledge of which remains uncertain (Chang, 1993). While prebiotic routes are typically thought to be substantially different from those in extant biosyntheses (Miller and Orgel, 1974), the tetrapyrroles have long been regarded as an important exception where, in at least some steps, biosynthesis may recapitulate biogenesis (Granick, 1950; Mauzerall, 1990, 1998; de Duve, 1991). Thus, it is expected that some steps in a prebiotic route to tetrapyrrole macrocycles may mimic extant biosyntheses—where intrinsic structure-directed transformations are under enzymatic control—and other steps may proceed via pathways that have no present-day biosynthetic counterpart.
In this regard, ALA (2-PrOH) is produced biosynthetically by reaction of succinyl-coenzyme A (CoA) and glycine, or by transformation of glutamic acid (Avissar and Moberg, 1995). In chemical studies, nonenzymatic transamination of 4,5-dioxovaleric acid with glycine afforded ALA and glyoxylic acid (Beale et al., 1979). A prebiotic route to the alkylthio precursor of CoA has been reported (Keefe et al., 1995). The five-carbon compound 4,5-dioxovaleric acid is a reduction product of α-ketoglutaric acid, an intermediate in the tricarboxylic acid (TCA) cycle (Owen et al., 2002). Thus, an early promiscuous chemistry that generates TCA intermediates (Eschenmoser, 2007b; Guzman and Martin, 2008) and analogues thereof—regardless of whether the full cycle was operative (Orgel, 2008)—would be expected to lead to a corresponding collection of α-aminoketones such as 2-Et and 2-PrOH.
The reactants 1-Me and 1-AcOH contain three structural features: a β- or 1,3-diketone, terminal methoxy substituents attached thereto, and a substituent (methyl or acetic acid) at the central carbon flanked by the two ketones. Analogous β-dicarbonyl compounds are prevalent intermediates in fatty acid biosynthesis, fatty acid degradation, and microbial polyketide synthesis. An acyl carrier protein (ACP) that bears a β-ketothioester moiety is a common intermediate in such metabolic processes (Smith and Tsai, 2007), as is the presence of substituents (typically methyl) at the carbon flanked by two ketones in the nascent polyketide chain. In addition, α-methoxymalonyl-ACP (derived from 1,3-bisphoshoglycerate) is employed as an extender unit via decarboxylative Claisen condensation to introduce the methoxyacetyl unit in polyketide synthesis (Chan et al., 2009). The formation of diketones such as 1-AcOH and 1-Me would likely entail condensation of analogous thioesters of α-substituted malonic acids. An acetyl-CoA–based metabolism via metal–sulfide chemistry has been proposed at volcanic sites (Wächtershäuser, 2006) or hydrothermal vents (Martin and Russell, 2007), although the practical availability of such products has been questioned from a quantitative perspective (Ross, 2008). In practice, the availability of β-diketones suited for pyrrole formation would depend not only on the synthesis of such reactants but also on the competition with reactions characteristic of β-diketones leading to pyridines (Hantzsch-like reaction), pyrimidines (Biginelli-like reaction), and arenes (polyketide-like self-condensations), notwithstanding that such products may also be beneficial in prebiotic chemistry.
5. Outlook
The results described herein illustrate a very simple pathway from acyclic reactants (1-Me + 2-Et) to give the very hydrophobic products, etioporphyrinogens. One of the reactants (2-Et) is a hydrophobic analogue of the universal precursor δ-aminolevulinic acid (ALA=2-PrOH) of the extant biosynthesis of tetrapyrrole macrocycles. Upon oxidation, etioporphyrinogens afford etioporphyrins. The polarity of etioporphyrins rivals that of the most hydrophobic pigments afforded by the extant biosynthesis, namely, chlorophylls and bacteriochlorophylls. In this regard, the structure-directed transformation of relatively simple acyclic reactants to give functional tetrapyrrole macrocycles raises questions about how biosynthetic pathways may have originated (Eschenmoser, 1988; Holliday et al., 2007; Lindsey et al., 2011; Taniguchi et al., 2012). Although whether species such as 1-Me and 2-Et or analogues thereof were present in the prebiotic era is not known (and may never be known), the chemistry described herein may provide a chemical model for the spontaneous formation of valuable hydrophobic molecules that rival advanced products of present-day biosynthesis.
Supplementary Material
Acknowledgments
This work was supported by a grant from the NSF Chemistry of Life Processes Program (NSF CHE-0953010). Mass spectra were obtained at the Mass Spectrometry Laboratory for Biotechnology at North Carolina State University. Partial funding for the facility was obtained from the North Carolina Biotechnology Center and the National Science Foundation.
Author Disclosure Statement
The authors (Ana R. M. Soares, Masahiko Taniguchi, Vanampally Chandrashaker, and Jonathan S. Lindsey) have no conflict of interest or competing financial interests concerning the studies and results reported herein.
Abbreviations
ACP, acyl carrier protein; ALA, δ-aminolevulinic acid; CoA, succinyl-coenzyme A; DMSO, dimethylsulfoxide; ESI-MS, electrospray ionization mass spectrometry; fwhm, full width at half-maximum; 1H NMR, proton nuclear magnetic resonance; TCA, tricarboxylic acid.
References
- Adam I. Orain D. Meier P. Concise synthesis of 1H-pyrazin-2-ones and 2-aminopyrazines. Synlett. 2004;2004:2031–2033. [Google Scholar]
- Apel C.L. Deamer D.W. Mautner M.N. Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim Biophys Acta. 2002;1559:1–9. doi: 10.1016/s0005-2736(01)00400-x. [DOI] [PubMed] [Google Scholar]
- Avissar Y.J. Moberg P.A. The common origins of the pigments of life—early steps of chlorophyll biosynthesis. Photosynth Res. 1995;44:221–242. doi: 10.1007/BF00048596. [DOI] [PubMed] [Google Scholar]
- Beale S.I. Gold M.H. Granick S. Chemical synthesis of 4,5-dioxovaleric acid and its nonenzymatic transamination to 5-aminolevulinic acid. Phytochemistry. 1979;18:441–444. [Google Scholar]
- Bothner-By A.A. Dadok J. Johnson T.E. Lindsey J.S. Molecular dynamics of covalently-linked multi-porphyrin arrays. J Phys Chem. 1996;100:17551–17557. [Google Scholar]
- Chan Y.A. Podevels A.M. Kevany B.M. Thomas M.G. Biosynthesis of polyketide synthase extender units. Nat Prod Rep. 2009;26:90–114. doi: 10.1039/b801658p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. Prebiotic synthesis in planetary environments. In: Greenberg J.M., editor; Mendoza-Gómez C.X., editor; Pirronello V., editor. The Chemistry of Life's Origins. NATO ASI Series C: Mathematical and Physical Sciences. Vol. 416. Kluwer Academic Publishers; Dordrecht: 1993. pp. 259–299. [Google Scholar]
- Cleaves H.J. Miller S.L. Oceanic protection of prebiotic organic compounds from UV radiation. Proc Natl Acad Sci USA. 1998;95:7260–7263. doi: 10.1073/pnas.95.13.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster H.G.L. Dielectric and electrical properties of lipid bilayers in relation to their structure. In: Tien H.T., editor; Ottova-Leitmannova A., editor. Planar Lipid Bilayers (BLM) and their Applications. Elsevier; Amsterdam: 2003. pp. 75–108. [Google Scholar]
- de Duve C. Blueprint for a Cell: The Nature and Origin of Life. Carolina Biological Supply Company, Neil Patterson Publishers; Burlington, NC: 1991. pp. 133–136. [Google Scholar]
- Dobson C.M. Ellison G.B. Tuck A.F. Vaida V. Atmospheric aerosols as prebiotic chemical reactors. Proc Natl Acad Sci USA. 2000;97:11864–11868. doi: 10.1073/pnas.200366897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfreund P. Rasmussen S. Cleaves J. Chen L. Experimentally tracing the key steps in the origin of life: the aromatic world. Astrobiology. 2006;6:490–520. doi: 10.1089/ast.2006.6.490. [DOI] [PubMed] [Google Scholar]
- Eschenmoser A. Vitamin B12: experiments concerning the origin of its molecular structure. Angew Chem Int Ed Engl. 1988;27:5–39. [Google Scholar]
- Eschenmoser A. The search for the chemistry of life's origin. Tetrahedron. 2007a;63:12821–12844. [Google Scholar]
- Eschenmoser A. On a hypothetical generational relationship between HCN and constituents of the reductive citric acid cycle. Chem Biodivers. 2007b;4:554–573. doi: 10.1002/cbdv.200790050. [DOI] [PubMed] [Google Scholar]
- Geier G.R., III Lindsey J.S. Investigation of porphyrin-forming reactions. Part 2. Examination of the reaction course in two-step, one-flask syntheses of meso-substituted porphyrins. J Chem Soc Perkin Trans. 2001;2:687–700. [Google Scholar]
- Granick S. The structural and functional relationships between heme and chlorophyll. Harvey Lect. 1950;44:220–245. [PubMed] [Google Scholar]
- Guzman M.I. Martin S.T. Oxaloacetate-to-malate conversion by mineral photoelectrochemistry: implications for the viability of the reductive tricarboxylic acid cycle in prebiotic chemistry. Int J Astrobiol. 2008;7:271–278. [Google Scholar]
- Holliday G.L. Thornton J.M. Marquet A. Smith A.G. Rébeillé F. Mendel R. Schubert H.L. Lawrence A.D. Martin M.J. Evolution of enzymes and pathways for the biosynthesis of cofactors. Nat Prod Rep. 2007;24:972–987. doi: 10.1039/b703107f. [DOI] [PubMed] [Google Scholar]
- Huang Y. Wang Y. Alexandre M.R. Lee T. Rose-Petruck C. Fuller M. Pizzarello S. Molecular and compound-specific isotopic characterization of monocarboxylic acids in carbonaceous meteorites. Geochim Cosmochim Acta. 2005;69:1073–1084. [Google Scholar]
- Hud N.V. Anet F.A.L. Intercalation-mediated synthesis and replication: a new approach to the origin of life. J Theor Biol. 2000;205:543–562. doi: 10.1006/jtbi.2000.2084. [DOI] [PubMed] [Google Scholar]
- Jaffé H.H. Orchin M. Theory and Applications of Ultraviolet Spectroscopy. John Wiley and Sons, Inc.; New York: 1962. pp. 178–182. [Google Scholar]
- Jeandon C. Callot H.J. Cyclotetramerization of modified Knorr pyrroles into porphyrins. A reinvestigation. Bull Soc Chim Fr. 1993;13:625–629. [Google Scholar]
- Keefe A.D. Newton G.L. Miller S.L. A possible prebiotic synthesis of pantetheine, a precursor to coenzyme A. Nature. 1995;373:683–685. doi: 10.1038/373683a0. [DOI] [PubMed] [Google Scholar]
- Komiya M. Shimoyama A. Organic compounds from insoluble organic matter isolated from the Murchison carbonaceous chondrite by heating experiments. Bull Chem Soc Jpn. 1996;69:53–58. [Google Scholar]
- Lahav N. Biogenesis—Theories of Life's Origin. Oxford University Press; New York: 1999. pp. 160–162. [Google Scholar]
- Lasaga A.C. Holland H.D. Dwyer M.J. Primordial oil slick. Science. 1971;174:53–55. doi: 10.1126/science.174.4004.53. [DOI] [PubMed] [Google Scholar]
- Lawless J.G. Yuen G.U. Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature. 1979;282:396–398. [Google Scholar]
- Lee C.-H. Li F. Iwamoto K. Dadok J. Bothner-By A.A. Lindsey J.S. Synthetic approaches to regioisomerically pure porphyrins bearing four different meso-substituents. Tetrahedron. 1995;51:11645–11672. [Google Scholar]
- Li F. Yang K. Tyhonas J.S. MacCrum K.A. Lindsey J.S. Beneficial effects of salts on an acid-catalyzed condensation leading to porphyrin formation. Tetrahedron. 1997;53:12339–12360. [Google Scholar]
- Lindsey J.S. Synthesis of meso-substituted porphyrins. In: Kadish K.M., editor; Smith K.M., editor; Guilard R., editor. The Porphyrin Handbook. Vol. 1. Academic Press; San Diego, CA: 2000. pp. 45–118. [Google Scholar]
- Lindsey J.S. Wagner R.W. Investigation of the synthesis of ortho-substituted tetraphenylporphyrins. J Org Chem. 1989;54:828–836. [Google Scholar]
- Lindsey J.S. Schreiman I.C. Hsu H.C. Kearney P.C. Marguerettaz A.M. Rothemund and Adler-Longo reactions revisited: synthesis of tetraphenylporphyrins under equilibrium conditions. J Org Chem. 1987;52:827–836. [Google Scholar]
- Lindsey J.S. MacCrum K.A. Tyhonas J.S. Chuang Y.-Y. Investigation of a synthesis of meso-porphyrins employing high concentration conditions and an electron transport chain for aerobic oxidation. J Org Chem. 1994;59:579–587. [Google Scholar]
- Lindsey J.S. Ptaszek M. Taniguchi M. Simple formation of an abiotic porphyrinogen in aqueous solution. Orig Life Evol Biosph. 2009;39:495–515. doi: 10.1007/s11084-009-9168-3. [DOI] [PubMed] [Google Scholar]
- Lindsey J.S. Chandrashaker V. Taniguchi M. Ptaszek M. Abiotic formation of uroporphyrinogen and coproporphyrinogen from acyclic reactants. New J Chem. 2011;35:65–75. [Google Scholar]
- Martin W. Russell M.J. On the origin of biochemistry at an alkaline hydrothermal vent. Philos Trans R Soc Lond B Biol Sci. 2007;362:1887–1925. doi: 10.1098/rstb.2006.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. The thermodynamic stability of porphyrinogens. J Am Chem Soc. 1960;82:2601–2605. [Google Scholar]
- Mauzerall D. The photoreduction of porphyrins: structure of the products. J Am Chem Soc. 1962;84:2437–2445. [Google Scholar]
- Mauzerall D. Chlorophyll and photosynthesis. Philos Trans R Soc Lond B Biol Sci. 1976;273:287–294. [Google Scholar]
- Mauzerall D. Granick S. Porphyrin biosynthesis in erythrocytes III. Uroporphyrinogen and its decarboxylase. J Biol Chem. 1958;232:1141–1162. [PubMed] [Google Scholar]
- Mauzerall D. Sun K. Photoinduced charge separation in lipid bilayers. In: Tien H.T., editor; Ottova-Leitmannova A., editor. Planar Lipid Bilayers (BLM) and Their Applications. Elsevier; Amsterdam: 2003. pp. 963–979. [Google Scholar]
- Mauzerall D.C. The photochemical origins of life and photoreaction of ferrous ion in the Archaean oceans. Orig Life Evol Biosph. 1990;20:293–302. [Google Scholar]
- Mauzerall D.C. Evolution of porphyrins. Clin Dermatol. 1998;16:195–201. doi: 10.1016/s0738-081x(97)00200-9. [DOI] [PubMed] [Google Scholar]
- Mercer-Smith J.A. Mauzerall D.C. Photochemistry of porphyrins: a model for the origin of photosynthesis. Photochem Photobiol. 1984;39:397–405. doi: 10.1111/j.1751-1097.1984.tb08197.x. [DOI] [PubMed] [Google Scholar]
- Mercer-Smith J.A. Raudino A. Mauzerall D.C. A model for the origin of photosynthesis–III. The ultraviolet photochemistry of uroporphyrinogen. Photochem Photobiol. 1985;42:239–244. doi: 10.1111/j.1751-1097.1985.tb08937.x. [DOI] [PubMed] [Google Scholar]
- Miller S.L. Orgel L.E. The Origins of Life on the Earth. Prentice-Hall, Inc.; Englewood Cliffs, NJ: 1974. pp. 185–188. [Google Scholar]
- Morton M.A. Rosney W.C.V. XCVII.–Absorption spectra and tautomerism. Part I. Keto-enol tautomerisation. Ethyl acetoacetate, acetylacetone, and α-benzoylcamphor. J Chem Soc. 1926;129:706–713. [Google Scholar]
- Moss G.P. Nomenclature of tetrapyrroles. Pure Appl Chem. 1987;59:779–832. [Google Scholar]
- Nguyen L.T. Smith K.M. Syntheses of type-I porphyrins via monopyrrole tetramerization. Tetrahedron Lett. 1996;37:7177–7180. [Google Scholar]
- Nilson F.P.R. Possible impact of a primordial oil slick on atmospheric and chemical evolution. Orig Life Evol Biosph. 2002;32:247–253. doi: 10.1023/a:1016577923630. [DOI] [PubMed] [Google Scholar]
- Olson J.M. Pierson B.K. Origin and evolution of photosynthetic reaction centers. Orig Life. 1987;17:419–430. [Google Scholar]
- Orgel L.E. The implausibility of metabolic cycles on the prebiotic Earth. PLoS Biol. 2008;6:e18. doi: 10.1371/journal.pbio.0060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O.E. Kalhan S.C. Hanson R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- Pichon-Santander C. Scott A.I. Studies on the tetramerization of substituted monopyrroles to type I porphyrins. Tetrahedron Lett. 2002;43:6967–6969. [Google Scholar]
- Riddick J.A. Bunger W.B. Physical Properties and Methods of Purification. 3rd. Wiley-Interscience; New York: 1970. Organic Solvents. [Google Scholar]
- Rimington C. Spectral-absorption coefficients of some porphyrins in the Soret-band region. Biochem J. 1960;75:620–623. doi: 10.1042/bj0750620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimington C. Benson A. Partition of porphyrins between cyclohexanone and aqueous sodium acetate as a function of pH. Biochem J. 1967;105:1085–1090. doi: 10.1042/bj1051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D.S. A quantitative evaluation of the iron-sulfur world and its relevance to life's origins. Astrobiology. 2008;8:267–272. doi: 10.1089/ast.2007.0199. [DOI] [PubMed] [Google Scholar]
- Scheer H. The pigments. In: Green B.R., editor; Parson W.W., editor. Light-Harvesting Antennas in Photosynthesis. Kluwer Academic Publishers; Dordrecht: 2003. pp. 29–81. [Google Scholar]
- Scheer H. An overview of chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications. In: Grimm B., editor; Porra R.J., editor; Rüdiger W., editor; Scheer H., editor. Chlorophylls and Bacteriochlorophylls. Biochemistry, Biophysics, Functions and Applications. Vol. 25. Advances in Photosynthesis and Respiration, Springer; Dordrecht: 2006. pp. 1–26. [Google Scholar]
- Sephton M.A. Organic compounds in carbonaceous meteorites. Nat Prod Rep. 2002;19:292–311. doi: 10.1039/b103775g. [DOI] [PubMed] [Google Scholar]
- Sivash A.A. Masinovsky Z. Lozovaya G.I. Surfactant micelles containing protoporphyrin IX as models of primitive photocatalytic systems: a spectral study. Biosystems. 1991;25:131–140. doi: 10.1016/0303-2647(91)90001-2. [DOI] [PubMed] [Google Scholar]
- Smith S. Tsai S.-C. The type I fatty acid and polyketide synthases: a tale of two megasynthases. Nat Prod Rep. 2007;24:1041–1072. doi: 10.1039/b603600g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares A.R.M. Taniguchi M. Chandrashaker V. Lindsey J.S. Self-organization of tetrapyrrole constituents to give a photoactive protocell. Chem Sci. 2012;3:1963–1974. [Google Scholar]
- Sun K. Mauzerall D. A simple light-driven transmembrane proton pump. Proc Natl Acad Sci USA. 1996;93:10758–10762. doi: 10.1073/pnas.93.20.10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M. Lindsey J.S. Diversity, isomer composition, and design of combinatorial libraries of tetrapyrrole macrocycles. J Porphyr Phthalocyanines. 2012a;16:1–13. [Google Scholar]
- Taniguchi M. Lindsey J.S. Enumeration of isomers of substituted tetrapyrrole macrocycles: from classical problems in biology to modern combinatorial libraries. In: Kadish K.M., editor; Smith K.M., editor; Guilard R., editor. Handbook of Porphyrin Science. Vol. 23. World Scientific; Singapore: 2012b. pp. 1–80. [Google Scholar]
- Taniguchi M. Du H. Lindsey J.S. Virtual libraries of tetrapyrrole macrocycles. Combinatorics, isomers, product distributions, and data mining. J Chem Inf Model. 2011;51:2233–2247. doi: 10.1021/ci200240e. [DOI] [PubMed] [Google Scholar]
- Taniguchi M. Soares A.R.M. Chandrashaker V. Lindsey J.S. Tandem combinatorial model for the prebiogenesis of diverse tetrapyrrole macrocycles. New J Chem. 2012;36:1057–1069. [Google Scholar]
- Tien H.T. Bilayer Lipid Membranes (BLM): Theory and Practice. Marcel Dekker; New York: 1974. [Google Scholar]
- Tuck A. The role of atmospheric aerosols in the origin of life. Surveys in Geophysics. 2002;23:379–409. [Google Scholar]
- Wächtershäuser G. From volcanic origins of chemoautotrophic life to Bacteria, Archaea and Eukarya. Philos Trans R Soc Lond B Biol Sci. 2006;361:1787–1808. doi: 10.1098/rstb.2006.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.