Abstract
We identified four anti-inflammatory sulfur-containing compounds from garlic, and their chemical structures were identified as Z- and E-ajoene and oxidized sulfonyl derivatives of ajoene. The sulfur compounds inhibited the production of nitric oxide (NO) and prostaglandin E2 (PGE2) and the expression of the pro-inflammatory cytokines tumor necrosis factor-α, interleukin-1β, and interleukin-6 in lipopolysaccharide (LPS)-activated macrophages. Western blotting and reverse transcription–polymerase chain reaction analysis demonstrated that these sulfur compounds attenuated the LPS-induced expression of the inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) proteins and mRNA. Moreover, these sulfur-containing compounds suppressed the nuclear factor-κB (NF-κB) transcriptional activity and the degradation of inhibitory-κBα in LPS-activated macrophages. Furthermore, we observed that they markedly inhibited the LPS-induced phosphorylations of p38 mitogen-activated protein kinases and extracellular signal-regulated kinases (ERK) at 20 μM. These data demonstrate that the sulfur compounds from garlic, (Z, E)-ajoene and their sulfonyl analogs, can suppress the LPS-induced production of NO/PGE2 and the expression of iNOS/COX-2 genes by inhibiting the NF-κB activation and the phosphorylations of p38 and ERK. Taken together, these data show that Z- and E-ajoene and their sulfonyl analogs from garlic might have anti-inflammatory therapeutic potential.
Key Words• Allium compounds, anti-inflammatory, garlic, nitric oxide, nuclear factor-κB
Introduction
Garlic (Allium sativum L.) is a pungent spice with a long history of use throughout the world for both its culinary and therapeutic properties.1 It has been reported that garlic as a dietary component may reduce the risk of cardiovascular disease.2 Garlic has been also known to possess antithrombotic,1 lipid-lowering,3 antioxidative,4 antihypercholesterolemia,5 anticancer,6,7 and anti-inflammatory8,9 activities. It has been reported that garlic extract suppresses the production of leukocyte inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1α, IL-6, and interferon-γ in vitro and shows a therapeutic potential in the treatment of inflammatory bowel disease.10 Its characteristic flavor and potent biological activities are due to its organosulfur compounds.11
Garlic contains diverse organosulfur compounds such as S-allyl-l-cysteine, diallyl disulfide, diallyl trisulfide, ajoene, and allicin, which have potent antioxidative, antibacterial, antiviral, and anticancer properties.11–13 The major water-soluble sulfur compound, S-allyl-l-cysteine, in garlic extract seems to have direct inhibitory effect on nuclear factor-κB (NF-κB) and indirect inhibitory effect on lipopolysaccharide (LPS)-induced IL-1β and TNF-α in human whole blood.14,15 The lipid-soluble sulfur compounds allicin and diallyl disulfide also inhibit NF-κB and reduce the expression of inducible nitric oxide (NO) synthase (iNOS) in LPS-stimulated macrophages.16,17 Allicin is a very unstable compound that is formed from alliin by alliinase upon crushing fresh garlic. It can be rearranged to produce diallyl sulfides, dithiins, and ajoene.18 In particular, ajoene is a fairly stable sulfoxide compound that can be produced during heat treatment of crushed garlic. Ajoene, a mixture of Z and E isomers, possesses a broad spectrum of biological activities, including antithrombotic,19 antimicrobial,20 anticancer,21 and anti-inflammatory activities.22
We previously showed that garlic extract exerts anti-inflammatory activity by inhibiting the LPS-induced Toll-like receptor-4 dimerization followed by the suppression of NF-κB transcriptional activity and the expression of iNOS and cyclooxygenase (COX)-2.23 NO synthase (NOS) catalyzes the oxidative deamination of l-arginine to produce NO. Three isoforms of NOS have been identified: endothelial NOS, neuronal NOS, and iNOS.24 Of these three isoforms, iNOS can be induced by LPS or cytokines in a variety of immune cells, including macrophages, to produce a large amount of NO as a pro-inflammatory mediator.25 COX-2 catalyzes the rate-limiting step in the synthesis of prostaglandins (PGs) that play a major role as mediators of the inflammatory response. Two isoforms of COX have been found: COX-1 and COX-2. COX-1 is a housekeeping enzyme and is constitutively expressed in most mammalian tissues. COX-2 is induced by several stimuli and is responsible for the production of large amounts of pro-inflammatory PGs at the inflammatory site.26
In this study, we identified (Z, E)-ajoenes and their oxidized analogs as anti-inflammatory principles of garlic (A. sativum L.) through the activity-guided purification procedure. Although the effect of the mixture of Z- and E-ajoene on the production of inflammatory mediators in LPS-induced macrophages has been investigated,17,22 little is known about the effect of pure Z- and E-ajoene and oxidized (sulfonyl) derivatives of ajoene on inflammatory responses. The present study compared the efficacy of Z- and E-ajoene and their analogs for inhibiting production of NO/PGE2 and the expression of iNOS/COX-2 in LPS-stimulated macrophages. We also disclosed the possible molecular mechanisms of these actions.
Materials and Methods
Test material
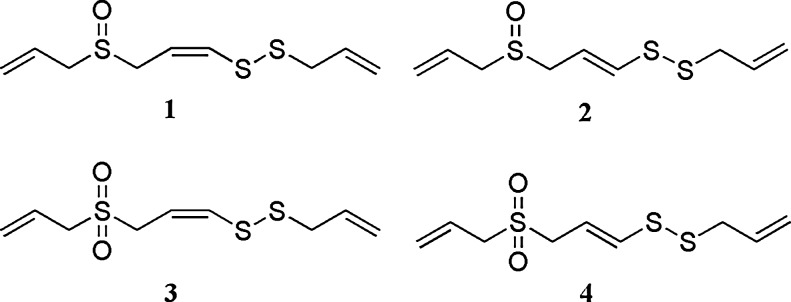
Fresh Korean garlic (A. sativum L.) (2 kg) was purchased from a Korean market in January 2007. Authentication of plant material was carried out by Prof. K.S. Yang at Sookmyung Women's University (Seoul, Korea). A voucher specimen (number 0070108) was deposited in the Herbarium of Sookmyung Women's University. Four sulfur-containing compounds, 1–4 (Fig. 1), as active principles from garlic (A. sativum L.) were isolated by repeated column chromatography and analyzed for purity by high-performance liquid chromatography (Shimadzu [Kyoto, Japan] high-performance liquid chromatography system with ultraviolet monitoring at 254 nm; μ-Bondapak C18 column, 10 μm, 10 mm ×300 mm; 70% aqueous methanol as eluent; flow rate, 2.0 mL/min): (Z)-4,5,9-trithiadodeca-1,6,11-triene 9-oxide (Z-ajoene) (1), (E)-4,5,9-trithiadodeca-1,6,11-triene 9-oxide (E-ajoene) (2), (Z)-4,5,9-trithiadodeca-1,6,11-triene 9,9-dioxide (3), and (E)-4,5,9-trithiadodeca-1,6,11-triene 9,9-dioxide (4). The structures of sulfur–containing compounds 1–4 were identified by 1H- and 13C-nuclear magnetic resonance spectra that were consistent with previous data.27–29 All test concentrations of active compounds showed no significant effect on cell viability. In the present study, curcumin (20 μM), a representative naturally occurring anti-inflammatory compound, was used as a positive control for the evaluation of anti-inflammatory activities.
FIG. 1.
The structures of sulfur compounds 1–4 from garlic.
Cell culture
RAW 264.7 cells (a murine macrophage cell line) (American Type Culture Collection, Rockville, MD, USA) were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA). T-RAW cells, stably transfected RAW 264.7 cells with a reporter construct of pNF-κB-SEAP-NPT encoding four copies of NF-κB binding κB sequence and secretory alkaline phosphatase (SEAP) as a reporter, were the kind gift of Prof. Yeong Shik Kim (Seoul National University, Seoul). T-RAW 264.7 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 500 μg/mL geneticin. All cells were incubated at 37°C in 5% CO2 in a humidified atmosphere.
Measurements of NO in LPS-activated RAW 264.7 cells
Cells (6×104 cells/mL in a 48-well plate) were incubated for 20 h in the absence or presence of test samples with LPS (1 μg/mL). NO was assessed by measuring the accumulated nitrite by the Griess method.30 In brief, samples (100 μL) of culture medium were incubated with 150 μL of Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamine in 2.5% phosphoric acid solution) at room temperature for 10 min in a 96-well microplate. Absorbance at 540 nm was measured by using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). The concentration of NO was determined by the sodium nitrite standard curve.
Measurement of PGE2
The accumulated PGE2 in culture medium was determined by using an enzyme immunoassay kit from Cayman Chemical Co. (Ann Arbor, MI, USA) according to the manufacturer's instruction. A standard curve was prepared simultaneously with PGE2 standards ranging from 0.06 to 6 ng/mL.
Western blot analysis
Whole-cell lysates and cytosol and nuclear extracts were prepared and subjected to western blotting as described previously.31 In brief, RAW 264.7 cells (8×105 cells per 60-mm-diameter dish) were treated with 1 μg/mL LPS in the presence or absence of test compounds. Following 20-h treatment, cells were harvested and gently lysed with cell lysis buffer (Cell Signaling Technologies, Beverly, MA, USA). Cell lysates were then centrifuged at 10,000 g for 20 min at 4°C. Supernatants were collected, and protein concentrations were determined by the Bradford method.
To prepare cytosol and nuclear extracts, cells were treated with test compounds for 30 min prior to activation with 1 μg/mL LPS. Following a 15-min treatment with LPS, cells were harvested by using NE-PER nuclear and cytoplasmic extraction reagents (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer's instructions. Antibodies against iNOS (BD Biosciences, Franklin Lakes, NJ, USA), COX-2 (Cayman Chemical), and inhibitory-κBα (I-κBα) and p65 (Santa Cruz Biotechnologies Inc., Santa Cruz, CA, USA) were used for immunoblot analysis.
Reverse transcription–polymerase chain reaction analysis
RAW 264.7 cells (1×106 cells per 60-mm-diameter dish) were stimulated for 6 h with LPS (1 μg/mL) in the presence or absence of test compounds. Total RNA was isolated by TRIzol® (Invitrogen) extraction according to the manufacturer's instructions and then reverse-transcribed into cDNA using reverse transcriptase (Invitrogen) and random hexamer (Cosmo, Seoul, Korea). Then polymerase chain reaction analyses were performed on the aliquots of the cDNA preparations to detect expression of the genes for iNOS, COX-2, IL-1β, IL-6, TNF-α, and β-actin using a recombinant Taq polymerase (Promega, Madison, WI, USA).
Measurement of NF-κB transcriptional activity
NF-κB transcriptional activity was measured by using the stably transfected RAW 264.7 cells with pNF-κB-SEAP-NPT (T-RAW 264.7 cells) as described previously with some modifications.32,33 In brief, T -RAW 264.7 cells were seeded on a 24-well plate and incubated for 24 h. Test compounds were added to cells 2 h before the treatment with LPS (1 μg/mL). After a 16-h incubation, aliquots of culture medium were heated at 65°C for 6 min, and then the activity of SEAP was measured. The transcriptional activity was expressed as fold induction over that of vehicle-treated cells.
Statistical analysis
The results were expressed as mean±SD values of three experiments, and statistical analysis was performed by one-way analysis of variance and Student's t test. A P value of < .01 was considered to indicate a significant difference.
Results
Effects of sulfur compounds on production of NO and PGE2 in LPS-stimulated RAW 264.7 cells
Compounds 1–4 inhibited the production of NO and PGE2 in a dose-dependent manner (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/jmf), whereas LPS treatment dramatically increased the concentrations of NO and PGE2. The concentrations of compounds 1–4 causing 50% inhibition are shown in Table 1. The overall pattern of the anti-inflammatory activity of the four compounds shows that the ajoenes (1, 2) are more potent than the oxidized forms (3, 4) and that the Z-isomer is more effective than the E-isomer in the inhibition of LPS-induced NO and PGE2 production. The most potent form is (Z)-ajoene, with concentrations causing 50% inhibition of 1.9 μM and 1.1 μM for NO and PGE2 production, respectively. These results guided us to examine the effects of compounds 1–4 on the expression of the iNOS and COX-2 enzymes, which produce NO and PGE2, as key mediators of inflammation.
Table 1.
Inhibition of Nitric Oxide and Prostaglandin E2 Production by Sulfur Compounds from Garlic in Lipopolysaccharide-Activated RAW 264.7 Cells
| |
IC50 (μM) |
|
|---|---|---|
| Compound | NOa | PGE2b |
| 1 | 1.9±0.2 | 1.1±0.2 |
| 2 | 4.0±0.2 | 1.8±0.3 |
| 3 | 3.3±0.2 | 2.1±0.2 |
| 4 | 4.2±0.1 | 4.0±0.3 |
RAW 264.7 cells were cultured in 48-well plates and activated with 1 μg/mL lipopolysaccharide in the presence or absence of various concentrations (0.1–20 μM) of compounds 1–4. After a 24-h treatment, cell culture supernatants were analyzed for nitrite (NO2−) by the Griess reaction. In brief, an equal volume of Griess reagent (1% sulfanilamide/0.1% naphthylethyenediamine dihydrochloride in 2.5% H3PO4) was mixed with cell culture supernatants, and color development was assessed at λ=540 nm using a microplate reader. Fresh culture medium was used as the blank in all experiments. The amount of nitrite was calculated from a sodium nitrite standard curve.
The accumulated prostaglandin E2 (PGE2) in culture medium was determined by using an enzyme immunoassay kit from Cayman Chemical according to the manufacturer's instruction. A standard curve was prepared simultaneously with PGE2 standard ranging from 0.06 to 6 ng/mL.
IC50, concentration causing 50% inhibition; NO, nitric oxide.
Effects of sulfur compounds on expression of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells
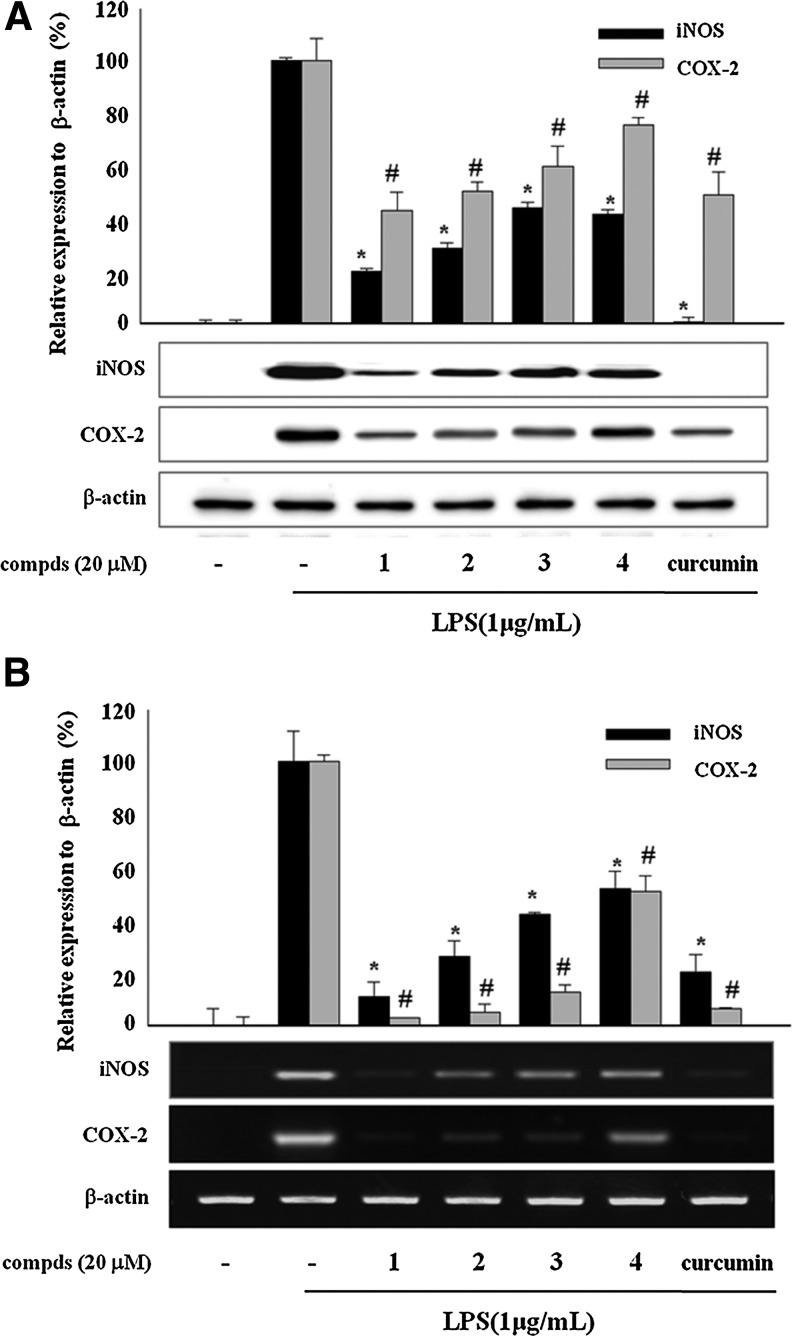
To elucidate the mechanism of active compounds for the inhibition of NO and PGE2 production, we examined the effects of sulfur compounds on the expression of iNOS/COX-2 protein and mRNA. As shown in Figure 2A, sulfur compounds 1–4 from garlic attenuated the expression of iNOS and COX-2 protein levels, whereas the protein levels of iNOS and COX-2 were markedly up-regulated by LPS treatment of RAW 264.7 cells. Furthermore, reverse transcription–polymerase chain reaction analysis showed down-regulation of the mRNA level of iNOS and COX-2 by the treatment with the compounds (Fig. 2B). As expected, (Z)-ajoene (1) showed the most potent activity in these experiments. These results suggested that sulfur compounds 1–4 control the LPS-induced expression of iNOS and COX-2 at the transcriptional level (see also Supplementary Fig. S2).
FIG. 2.
Effects of sulfur compounds (compds) 1–4 on the expression of lipopolysaccharide (LPS)-induced inducible NO synthase (iNOS)/cyclooxygenase-2 (COX-2) protein and mRNA in RAW 264.7 macrophages. (A) Cells were treated with compds for 20 h during LPS (1 μg/mL) activation. Cell lysates were prepared, and the iNOS, COX-2, and β-actin protein levels were determined by western blotting. (B) Cells were treated with compds for 6 h during LPS (1 μg/mL) activation. The mRNA levels for iNOS, COX-2, and β-actin were determined by reverse transcription–polymerase chain reaction from total RNA extracts. The relative intensity of iNOS/COX-2 to β-actin bands was measured by densitometry. Data are mean±SD values of three individual experiments. P<.01, significant difference from LPS alone for *iNOS and #COX-2.
Effects of sulfur compounds on mRNA expression of pro-inflammatory cytokines in LPS-stimulated RAW 264.7 cells
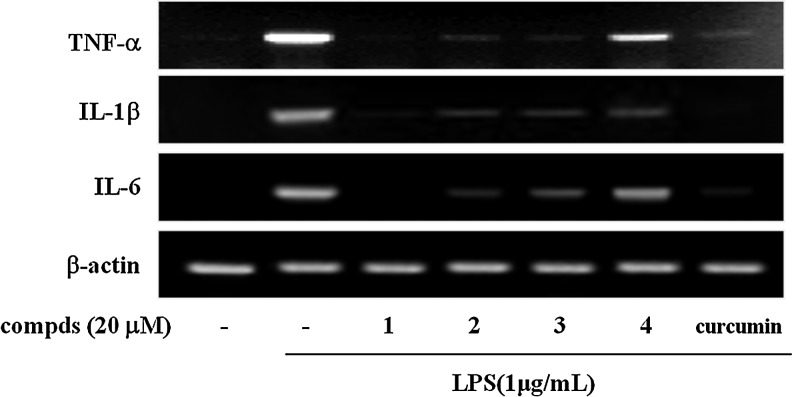
To examine the anti-inflammatory potential of these sulfur compounds from garlic, we investigated the effect of the compounds 1–4 on the LPS-induced mRNA expression of TNF-α, IL-1β, and IL-6 in RAW 264.7 cells. Cells were treated with 20 μM sulfur compounds 1–4 in the presence of LPS (1 μg/mL) for 6 h. Compounds 1–4 significantly suppressed the expression of IL-1β, IL-6, and TNF-α mRNAs in LPS-stimulated macrophages (Fig. 3). Here, Z-ajoene (1) was the most effective among the four sulfur compounds, and the Z isomers of ajoene and oxidized ajoene were more potent than the E isomers.
FIG. 3.
Effects of sulfur compds 1–4 on the LPS-induced inflammatory cytokines in RAW 264.7 macrophages. Cells were stimulated with LPS in the presence or absence of compds for 8 h. The levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) mRNAs were determined by reverse transcription–polymerase chain reaction analysis. β-Actin was used as an internal control. Images are representative of three independent experiments that showed similar results.
Inhibition of NF-κB transcriptional activity by sulfur compounds via the suppression of I-κBα degradation and nuclear translocation of p65 in LPS-stimulated macrophages
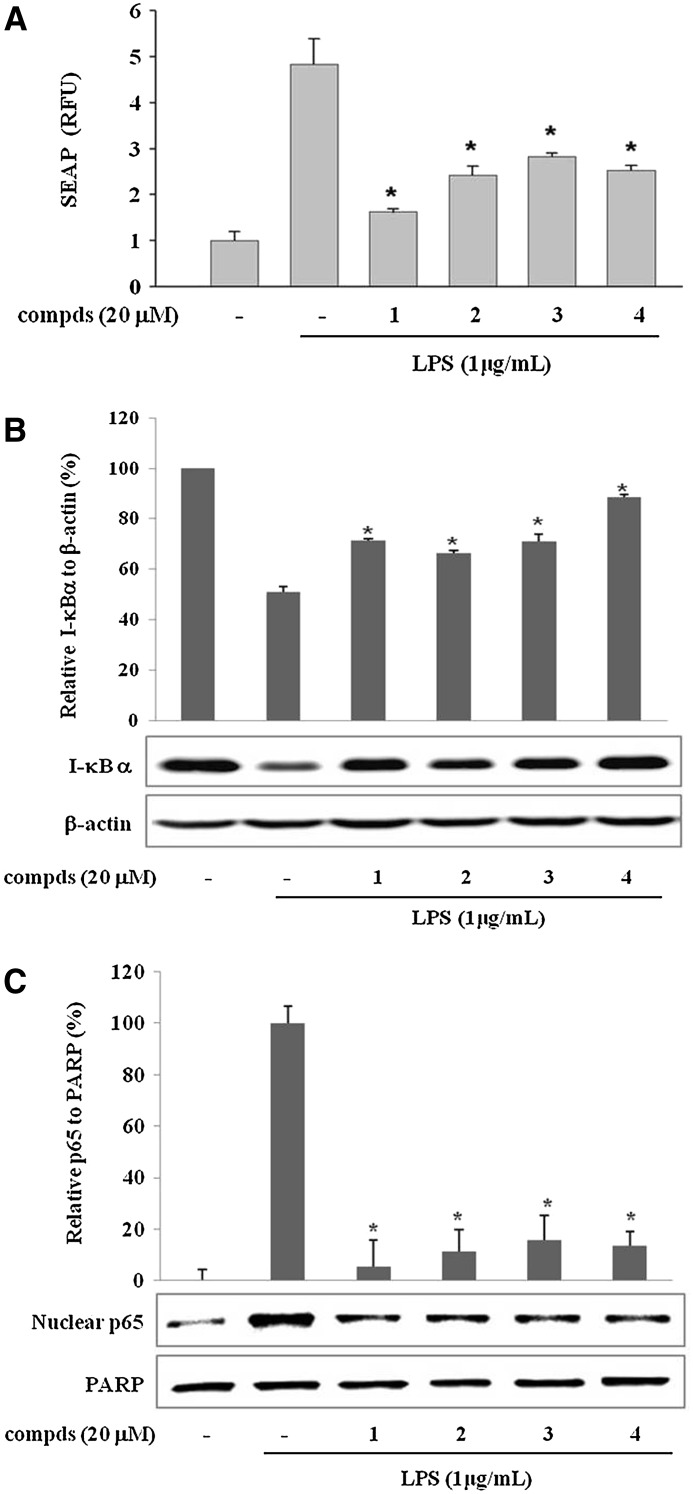
To reveal the molecular mechanism of the suppression of LPS-induced pro-inflammatory enzyme and cytokine expressions by the sulfur compounds 1–4, the transcriptional activity of NF-κB was determined by using a reporter gene assay system. NF-κB is an important transcription factor complex controlling the expression of cell survival genes as well as pro-inflammatory enzymes and cytokines such as iNOS, COX-2, TNF-α, IL-1β, and IL-6.34 T-RAW 264.7 cells, a murine macrophage cell line transfected by pNF-κB-SEAP-NPT reporter construct, were treated with compounds 1–4 and/or LPS (1 μg/mL) for 16 h. As shown in Figure 4A, LPS treatment increased the SEAP activity by the transcriptional activation of NF-κB in T-RAW 264.7 cells. Compounds 1–4 significantly suppressed the LPS-induced NF-κB activation at 20 μM concentration. This result suggests that sulfur compounds 1–4 have a suppressive effect on the activation of NF-κB, which regulates the expression of pro-inflammatory genes in activated macrophages.
FIG. 4.
Sulfur compounds 1–4 from garlic suppress the activation of nuclear factor-κB in LPS-stimulated macrophages. (A) Effect of sulfur compounds on LPS-induced nuclear factor-κB transcriptional activation in T-RAW cells. T-RAW cells were treated with compds for 2 h prior to stimulation by LPS for 16 h. Data are mean±SD values of three individual experiments. *P<.01, significant difference from LPS alone. SEAP, secretory alkaline phosphatase. (B, C) Effect of sulfur compounds on (B) inhibitory-κBα (I-κBα) degradation and (C) p65 translocation to the nucleus in LPS-stimulated RAW 264.7 macrophages. Cells were pretreated with compds for 30 min prior to LPS treatment for 15 min. Cytoplasmic and nuclear extracts were prepared for the western blotting of I-κBα and p65, respectively. Images are representative of three independent experiments that showed similar results. The relative intensity of (B) I-κBα/β-actin bands and (C) p65/poly(ADP-ribose) polymerase (PARP) bands was measured by densitometry. Data are mean±SD values of three individual experiments. *P<.01, significant difference from LPS alone.
Next, we checked whether the active sulfur compounds affect the LPS-induced I-κBα degradation. NF-κB, composed of p50 and p65 subunits, is located in the cytoplasm as an inactive p50/p65 dimer that is physically combined with I-κB.35 In response to pro-inflammatory stimuli, I-κB is phosphorylated, ubiquitinated, and rapidly degraded to release and activate p50/p65. Active NF-κB (p50/p65 dimer) translocates to the nucleus and induces the expression of pro-inflammatory genes.35 The I-κBα was fully degraded by a 15-min incubation with LPS (1 μg/mL) and regenerated gradually afterwards (data not shown). As shown in Figure 4B, the LPS-induced degradation of I-κBα was suppressed by the treatment of 20 μM compounds 1–4 for 15 min. We also investigated whether the compounds prevented the nuclear translocation of the p65 subunit of NF-κB after its release from I-κBα. Treatment with compounds 1–4 decreased the level of nuclear p65 as shown in Figure 4C. Poly(ADP-ribose) polymerase was used as a loading control of nuclear extract in the immunoblot experiment. Taken together, these observations indicate that sulfur compounds 1–4 decrease LPS-induced NF-κB activation by inhibiting the I-κBα degradation and nuclear translocation of NF-κB (see also Supplementary Fig. S3).
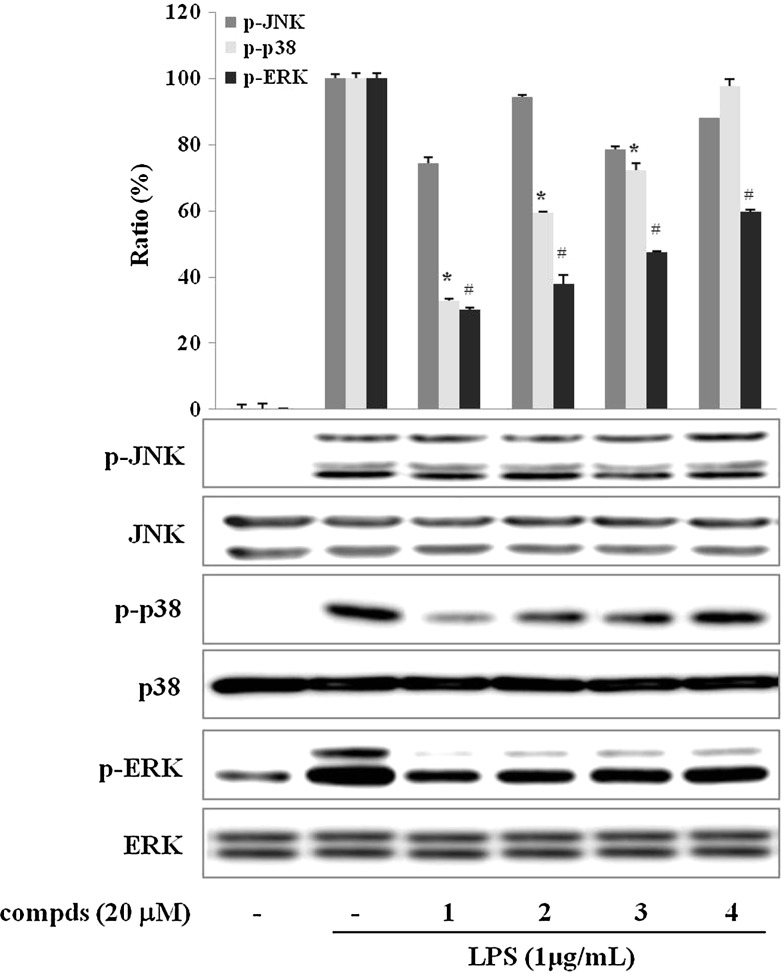
Suppression of phosphorylation of mitogen-activated protein kinases by sulfur compounds in LPS-stimulated RAW 264.7 cells
The mitogen-activated protein kinase (MAPKs) pathways are implicated in the up-regulation of LPS-induced expression of pro-inflammatory mediators in macrophages.36,37 To investigate whether the inhibition of compounds 1–4 against NF-κB activation is mediated through the MAPKs pathway, we observed their effects on the LPS-induced phosphorylation of MAPKs such as p38, c-Jun amino-terminal kinases (JNKs), and extracellular signal-regulated kinases (ERKs). Cells were pretreated with compounds 1–4 for 30 min and then stimulated with LPS (1 μg/mL) for 15 min. As shown in Figure 5, the phosphorylations of p38, JNK, and ERK were increased by the LPS stimulation. Treatment with compounds 1–4 reduced the LPS-induced phosphorylation of p38 and ERK, whereas phosphorylation of JNK was not changed. Nonphosphorylated p38, JNK, and ERK were not affected by the treatment of LPS and/or sulfur compounds from garlic. The data above suggest that the suppressed phosphorylation of p38 and ERK by sulfur compounds 1–4 may play a part in their inhibition of LPS-induced NF-κB activation and pro-inflammatory responses in RAW 264.7 cells.
FIG. 5.
Effect of sulfur compds 1–4 on LPS-induced activation of mitogen-activated protein kinases in LPS-stimulated RAW 264.7 cells. Cells were pretreated with compds for 30 min prior to LPS stimulation. After treatment with LPS for an additional 15 min, proteins were extracted, and the levels of phosphorylated p38, c-Jun amino-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK) were analyzed by western blot. Images are the representative of three independent experiments that shows similar results. The relative intensity of phospho-p38 (p-p38)/p38, phospho-JNK (p-JNK)/JNK, and phospho-ERK (p-ERK)/ERK bands was measured by densitometry. Data are mean±SD values of three individual experiments. P<.01, significant difference from LPS alone for *p-p38 and #p-ERK.
Discussion
Garlic (A. sativum L.) is a common spice for cooking and also a popular herbal remedy for the treatment of wide variety of health problems, including infection, cancer, and cardiovascular and inflammatory diseases. Garlic is rich in sulfur-containing compounds, which are responsible for the most of its biological activities.38
Our previous study showed that garlic extract exerted anti-inflammatory activity by inhibiting Toll-like receptor-mediated signaling pathways at the receptor level.23 In the present study, we isolated four sulfur compounds, 1–4 (Fig. 1), exhibiting anti-inflammatory properties through activity-guided procedures. The structures were identified as Z and E isoforms of ajoene and oxidized sulfonyl ajoene with a diallyl disulfide backbone. We investigated their inhibitory effects on inflammatory responses in LPS-activated RAW 264.7 macrophages.
During inflammatory responses, the activation of macrophages contributes to host damage by excessive release of various inflammatory mediators such as NO and PGE2 and of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. Many studies have demonstrated that the overexpressions of these inflammatory mediators and cytokines are responsible for many chronic inflammatory diseases such as diabetes, atherosclerosis, and rheumatoid arthritis.34,39–41 We demonstrated the inhibitory effect of sulfur compounds 1–4 on the production of the pro-inflammatory mediators NO and PGE2 (Table 1) and also their suppression of iNOS and COX-2 expression (Fig. 2) in the LPS-activated macrophage culture system. Moreover, the levels of IL-1β, IL-6, and TNF-α mRNAs were attenuated by compounds 1–4 in activated RAW 264.7 macrophages (Fig. 3). These data suggest that sulfur compounds 1–4 are the major anti-inflammatory principles of garlic. In the present study, we found that ajoenes as well as their oxidized derivatives showed strong anti-inflammatory activity and that Z-ajoene (1) is the most potent among them.
Yoshida et al.42 demonstrated that Z-ajoene exhibits an antimicrobial activity at least twofold higher than that of the E isomer. The Z-ajoene isomer is also reported to be more potent than its E isomer for antithrombotic activity and the inhibition of cancer cell proliferation.28,43–45 The study of Block et al.28 was undertaken to compare the antithrombotic activity of two ajoene isomers and its homologs. It has been suggested that Z-ajoene and its homologs are more active than E isomers, and these activities are retained upon the oxidation of the sulfonyl derivatives of ajoene but are lost upon reduction of the sulfinyl group to a sulfide group. These trends are also observed in the present study, suggesting that Z-ajoene exhibits more potent anti-inflammatory activity than the E isomer and that the oxidized derivatives of ajoene also possess significant anti-inflammatory activity.
NF-κB is a master switch for the regulation of pro-inflammatory genes, including IL-1β, IL-6, and TNF-α, as well as the hallmarks of inflammation, NO and PGE2.34 NF-κB is present in cytoplasm as a heterodimer consisting of p50 and p65, which are bound by I-κBα in resting macrophages. Upon activation by proper stimulation, phosphorylation and degradation of I-κBα release the p50/p65 complex. The released NF-κB translocates to the nucleus and regulates the transcription of target genes through the binding to specific sequences in the DNA.35 We observed that sulfur compounds 1–4 from garlic decreased the LPS-induced nuclear accumulation of the p65 subunit of NF-κB through the inhibition of I-κBα degradation (Fig. 4B and C). This suggests that garlic compounds inhibit the transcriptional activity of NF-κB and stabilize I-κBα, thereby suppressing the nuclear translocation of NF-κB (Fig. 4A).
The MAPKs signaling pathways are involved in LPS-induced iNOS and COX-2 expression in activated macrophages. Moreover, it has been demonstrated that MAPKs play critical roles in the activation of NF-κB.46 However, they seems be differently involved in the response of anti-inflammatory compounds in macrophages.47,48 In the present study, the four sulfur compounds were found to inhibit the phosphorylation of p38 and ERK, but not that of JNK, in LPS-activated RAW 264.7 macrophages (Fig. 5).
When the data are taken as a whole, the four sulfur compounds 1–4 from garlic significantly suppressed the production of inflammatory mediators such as NO and PGE2 and the expression of IL-1β, IL-6, and TNF-α mRNAs. They also decreased mRNA and protein levels of iNOS and COX-2 by the suppression of LPS-induced NF-κB activation, and they also decreased the phosphorylation of p38 and ERK. These sulfur compounds, the ajoenes and their oxidized analogs from garlic, may be promising therapeutic agents for the treatment of inflammation-related diseases.
Supplementary Material
Acknowledgments
This work was supported by Sookmyung Women's University Research Grants in 2010 and by the MRC program (2011-0030699) through the National Research Foundation of Korea funded by the Korean Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 2.Ginter E. Simko V. Garlic (Allium sativum L.) and cardiovascular diseases. Bratisl Lek Listy. 2010;111:452–456. [PubMed] [Google Scholar]
- 3.Silagy C. Neil A. Garlic as a lipid lowering agent—a meta-analysis. J R Coll Physicians Lond. 1994;28:39–45. [PMC free article] [PubMed] [Google Scholar]
- 4.Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001;131:1010S–1015S. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- 5.El-Sayyad HI. Abou-El-Naga AM. Gadallah AA. Bakr IH. Protective effects of Allium sativum against defects of hypercholesterolemia on pregnant rats and their offspring. Int J Clin Exp Med. 2010;3:152–163. [PMC free article] [PubMed] [Google Scholar]
- 6.Lamm DL. Riggs DR. Enhanced immunocompetence by garlic: role in bladder cancer and other malignancies. J Nutr. 2001;131:1067S–1070S. doi: 10.1093/jn/131.3.1067S. [DOI] [PubMed] [Google Scholar]
- 7.Kyo E. Uda N. Kasuga S. Itakura Y. Immunomodulatory effects of aged garlic extract. J Nutr. 2001;131:1075S–1079S. doi: 10.1093/jn/131.3.1075S. [DOI] [PubMed] [Google Scholar]
- 8.Hobauer R. Frass M. Gmeiner B. Kaye AD. Frost EA. Garlic extract (Allium sativum) reduces migration of neutrophils through endothelial cell monolayers. Middle East J Anesthesiol. 2000;15:649–658. [PubMed] [Google Scholar]
- 9.Hofbauer R. Frass M. Gmeiner B. Kaye AD. Frost EA. Effects of garlic extract (Allium sativum) on neutrophil migration at the cellular level. Heart Dis. 2001;3:14–17. doi: 10.1097/00132580-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Hodge G. Hodge S. Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. 2002;48:209–215. doi: 10.1002/cyto.10133. [DOI] [PubMed] [Google Scholar]
- 11.Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136:716S–725S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 12.Jacob C. A scent of therapy: pharmacological implications of natural products containing redox-active sulfur atoms. Nat Prod Rep. 2006;23:851–863. doi: 10.1039/b609523m. [DOI] [PubMed] [Google Scholar]
- 13.Keiss HP. Dirsch VM. Hartung T, et al. Garlic (Allium sativum L.) modulates cytokine expression in lipopolysaccharide-activated human blood thereby inhibiting NF-kappaB activity. J Nutr. 2003;133:2171–2175. doi: 10.1093/jn/133.7.2171. [DOI] [PubMed] [Google Scholar]
- 14.Ide N. Lau BH. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. J Nutr. 2001;131:1020S–1026S. doi: 10.1093/jn/131.3.1020S. [DOI] [PubMed] [Google Scholar]
- 15.Ho SE. Ide N. Lau BH. S-Allyl cysteine reduces oxidant load in cells involved in the atherogenic process. Phytomedicine. 2001;8:39–46. doi: 10.1078/0944-7113-00005. [DOI] [PubMed] [Google Scholar]
- 16.Liu KL. Chen HW. Wang RY, et al. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-kappaB activation in RAW 264.7 macrophages. J Agric Food Chem. 2006;54:3472–3478. doi: 10.1021/jf060043k. [DOI] [PubMed] [Google Scholar]
- 17.Dirsch VM. Kiemer AK. Wagner H. Vollmar AM. Effect of allicin and ajoene, two compounds of garlic, on inducible nitric oxide synthase. Atherosclerosis. 1998;139:333–339. doi: 10.1016/s0021-9150(98)00094-x. [DOI] [PubMed] [Google Scholar]
- 18.Amagase H. Petesch BL. Matsuura H. Kasuga S. Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 19.Apitz-Castro R. Badimon JJ. Badimon L. Effect of ajoene, the major antiplatelet compound from garlic, on platelet thrombus formation. Thromb Res. 1992;68:145–155. doi: 10.1016/0049-3848(92)90030-e. [DOI] [PubMed] [Google Scholar]
- 20.Naganawa R. Iwata N. Ishikawa K, et al. Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic. Appl Environ Microbiol. 1996;62:4238–4242. doi: 10.1128/aem.62.11.4238-4242.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor P. Noriega R. Farah C, et al. Ajoene inhibits both primary tumor growth and metastasis of B16/BL6 melanoma cells in C57BL/6 mice. Cancer Lett. 2006;239:298–304. doi: 10.1016/j.canlet.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Dirsch VM. Vollmar AM. Ajoene, a natural product with non-steroidal anti-inflammatory drug (NSAID)-like properties? Biochem Pharmacol. 2001;61:587–593. doi: 10.1016/s0006-2952(00)00580-3. [DOI] [PubMed] [Google Scholar]
- 23.Youn HS. Lim HJ. Lee HJ, et al. Garlic (Allium sativum) extract inhibits lipopolysaccharide-induced Toll-like receptor 4 dimerization. Biosci Biotechnol Biochem. 2008;72:368–375. doi: 10.1271/bbb.70434. [DOI] [PubMed] [Google Scholar]
- 24.Lowenstein CJ. Hill SL. Lafond-Walker A, et al. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Invest. 1996;97:1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH. Soyoola E. Chanmugam P, et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- 27.Block E. Ahamad S. (E,Z)-Ajoene a potent antithrombotic agent from garlic. J Am Chem Soc. 1984;106:8295–8296. [Google Scholar]
- 28.Block E. Ahmad S. Catalfamo JL. Jain MK. Apitz-Castro R. Antithrombotic organosulfur compounds from garlic: structural, mechanistic and synthetic studies. J Am Chem Soc. 1986;108:7045–7055. [Google Scholar]
- 29.Lawson LD. Wang Z-YJ. Hughes BG. Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products. Planta Med. 1991;57:363–370. doi: 10.1055/s-2006-960119. [DOI] [PubMed] [Google Scholar]
- 30.Green LC. Wagner DA. Glogowski J. Skipper PL. Wishnok JS. Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 31.Lee HJ. Lim HJ. Lee DY, et al. Carabrol suppresses LPS-induced nitric oxide synthase expression by inactivation of p38 and JNK via inhibition of I-kappaB alpha degradation in RAW 264.7 cells. Biochem Biophys Res Commun. 2010;391:1400–1404. doi: 10.1016/j.bbrc.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 32.Ahn KS. Noh EJ. Zhao HL, et al. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-kappaB activation in RAW 264.7 cells. Life Sci. 2005;76:2315–2328. doi: 10.1016/j.lfs.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 33.Li H. Kim JY. Hyeon J, et al. In vitro antiinflammatory activity of a new sesquiterpene lactone isolated from Siegesbeckia glabrescens. Phytother Res. 2011;25:1323–1327. doi: 10.1002/ptr.3420. [DOI] [PubMed] [Google Scholar]
- 34.Surh YJ. Chun KS. Cha HH, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001:480–481. doi: 10.1016/s0027-5107(01)00183-x. 243–268. [DOI] [PubMed] [Google Scholar]
- 35.Karin M. Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 36.Chen C. Chen YH. Lin WW. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999;97:124–129. doi: 10.1046/j.1365-2567.1999.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SH. Kim J. Sharma RP. Inhibition of p38 and ERK MAP kinases blocks endotoxin-induced nitric oxide production and differentially modulates cytokine expression. Pharmacol Res. 2004;49:433–439. doi: 10.1016/j.phrs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Liu CT. Sheen LY. Lii CK. Does garlic have a role as an antidiabetic agent? Mol Nutr Food Res. 2007;51:1353–1364. doi: 10.1002/mnfr.200700082. [DOI] [PubMed] [Google Scholar]
- 39.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH. Soyoola E. Chanmugam P, et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- 41.Lin WW. Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida H. Iwata N. Katsuzaki H, et al. Antimicrobial activity of a compound isolated from an oil-macerated garlic extract. Biosci Biotechnol Biochem. 1998;62:1014–1017. doi: 10.1271/bbb.62.1014. [DOI] [PubMed] [Google Scholar]
- 43.Li M. Ciu JR. Ye Y, et al. Antitumor activity of Z-ajoene, a natural compound purified from garlic: antimitotic and microtubule-interaction properties. Carcinogenesis. 2002;23:573–579. doi: 10.1093/carcin/23.4.573. [DOI] [PubMed] [Google Scholar]
- 44.Hunter R. Kaschula CH. Parker IM, et al. Substituted ajoenes as novel anti-cancer agents. Bioorg Med Chem. 2008;18:5277–5279. doi: 10.1016/j.bmcl.2008.08.056. [DOI] [PubMed] [Google Scholar]
- 45.Kaschula CH. Hunter R. Parker MI. Garlic-derived anticancer agents: structure and biological activity of ajoene. Biofactors. 2010;36:78–85. doi: 10.1002/biof.76. [DOI] [PubMed] [Google Scholar]
- 46.Carter AB. Knudtson KL. Monick MM. Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 47.Chi H. Barry SP. Roth RJ, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watters JJ. Sommer JA. Pfeiffer ZA, et al. A differential role for the mitogen-activated protein kinases in lipopolysaccharide signaling: the MEK/ERK pathway is not essential for nitric oxide and interleukin 1beta production. J Biol Chem. 2002;277:9077–9087. doi: 10.1074/jbc.M104385200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.