Abstract
This article presents major epidemiologic features of tick-borne encephalitis (TBE) in the Czech Republic, using data of laboratory-confirmed cases since 1970. A total of 17,053 cases of TBE were reported in the Czech Republic (population 10 million) in 1970–2008. The data show several important features. First, the pattern of TBE incidence changed over time. Until the end of the 1970s, TBE was characterized by periods of alternately higher and lower incidence (between 180 and 595 cases per year); the 1980s were a period of low incidence with minimum variability; since the beginning of the 1990s, there has been a steep rise in incidence, with marked year-to-year variation (e.g., 745 cases were registered in 1995, and a maximum of 1029 cases were registered in 2006). Second, the age distribution of TBE incidence has changed. Until the end of 1990s, incidence peaked among those 15–19 years of age, with a gradual decline with age. In the 2000s, however, TBE incidence has been rising in those aged 60–64 years, with a sharp decline in those older than 65 years. Third, the seasonal pattern of TBE has changed markedly over time. In the earlier period, incidence had a clear peak in July/August; since the 1990s, more cases have occurred in earlier and later months of the year. The proportion of cases occurring in April, May, October, and November increased from 9% in the 1970s to 23% in 2000–2008. Fourth, the geographical distribution of TBE also changed over time, with TBE increasingly occurring in the mountainous districts at higher altitudes. These changes in incidence patterns appear to be linked with changes in climatic and meteorological conditions. The link between climate change and TBE incidence is plausible, since TBE is a recreation-related infection associated with outdoor activities, and since climatic changes affect the life cycle of the vector.
Key Words: Climate variation, Epidemiology, Surveillance, Tick-borne encephalitis, Vector-borne
Introduction
Until the end of the 1970s, the incidence of tick-borne encephalitis (TBE) in the Czech Republic (population 10 million) was characterized by periods of relatively low incidence, but large year-to-year variations (from 180–595 cases annually). The 1980s were a period of low occurrence and slight variability. At the beginning of the 1990s, there was a steep rise in registered cases (since 1993), and this situation, with some year-to-year variation, continues to this day. A large number of cases (745) was registered in 1995, and the maximum number of cases reported to date (1029 cases) was registered in 2006 (Danielova and Benes 1997; Danielova et al. 2004; Daniel et al. 2008; Süss 2008; Randolph et al. 2008).
The following features of the changing epidemiology of TBE in the Czech Republic have previously been described: (1) increased numbers of cases in regularly-manifest natural foci of TBE infection (Danielova et al. 2004); (2) a renewed appearance of TBE in locations of former occurrence after 20 or more years, a phenomenon that also seems to be connected regionally with the problem of high SO2 pollution of the natural environment that has in the past negatively influenced ecosystems supporting TBE virus circulation (Kříž et al. 2004; Danielova et al. 2004); (3) the appearance of new regions where TBE did not previously occur (or occurred only sporadically), mainly at higher altitudes (Daniel et al. 2004; Danielova et al. 2004, 2008a, 2008b; Süss, 2008). The distribution of registered cases (by locality of infection) has been cartographically processed into the Atlas of TBE (Daniel and Kříž, 2002), which clearly depicts the major regions of TBE occurrence and its connections with certain types of landscape and vegetation characteristics. Socioeconomic analysis (Kříž et al. 2004) has shown that TBE is a recreation-related infection in the Czech Republic connected with outdoor activities.
The changes in the seasonal appearance of TBE (shifts to the early spring and late autumn months) were also accompanied by a second (autumn) peak in the annual incidence of TBE (Daniel et al. 2006a).
The change in the epidemiology of TBE seems to be associated with changes in climatic and meteorological conditions (Daniel et al. 2006a, 2008a; Hartelt et al 2008; Schwaiger and Bauer 2009; Süss 2008). Research has demonstrated an important role of climate-linked conditions in the ecology of the vector Ixodes ricinus, connected with changes in the I. ricinus population (Daniel et al. 2004; Danielova et al. 2008a). TBE occurrence in new areas (or higher incidences in places of formerly sporadic occurrence) is closely associated with wider distribution of I. ricinus (from the former 700 m to today's 1200 m a.s.l.), again linked to climate change (Danielova and Beneš 1997; Daniel et al. 2006a, 2008b; Holtzmann and Heinz, 2008). The risk assessment and prediction of I. ricinus tick questing activity and human TBE in the Czech Republic have also been studied and evaluated (Daniel et al. 2006b, Daniel et al. 2010).
The primary aim of this article is to review and describe the changes in the incidence of TBE in the Czech Republic, and the major factors involved in these changes. Specifically, we describe the variations of incidence in the general population, age- and sex-specific incidence, case fatality rates, seasonality, and geographic distribution.
Materials and Methods
This study used routinely collected data described in detail below.
Data on population
The population data used for the calculation of the incidence rates were obtained from the Czech Statistical Office. Population size (by age, sex, and geographic area) is estimated from census data (conducted every 10 years); mortality is collected by a nationwide death register.
Data on TBE
The system of reporting TBE in the Czech Republic is as follows. Laboratory diagnostics of TBE are carried out by determining IgM antibodies in the serum or cerebrospinal fluid (CSF) of patients with the aid of enzyme-linked immunosorbent assay (ELISA), by the indirect immunofluorescence technique, by the determination of seroconversion or a significant rise (at least fourfold) in class IgG antibodies, or of an increase in total antibodies with the aid of ELISA, indirect immunofluorescence, or complement fixation reaction (CFR). In patients recently vaccinated against yellow fever or Japanese encephalitis, and in subjects returning from endemic regions of those viruses, or exposed to the dengue and West Nile viruses, serological results have to be confirmed by the virus neutralizing test.
According to a ministerial decree of 1970, only laboratory-confirmed cases of TBE should be reported to the central surveillance center. The number of variables collected for TBE cases has gradually increased since 1982. Since 1993, the national reporting system (EPIDAT) has been computerized.
Each case of TBE is reported by the diagnosing physician to public health authorities, who carry out epidemiological investigations. Through an interview with the patient, the medical epidemiologist or infection physician obtains the medical history, including the probable time and place of infection and the possible route of transmission. The data are recorded in a standardized questionnaire, which includes the location (GIS grid) and route of transmission. These data are electronically transferred on a weekly basis to a safeguarded data depository of the Ministry of Health, from which they are transmitted to the National Institute of Public Health.
Statistical analysis
Data presented in this report have been analyzed as follows. The analysis of continuous variables was based on models of analysis of variance and linear regression; aggregate data on the incidence of TBE have been analyzed by Poisson regression. Where appropriate, autocorrelation of residuals was taken into account using the Newey-West standard errors estimate. Categorical data were tested with the Fisher exact test and the Armitage test for trend in proportions. All statistical tests have been carried out at the 0.05 level of significance. The data were analyzed by Epi-Info 6.04 (Centers for Disease Control and Prevention, Atlanta, GA), and Stata version 9.2 (StataCorp LP, College Station, TX).
Results
Incidence of TBE cases in the general population
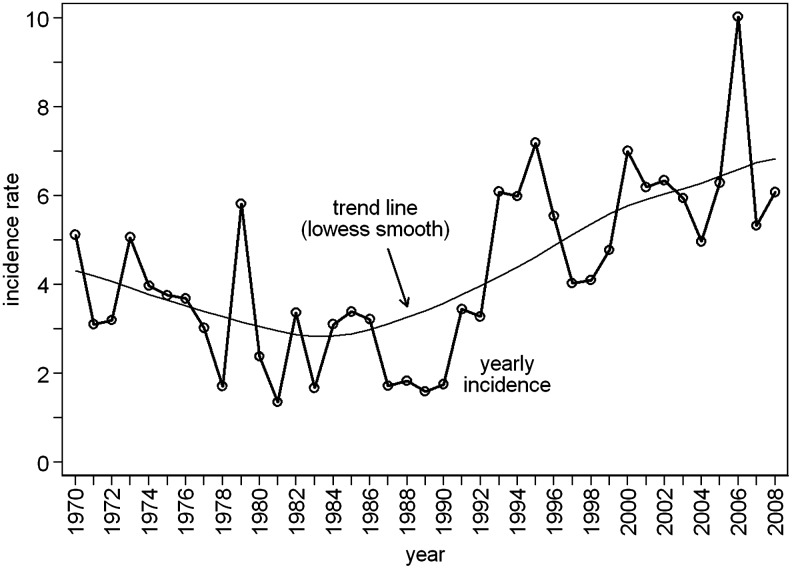
A total of 17,053 cases of tick-borne encephalitis were reported in the Czech Republic between 1970 and 2008. Three periods with different patterns of TBE incidence are apparent: a relatively large variation in incidence in the 1970s, lower occurrence in the 1980s, and a rising trend in the following period up to the present. The decrease in incidence of TBE between 1970 and 1989, and the increase seen between 1990 and 2008, were statistically significant (p<0.001 and p=0.025, respectively).
Following the peak incidence of 10.0/100,000 in 2006, the incidence declined to 5.3/100,000 in 2007, and rose again to 6.1/100,000 in 2008 (Fig. 1). Men have been affected more than women, with a male:female ratio of 1.5 (range 1.3–1.9) over the entire study period. The trends of standardized incidence during the period from 1982–2008 were similar in men and women, and the male:female ratio remained stable over time (p=0.572 for the interaction between age and time period).
FIG. 1.
Tick-borne encephalitis in the Czech Republic 1971–2008, trend in yearly incidence per 100,000 population.
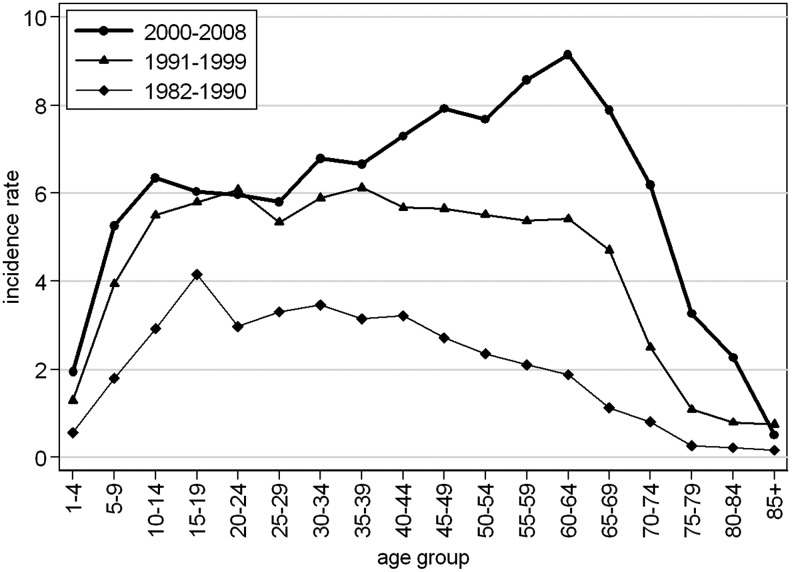
Age-specific incidence of TBE cases
The age-specific incidence changed substantially over time. The age distribution of cases in three time periods (1982–1990, 1991–1999, and 2000–2008) is shown in Figure 2. In the first period, the incidence was highest in those aged 15–19 years. The pattern in the second period was similar. In the third period, however, the pattern was different: following a steep rise in incidence in the younger age groups, with a peak in 5- to 14-year-olds, the incidence rose in those aged 65–69 years, followed by a steep decrease in this group (Fig. 2). The difference in the distribution of incidence by age group between the three periods was statistically significant (p<0.001).
FIG. 2.
Tick-borne encephalitis in the Czech Republic, 1982–2008, incidence per 100,000 population by age group and time period.
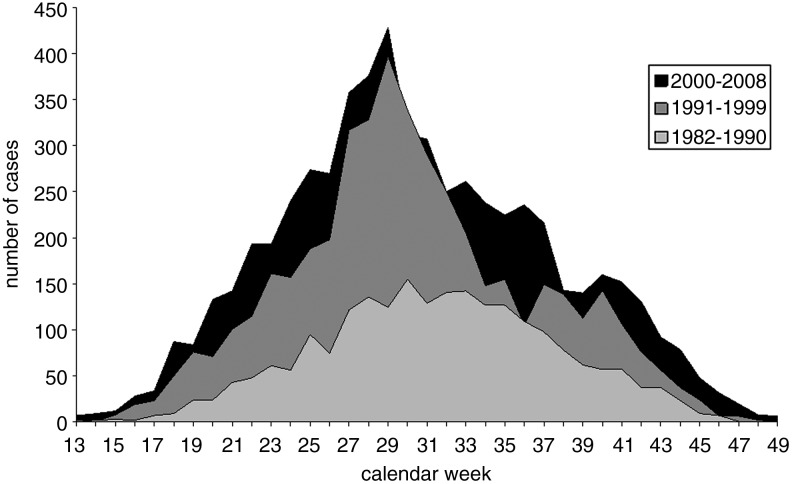
Seasonality of TBE incidence
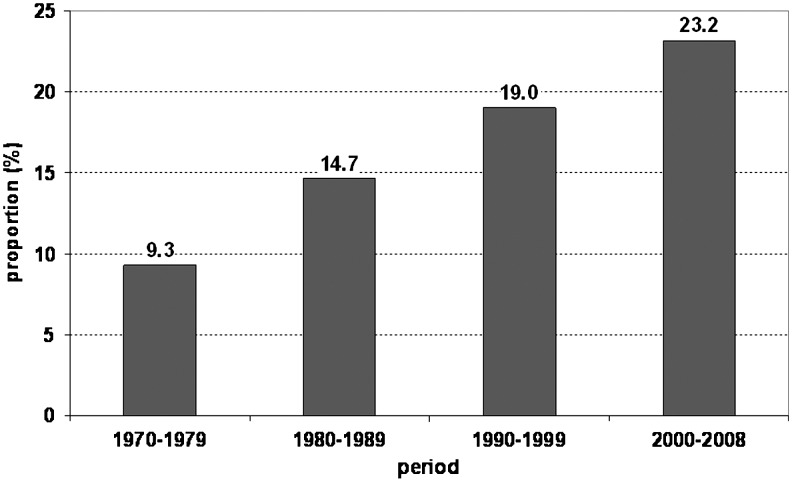
The seasonal distribution (absolute frequencies by week of onset) of TBE cases in the three time periods is shown in Figure 3. In the first period (1982–1990), the seasonal curve was relatively flat, reaching a maximum in weeks 30–34 (i.e., towards the end of July and throughout August). The two later periods show a more pronounced peak, around calendar week 29 (mid-July). The total numbers of cases were higher in 1991–1999 and 2000–2008 than in the period from 1982–1990. In addition, since the 1990s the incidence spreads into the earlier and later calendar weeks (Fig. 4). This resulted in a higher proportion of TBE occurrence in the spring and autumn months over time as well. In 1970–1979, the proportion of cases occurring in the months of April, May, October, and November of the total number of cases was 9.3%; in the years 2000–2008 it was 23.2%. The Armitage test for linear trends demonstrates the significantly increasing proportion of cases in those months in the four periods (p<0.001).
FIG. 3.
Tick-borne encephalitis in the Czech Republic, 1982–2008, by week of onset and period.
FIG. 4.
Tick-borne encephalitis in the Czech Republic, 1970–2008, proportion of cases with onset in the months April, May, October, and November, of the total in each period (based on 16,489 cases with known date of onset).
Geographic distribution of TBE cases
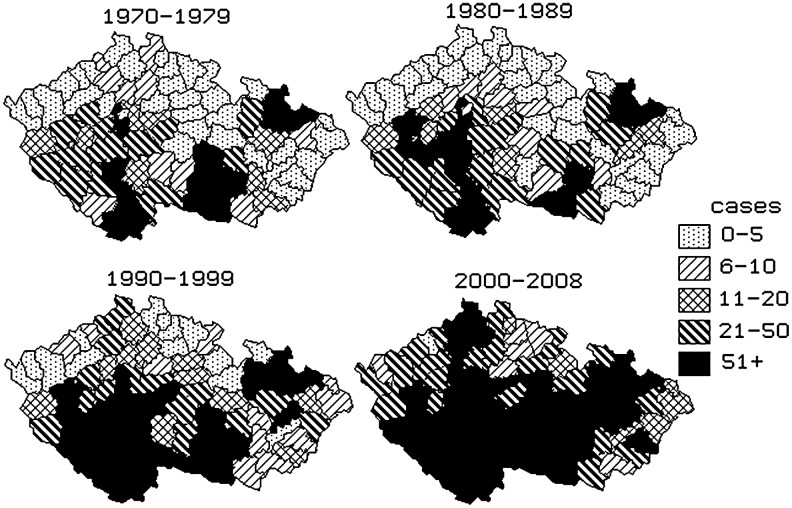
The dynamics of geographical distribution of TBE by district in the Czech Republic in the four decades is shown in Figure 5. The cartograms illustrate the gradual spread of TBE into formerly unaffected districts, namely in the border districts of the country at higher altitudes. Those regions are mostly hilly or mountainous.
FIG. 5.
Tick-borne encephalitis, Czech Republic, 1970–2008, reported cases by district of infection and period.
Case fatality rate
Over the period 1970–2008 there have been 90 deaths, representing an overall case fatality rate of 0.55%. More than half of the fatal cases occurred in the age group 50–69 years. The highest mortality, 0.83%, was found in the period from 1970–1979; overall, however, the case-fatality rate remained stable (Table 1).
Table 1.
Mortality and Case Fatality Rates of Tick-Borne Encephalitis Cases in 1970–2008
| |
No.of deaths by age group |
|
|
||||
|---|---|---|---|---|---|---|---|
| Period | Total (%) | 1–19 | 20–49 | 50–69 | 70+ | No.of cases | Case fatality rate % |
| 1970–1979 | 32 (36.78) | 2 | 8 | 18 | 4 | 3856 | 0.83 |
| 1980–1989 | 9 (10.34) | 0 | 3 | 2 | 4 | 2460 | 0.37 |
| 1990–1999 | 20 (22.99) | 1 | 5 | 12 | 2 | 4776 | 0.42 |
| 2000–2008 | 26 (29.89) | 1 | 2 | 14 | 9 | 5961 | 0.44 |
| Total | 87 | 4 | 18 | 46 | 19 | 17053 | 0.51 |
| % | 100.00 | 4.60 | 20.69 | 52.87 | 21.84 | ||
Discussion
TBE laboratory data confirmed that cases registered in the Czech Republic present a unique and reliable dataset that allows the evaluation of temporal changes in the epidemiology of TBE over several decades, which coincide with major changes in climatic variables. Our results suggest major changes in a number of fundamental features of TBE epidemiology: an increase in the overall incidence rate, a shift in the peak of age-specific incidence rates to older age groups, changes in the seasonal pattern towards earlier and later months of the year, and changes of the geographical distribution and spread of TBE to mountainous areas.
These profound and rapid changes in the epidemiological patterns are likely associated with climatic changes and environmental factors affecting the transmission of TBE and the life cycle of its vector.
We have previously found that TBE incidence was associated with climate changes observed during the same periods. The impact of these changes on the incidence of TBE appeared to be primarily mediated by the effect of temperature changes on the ecology of the tick I. ricinus, the main vector of the TBE virus, and to some extent, also on human behavior and outdoor recreational activities during tick host-seeking activity (Danielova et al. 2008a).
The natural conditions of the Czech Republic are favorable for the survival and development of the I. ricinus tick and the reservoir animals. The configuration of the landscape, where fields alternate with deciduous and coniferous woods and forests, with an abundance of game animals, which also participate in the transmission of TBE, is favorable for the maintenance of TBE. The mean annual temperature of 7.5°C (range 6.3–9.1°C) over the study period, and relatively small geographical differences between different regions of the country (the mountains at the borders of the Czech Republic rarely exceed 1250 m in height) present suitable conditions for both the vector and the reservoir.
Since the 1990s, an increase in TBE incidence was reported in several other countries of central and western Europe, including Germany, Slovakia, Switzerland, Slovenia, and Poland (Süss et al. 2004; Kunze 2007; Sumilo et al. 2008; Donoso Mantke 2008). In Austria, this increase has been less pronounced due to a high vaccination rate. In the Czech Republic, the vaccination rate in the population remains relatively low at around 17%, despite public education campaigns (GFK Gruppe 2008). The gradual improvement in public awareness about the increasing incidence of TBE in some countries in the 1990s has led to increased interest in this issue and increased vaccination rates in other countries. This could explain the delayed increase in TBE incidence seen in countries of northern Europe and the Baltic States (Kunze 2007).
The increase in incidence in higher altitudes has been observed since the beginning of the 1990s. This has mainly affected the mountainous regions at the borders with Austria, Germany, and Poland, as well as in the Highland Region within the Czech Republic between Bohemia and Moravia, which are characterized by a cooler climate (Danielova et al. 2008b).
In the mountain region of Krkonoše, at 1250 m a.s.l. (N.E. Bohemia), all developmental stages of I. ricinus have been found, and it has also been shown that ticks were able to complete their development up to at least 1000 m a.s.l. during 2006 (Materna et al. 2005, 2008). It is likely that in the future ticks may become more common at higher altitudes in response to climate change (Gilbert 2010).
The maps of TBE incidence in Czech regions clearly illustrate the dynamics of the changes in TBE occurrence. Over the study period, land use has not changed substantially. The collectivized mode of farming introduced by the Communist regime in the 1950s continues today, and the tradition of individual family farms has not been renewed.
Instead, the land owners have become members or shareholders in collective farms. Farming of small animals, such as goats and sheep, in the border regions plays an insignificant role in the spread of TBE in the Czech Republic (Kříž et al. 2004). Previous research suggests that the spread of I. ricinus ticks to new regions cannot be explained solely by changes in the economic exploitation of the land, as was anticipated in the Baltic States (Sumilo et al. 2008).
The effects of air pollution with sulfur oxides have been paradoxical. Air pollution was extreme in the 1970s and 1980s in the northwest region of Bohemia (Krušné Hory) at the border with the former East Germany. The burning of poor-quality coal containing high concentrations of sulfur in power plants resulted in a complete degradation of the entire forest ecosystem. SO2 air concentrations of over 60 μg/m3, acid rain, and snow depressed the I. ricinus population, and nearly extinguished existing TBE virus foci. However, within 10 years after the change of the technology in the power stations, which led to 10-fold decline in air pollution, there was a resurgence of I. ricinus ticks and their reservoir animals. In fact, this region now constitutes 10% of all cases of TBE in the Czech Republic (Kříž et al. 2004).
The shift of disease incidence into the spring and autumn months has been repeatedly demonstrated in the Czech Republic. It has been predicted that warmer winters and hotter summers will further modify the patterns of seasonal activity in the later part of the year (Gray et al. 2009). The seasonal distribution of TBE is similar in different countries of central and western Europe. Cases of TBE are reported in the period from April to November, and only rarely in the other spring and autumn months, and the maximum is seen in July. In Germany, rates are highest from April to November with a maximum in July (Kaiser, 1999); in Slovenia rates are highest from March through November and December with a maximum in July (Logar et al. 2006). In the Czech Republic the first symptoms of TBE are found from late March or early April to November with a maximum in July. The fact that a shift in the seasonal distribution has not been reported in other countries may be explained by a lack of adequate data; for example, in many countries only the date of reporting is available, rather than the date of the beginning of symptoms.
In conclusion, the data available in the Czech Republic suggest gradual but significant changes in several important features of the epidemiology of TBE, including changes in the overall incidence, age-specific distribution, seasonality, and geographic distribution. Climate change is the most likely explanation for these epidemiological shifts. It is possible that the incidence of TBE will increase even further, and if this occurs TBE will become an even more significant public health problem, requiring a more vigorous response from local governments.
Acknowledgement
This study was supported by the Czech Ministry of Health Project grant no. NT11425-5/2010.
Author Disclosure Statement
No competing financial interests exist
References
- Daniel M. Danielová V. Kříž B, et al. Tick- borne encephalitis. In: Menne B, editor; Ebi KL, editor. Climate Change and Adaptation Strategies for Human Health. Darmstadt: Springer; 2006a. pp. 189–205. [Google Scholar]
- Daniel M. Danielová V. Kříž B, et al. An attempt to elucidate the increased incidence of tick-borne encephalitis and spread to higher altitudes in the Czech Republic. Int J Med Microbiol. 2004;293(Suppl 37):55–62. doi: 10.1016/s1433-1128(04)80009-3. [DOI] [PubMed] [Google Scholar]
- Daniel M. Kříž B. Danielová V, et al. Sudden increase in tick-borne encephalitis cases in the Czech Republic 2006. Int J Med Microbiol. 2008;298:81–87. [Google Scholar]
- Daniel M. Kříž B. Danielová V, et al. Correlation between meteorological factors and tick-borne encephalitis incidence in the Czech Republic. Parasitol Res. 2008a;103(Suppl 1):S97–S107. doi: 10.1007/s00436-008-1061-x. [DOI] [PubMed] [Google Scholar]
- Daniel M. Kříž B. Tick-borne encephalitis in the Czech Republic. I. Predictive maps of Ixodes ricinus tick high occurrence habitats and tick-borne encephalitis risk assessment in Czech regions. In: Daniel M, editor; Kolář J, editor; Beneš Č, et al., editors; Pejčoch M, editor; Beneš Č, editor; Vymazal J, editor. Project Climate Change and Adaptation Strategies for Human Health in Europe, EVK2-2000-00670. National Institute of Public Health; Praha: 2002. II. Maps of tick-borne encephalitis incidence in the CR. [Google Scholar]
- Daniel M. Kříž B. Valter J, et al. The influence of meteorological conditions of the preceding winter on the incidence of tick-borne encephalitis a L. borreliosis in the Czech Republic. Int J Med Microbiol. 2008b;298:60–67. [Google Scholar]
- Daniel M. Vrábík J. Valter J, et al. Prediction of Ixodes ricinus host-seeking activity using TICKPRO computer program and warning system published on websites in the Czech Republic. Centr Eur J Publ Health. 2010;18:230–236. doi: 10.21101/cejph.a3620. [DOI] [PubMed] [Google Scholar]
- Daniel M. Zitek K. Danielová V, et al. Risk assessment and prediction of Ixodes ricinus tick questing activity and human tick-borne encephalitis infection in space and time in the Czech Republic. Int J Med Microbiol. 2006b;296:41–47. doi: 10.1016/j.ijmm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Danielová V. Beneš Č. Recent situation in tick-borne encephalitis in the Czech Republic. In: Süss J, editor; Kahl O, editor. 4th International Potsdam Symposium on Tick-Borne Diseases: Tick-borne encephalitis and Lyme borreliosis, 21.-22. 2. 1997. Pabst Science Publishers; Lengerich: 1997. pp. 47–56. [Google Scholar]
- Danielová V. Klingrová S. Daniel M, et al. Influence of climate warming on tick-borne encephalitis expansion to higher altitudes during the last decade (1997–2006), Region Highland (Czech Republic) Centr Eur J Publ Health. 2008b;16:4–11. doi: 10.21101/cejph.a3460. [DOI] [PubMed] [Google Scholar]
- Danielová V. Kříž B. Daniel M, et al. Effects of climate change on the incidence of tick-borne encephalitis in the Czech Republic in the past two decades. Epidemiol Mikrobiol Imunol. 2004;53:174–181. (in Czech with English summary). [PubMed] [Google Scholar]
- Danielová V. Schwarzová L. Materna J, et al. Tick-borne encephalitis virus expansion to higher altitudes correlated with climate warming. Int J Med Microbiol. 2008a;298:68–72. [Google Scholar]
- Donoso Mantke O. Schädler R. Niedrig M. A survey on cases of tick-borne encephalitis in European countries. Eurosurveillance. 2008;13:24. [PubMed] [Google Scholar]
- GFK Gruppe. Czech Republic: TBE Vacc Rate Study; 2008. Vaccination rate—household members. [Google Scholar]
- Gilbert L. Altitudinal patterns of tick and host abundance: a potential role for climate change in regulating tick-borne diseases? Oecologia. 2010;162:217–225. doi: 10.1007/s00442-009-1430-x. [DOI] [PubMed] [Google Scholar]
- Gray JS. Dautel H. Estrada-Peña A, et al. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis. 2009;2009:593232. doi: 10.1155/2009/593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartelt K. Pluta S. Oehme R, et al. Spread of ticks and tick-borne diseases in Germany due to global warming. Parasitol Res. 2008;103(Suppl 1):S109–S116. doi: 10.1007/s00436-008-1059-4. [DOI] [PubMed] [Google Scholar]
- Holzmann H. Heinz XH. Tick-borne encephalitis outbreak owing to consumption of fresh cheese from unpasteurized goat milk. Virusepidemiologische Information, Medizinische Universität Wien. 2008;17(8):2–4. [Google Scholar]
- Kaiser R. The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994–98: a prospective study of 656 patients. Brain. 1999;122:2067–2078. doi: 10.1093/brain/122.11.2067. [DOI] [PubMed] [Google Scholar]
- Kunze U. Tick-borne encephalitis: from epidemiology to vaccination recommendations in 2007. New issues—best practices. Wien Med Wochenschr. 2007;157:228–232. doi: 10.1007/s10354-007-0424-8. [DOI] [PubMed] [Google Scholar]
- Kříž B. Beneš Č. Danielová V, et al. Socio-economic conditions and other anthropogenic factors influencing tick-borne encephalitis incidence in the Czech Republic. Int J Med Microbiol. 2004;293(Suppl 37):63–68. doi: 10.1016/s1433-1128(04)80010-x. [DOI] [PubMed] [Google Scholar]
- Logar M. Bogovic P. Cerar D, et al. Tick-borne encephalitis in Slovenia from 2000 to 2004: comparison of the course in adult and elderly patients. Wien Klin Wochenschr. 2006;118:702–707. doi: 10.1007/s00508-006-0699-6. [DOI] [PubMed] [Google Scholar]
- Materna J. Daniel M. Danielová V. Altitudinal distribution limit of the tick Ixodes ricinus shifted considerably towards higher altitudes in Central Europe: results of the three years monitoring in the Krkonoše Mts. (Czech Republic) Centr Eur J Publ Health. 2005;13:24–28. [PubMed] [Google Scholar]
- Materna J. Daniel M. Metelka L, et al. The vertical distribution, density and the development of the tick Ixodes ricinus in mountain areas influenced by climate changes (The Krkonoše Mts., Czech Republic) Int J Med Microbiol. 2008;298:25–37. [Google Scholar]
- Randolph SE. Asokliene L. Avsic-Zupanc T, et al. Variable spikes in tick-borne encephalitis incidence in 2006 independent of variable tick abundance but related to weather. Parasit Vectors. 2008;1:44. doi: 10.1186/1756-3305-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger K. Bauer J. Epidemiology of emerging and resurging vector-borne diseases with special attention to climate change in Germany (Review) Berl Munch Tierarztl Wochenschr. 2009;122:141–160. [PubMed] [Google Scholar]
- Sumilo D. Bormane A. Asokliene L, et al. Socio-economic factors in the differential upsurge of tick-borne encephalitis in Central and Eastern Europe. Rev Med Virol. 2008;18:81–95. doi: 10.1002/rmv.566. [DOI] [PubMed] [Google Scholar]
- Süss J. Schräder C. Falk U, et al. Tick-borne encephalitis (TBE) in Germany—epidemiological data, development of risk areas and virus prevalence in field-collected ticks and in ticks removed from humans. Int J Med Microbiol. 2004;(Suppl 37):69–79. doi: 10.1016/s1433-1128(04)80011-1. [DOI] [PubMed] [Google Scholar]
- Süss J. Tick-borne encephalitis in Europe and beyond—the epidemiological situation as of 2007. Eurosurveillance. 2008;13:26. [PubMed] [Google Scholar]