Abstract
Human angiostrongyliasis results from accidental infection with Angiostrongylus, an intra-arterial nematode. Angiostrongylus cantonensis infections result in eosinophilic meningitis, and A. costaricensis infections cause eosinophilic enteritis. Immunological methodologies are critical to the diagnosis of both infections, since these parasites cannot be isolated from fecal matter and are rarely found in cerebrospinal fluid samples. A. costaricensis and A. cantonensis share common antigenic epitopes which elicit antibodies that recognize proteins present in either species. Detection of antibodies to a 31-kDa A. cantonensis protein present in crude adult worm extracts is a sensitive and specific method for immunodiagnosis of cerebral angiostrongyliasis. The objective of the present work was to isolate and characterize the 31-kDa proteins using soluble protein extracts derived from adult female worms using both one- (1DE) and two-dimensional (2DE) gel electrophoresis. Separated proteins were blotted onto nitrocellulose and probed using sera from infected and non-infected controls. The 31-kDa band present in 1DE gels and the 4 spots identified in 2DE gels were excised and analyzed by electrospray ionization mass spectrometry. Using the highest scores obtained following Mascot analysis, amino acid sequences were obtained that matched four unique proteins: tropomyosin, the 14-3-3 phosphoserine-binding protein, a protein containing a nascent polypeptide-associated complex domain, and the putative epsilon subunit of coatomer protein complex isoform 2. Oxidative cleavage of diols using sodium m-periodate demonstrated that carbohydrate moieties are essential for the antigenicity of all four spots of the 31-kDa antigen. In this article we describe the identification of the 31-kDa antigen, and provide DNA sequencing of the targets. In conclusion, these data suggest that reactivity to the 31-kDa proteins may represent antibody recognition of more than one protein, and recombinant protein-based assays for cerebral angiostrongyliasis diagnosis may require eukaryotic expression systems to maintain antigenicity.
Key Words: Abdominal angiostrongyliasis, Angiostrongylus, Eosinophilic meningitis, Immunodiagnosis, 31-kDa antigen
Introduction
The nematode Angiostrongylus cantonensis is the most common causative agent of eosinophilic meningoencephalitis (Graeff-Teixeira et al. 2009). Completion of its life cycle requires two hosts: an intermediate mollusk host and a definitive rodent host, typically Rattus norvegicus. The first stage larva (L1) is released in rat feces and mollusks become infected by ingesting organic debris contaminated with L1 larvae. Inside mollusk tissues, L1 larvae develop into the infective third-stage L3 larvae. Rats may ingest L3 larvae that penetrate the mucosa, invade blood vessels, and migrate to the meninges. In the central nervous system (CNS) the larvae mature into young adults (fifth-stage larvae) that complete their maturation inside the pulmonary arteries and right cardiac cavities. Humans can be accidentally infected by ingesting L3 larvae present in contaminated water, or food that is raw or undercooked. In humans, L3 larvae are incapable of completing the life cycle and die in the CNS, resulting in disease.
Cerebral angiostrongyliasis has been reported in Southeast Asia, Africa, Australia, America (Wang et al. 2008), and recently transmission foci have been identified in Brazil (Caldeira et al. 2007; Maldonado et al. 2010) and Ecuador (Pincay et al. 2009). In addition, angiostrongyliasis is considered an emerging public health problem in the United States (Diaz, 2008).
Confirmed diagnosis of cerebral angiostrongyliasis is seldom possible, since larvae are typically not found in cerebrospinal fluid (CSF; Yii, 1976). Several molecular targets have been identified as potential antigens for angiostrongyliasis immunodiagnosis (Eamsobhana and Yong, 2009). However, these targets are not widely available for independent evaluation or testing in either clinical or epidemiological investigations. Standardization of immunological tests requires their validation using various geographical isolates and sera collected from patients with different co-infections to rule out potentially cross-reactive responses. Preparation of large quantities of the target antigens is a complicated and laborious process. Molecular cloning and the expression of recombinant proteins represent a reliable alternative for generating sufficient amounts of well-defined antigens for use in immunodiagnostic assays.
Immuoblotting studies have identified an immunoreactive band with an estimated molecular weight of 31 kDa that has been considered a target for a highly-sensitive and specific antibody detection assay for A. cantonensis infections (Nuamtanong, 1996; Kirsch et al. 2008). Eamsobhana and associates demonstrated that the 31-kDa glycoprotein possessed sugar residues that did not affect antibody recognition (Eamsobhana et al. 1998); furthermore, this protein was purified and employed in enzyme-linked immunosorbent (ELISA) and dot-blot assays, resulting in 100% sensitivity and specificity (Eamsobhana et al. 2003; Eamsobhana and Yong, 2009). Nevertheless the identity of this 31-kDa antigen is unknown.
Heterologous antigens have been used in various immunodiagnostic assays, taking into account the various shared epitopes present between different helminth species. This approach has also been utilized in the diagnosis of angiostrongyliasis, since A. cantonensis and A. costaricensis possess cross-reactive antigens that can be used to diagnose infections with either pathogen (Dekumyoy et al. 2000; Ben et al. 2010). Since A. cantonensis is more easily maintained in the laboratory, proteins from this nematode may be used to identify antigenic targets with potential for use in the diagnosis of infections with either pathogen.
In the present study we characterized the makeup of the 31-kDa A. cantonensis antigen complex using one- (1DE) and two-dimensional (2DE) gel electrophoresis, which allowed the identification of various targets that can be used in the development of recombinant antigens for immunodiagnostic purposes.
Materials and Methods
Biological materials
Worms
Adult A. cantonensis worms were recovered from experimentally-infected rats. A. cantonensis worms were originally obtained from the Department of Parasitology, Akita Medical School, Akita City, Japan, and have been maintained in our laboratory since 1997. Wistar rats served as definitive hosts and Biomphalaria glabrata as intermediate hosts. Rats were infected with 104 larvae by gavage inoculation, and 42 days post-infection the animals were sacrificed and the worms collected.
Antigen preparation
Total extract (TE) was obtained from harvested female worms that were macerated in liquid nitrogen and homogenized in phosphate-buffered saline (PBS; pH 7.4). The suspension was centrifuged at 12,000 g for 1 h at 4°C, and the supernatants were used to derive the TE. Protein concentrations were determined by the Bradford assay using bovine serum albumin as a standard.
Two-dimensional electrophoresis (2DE)
An aliquot of TE that contained 60 μg of total protein was desalted using a 2-D Clean-Up Kit (GE Healthcare, Piscataway, NJ), followed by resolubilization in DeStreak Rehydration Solution (GE Healthcare), with 66 mM DTT and 0.5% carrier ampholytes (v/v). The samples were in-gel rehydrated on 11-cm pH 3–11 NL or 3–6 NL IPG strips (GE Healthcare), and isoeletric focusing was performed using an IPGphor Isoelectric Focusing System (GE Healthcare), with voltages increasing stepwise as follows: 500 V for 500 V h, a linear gradient from 500–8000 V for 6500 V h, followed by a hold at 6000 V for 22,000 V h.
After isoeletric focusing, the strips were soaked for 15 min in fresh equilibration buffer (20% v/v glycerol, 6 M urea, 1% DTT, and 2% SDS). IPG strips were run in the second dimension on 4–12% polyacrylamide Bis-Tris gels with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Bio-Rad, Hercules, CA). The gels were then stained with colloidal Coomassie blue or mass spectrometry-compatible silver stain (Mortz et al. 2001), or transferred to nitrocellulose membranes for immunological analyses.
Western blot analysis
Resolved proteins were electro-transferred onto nitrocellulose membranes using a semi-dry trans-blot apparatus (Bio-Rad). The membrane was washed three times with PBS-T (0.05% Tween), and blocked with 5% skim milk for 1 h at room temperature. The membranes were then incubated for 2 h with a pool of sera (1:200 dilution), prepared from either 20 patients histopathologically diagnosed with abdominal angiostrongyliasis, 20 patients positive for eosinophilic meningoencephalitis, or 20 pooled serum samples from uninfected controls. After three washes, the membranes were probed with a secondary peroxidase-conjugated anti-human IgG (diluted 1:8000; Sigma-Aldrich, St. Louis, MO) for 1 h at room temperature. Diaminobenzidine (DAB) (Sigma-Aldrich; 0.05% DAB and 0.015% H2O2 in PBS, pH 7.4) was added as developer reagent.
Tandem mass spectrometry (MS/MS) analysis
Immunoreactive spots were manually excised from 2DE gels and subjected to in-gel tryptic digestion (Promega, Madison, WI), and mass spectrometric analysis. Electrospray ionization (ESI) mass spectrometric analysis was performed using a Bruker model maXis ESI-Q-TOF instrument interfaced with an on-line nanospray source (Bruker Daltonics, Billerica, MA), to perform LC-MS/MS using a U3000 HPLC configured for nanoliter-per-minute flows. The Dionex U-3000 nanobore HPLC was configured with dual ternary pumps with the flow output of one pump split using a calibrated 1:1000 splitter with an active flow control. This system used a pulled-loop auto sampler configured with a 20-μL sample loop. A desalting trap column (0.3×5 mm, 5 μm C18 PepMap 120 A; Dionex, Sunnyvale, CA), and C18 PepMap (0.075×150 mm, 3 μm, 120 A; Dionex) were used. The solvents used were 0.1% formic acid in water, and 80% acetonitrile/0.1% formic acid. The gradient was 2–55% in 90 min. The eluent from the analytical column was introduced into the maXis using the Bruker on-line nanospray source. The source was operated at a spray voltage of 900 V with a drying gas of nitrogen flowing at 6 L/min. The capillary temperature was set to 150°C. The mass spectrometer was set to acquire line spectra of m/z 50–1900. MS/MS data were acquired in an automated fashion using the three most intense ions from the MS scan with precursor active exclusion for 90 sec after three spectra were acquired for each parent ion. MS data were acquired at a scan speed of 3 Hz and MS/MS data were acquired at a scan speed of 1–1.5 Hz, depending on the intensity of the parent ion. MS internal calibration was achieved by the use of a lock mass (HP-1222; Agilent Technologies, Santa Clara, CA).
Collected data were processed by Data Analysis (Bruker Daltonics), to produce deconvoluted and internally-calibrated data and saved as an xml peaklist, which was searched against the NCBInr database with the Mascot on-line program (http://www.matrixscience.com). The data were acquired in data-dependent mode (DDA), and multiple-charged peptide ions (+2 and +3) were automatically mass selected and dissociated in MS/MS experiments. Mascot search parameters allowed a maximum of one missed cleavage, carbamidomethylation of cysteine as fixed modifications, methionine oxidation as a variable modification, peptide tolerance of 0.2 Da, and MS/MS tolerance of 0.2 Da. The significance threshold was set at p<0.05, and identification required that each protein contain at least one peptide with an expected value <0.05.
Oxidation of the carbohydrates
Carbohydrate moieties were oxidized using sodium periodate to investigate their antigenicity. Proteins were electro-transferred onto nitrocellulose membranes, washed three times with PBS-T, and incubated for 30 min with 100 mM NaOAc (pH 5.0). The membranes were incubated with a sodium m-periodate solution (20 mM NaIO4 diluted in 100 mM NaOAc), and kept at 37°C for 1 h in the dark. After washing with 100 mM NaOAc, the membranes were incubated with 50 mM NaBH4 in PBS-T for 30 min at room temperature, and developed as described above (Western blot analysis).
Results
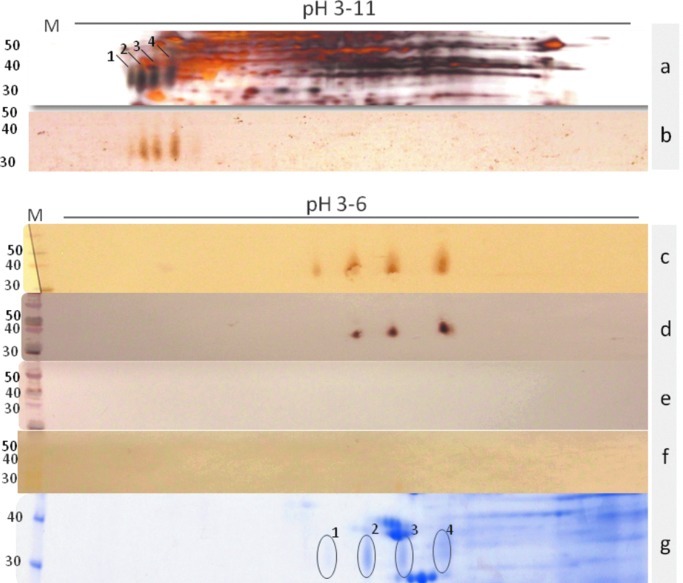
Immunodiagnostic targets were identified in crude female worm extracts using 1DE gel electrophoresis. This analysis identified a 31-kDa band (Fig. 1), which is consistent with previously published data (Eamsobhana et al. 1998). However, 2DE resolved the 31-kDa band into four distinct antigenic spots in the acidic region that appeared elongated and diffuse in shape. These spots were recognized by sera from angiostrongyliasis patients, but not by sera from uninfected controls (Fig. 2). A better separation of immunoreactive spots distributed in the pH range 4–5 was obtained when a 3–6 pH-NL strip was employed (Fig. 2). The sugar moieties of the 31-kDa glycoprotein are essential to maintain antigenicity of the protein, since carbohydrate oxidation revealed no recognition by infected sera (Fig. 2f).
FIG. 1.
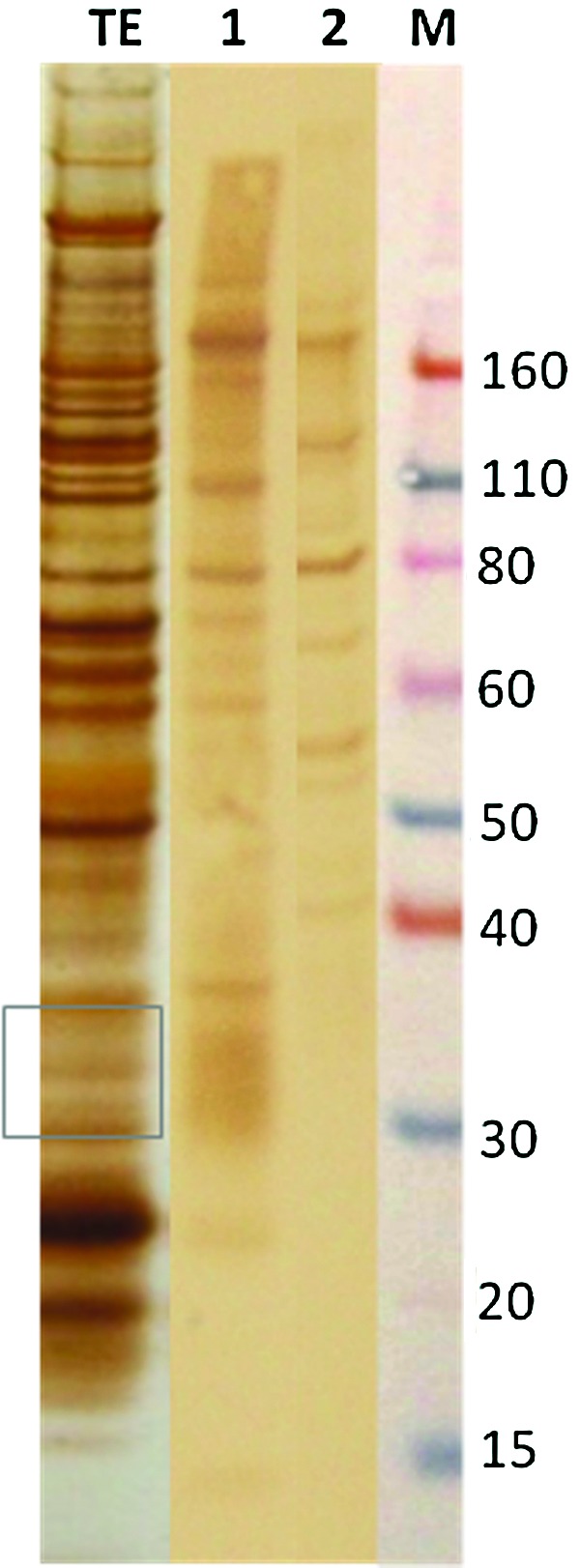
Identification of 31-kDa molecules on 1DE. Female worm total protein extract (TE) was resolved in 1DE gel and probed on Western Blot with: lane 1, pool of positive controls for abdominal angiostrongyliasis; lane 2, pool of normal human sera. The square represents the band excised from the gel for MS analyses. (Color image available at www.liebertpub.com/vbz).
FIG. 2.
Identification of the 31-kDa protein complex on 2DE. (a) TE pH range 3–11, silver staining. (b) TE pH range 3–11 Western blot using sera derived from pooled A. costaricensis and A. cantonensis infection. (c) TE pH range 3–6 sera derived from A. costaricensis infection. (d) TE pH range 3–6 sera derived from A. cantonensis infection. (e) Normal human sera. (f) Carbohydrate oxidation. (g) Coomassie blue staining. The four spots of the 31-kDa proteins are indicated on the pH 3–11 strip. Circles represent the spots excised for MS analyses. (Color image available at www.liebertpub.com/vbz).
The 31-kDa band detected on 1DE gels and the four antigenic spots identified on 2DE gels were excised and digested with trypsin for further analysis using MS/MS ESI-Q-TOF. The Mascot score was used to determine the probability that the observed matches between the experimental data and the database sequences were not random. Analysis of mass spectrometry data for 1DE (Table 1), and three of the spots from 2DE (Table 2), identified amino acid sequences that matched several unique proteins or protein domains in the database. Since there are few Angiostrongylus sequence data available, most protein identifications rely on homologous sequences from closely related organisms within the database. No peptide matches were obtained from spot 1. Amino acid sequences of two proteins, the 14-3-3 protein and the NAC domain-containing protein, were obtained from all three of the spots in which protein identifications were made. The highest Mascot scores for the 14-3-3 protein were detected with database sequences derived from the 14-3-3 proteins of Ancylostoma caninum, and a 28-kDa protein of Meloidogyne incognita, a plant nematode. Peptide sequences obtained from spot 4 matched sequences from the A. cantonensis putative epsilon subunit of the coatomer protein complex isoform 2 (33 kDa). Amino acid sequences from spots 2 and 3 matched heat shock proteins of Loa loa and Haemonchus contortus. Peptide sequences also matched sequences from two other A. cantonensis proteins (Table 2).
Table 1.
Proteins Identified Following One-Dimensional Gel Electrophoresis
| Peptide sequence | Protein name (Da) | Organism with homologous target | Scorea | Coverage % |
|---|---|---|---|---|
| R.ANTIEAQLK.E | Tropomyosin (33) | Heligmosomoides polygyrus | 262 | 36 |
| R.LEDELVHEK.E | ||||
| K.IVELEEELR.V | ||||
| K.LAMVEADLER.A | ||||
| K.EAQMLAEEADR.K | ||||
| R.MTLLEEELER.A | ||||
| K.VQEAEAEVAALNR.R | ||||
| K.EVDRLEDELVHEK.E | ||||
| K.AQEDLATATSQLEEK.D | ||||
| R.ALQASCLAK.W | Hypothetical protein CBG15316 (39) | Caenorhabditis briggsae | 172 | 9 |
| K.GILAADESTGSMEK.R | ||||
| R.GAAQNIIPAATGAAK.A | Glyceraldehyde-3-phosphate dehydrogenase (36) | Dictyocaulus viviparus | 131 | 10 |
| R.VPTPDVSVVDLTCR.L | ||||
| K.AGFAGDDAPR.A | Actin-2 (41) | Ascaris suum | 134 | 15 |
| K.DSYVGDEAQSK.R | ||||
| K.QEYDESGPSIVHR.K | ||||
| R.VAPEEHPVLLTEAPLNPK.A | ||||
| K.ITETVLSYCYR.A | Aldolase (39) | Haemonchus contortus | 88 | 6 |
| K.KPWALTFSYGR.A | ||||
| K.EPDWVQSER.E | CALUmenin (calcium-binding protein) homolog family member (36) | Caenorhabditis elegans | 87 | 12 |
| R.HLVGIADDNKDGK.L | ||||
| R.DWIMPVGFDHAEAEAR.H | ||||
| R.LLLEQMSQDPGAVR.E | TPR domain (31) | Brugia malayi | 78 | 7 |
| K.LMEFQR.A | ||||
| R.DYGVLKEDDGIAYR.G | Peroxiredoxin (21) | Ascaris suum | 75 | 17 |
| R.LVQAFQFVDK.H | ||||
| R.QITVNDLPVGR.S |
Mascot score is −10×log (P), where P is the probability that the observed match is a random event. Mass is molecular weight in kilodaltons.
The band on the area of 31-kDa was excised from the gel and subjected to trypsin digestion, and then analyzed by mass spectrometry for protein identification.
Table 2.
Identification of the 31-kDa Proteins Excised from A. cantonensis Two-Dimensional Gel Electrophoresis Preparations
| Spot no. | Peptide sequence | Protein identified (kDa) | Organism with homologous target | Scorea | Coverage (%) |
|---|---|---|---|---|---|
| 2 | K.ADLVNNLGTIAK.S | Heat shock protein 90 (80) | Loa loa | 201 | 7 |
| K.EDQTEVLEER.R | Heat shock protein 90 (81) | Haemonchus contortus | 144 | 5 | |
| R.ELISNSSDALDK.I | |||||
| K.TLTIMDTGIGMTK.A | |||||
| R.YQALTEPAELESGK.E | |||||
| R.VLSSIEQK.T | 14-3-3 product (29) | Meloidogyne incognita | 111 | 14 | |
| K.DSTLIMQLLR.D | |||||
| K.SQQSYQEAFDIAK.D | |||||
| K.SPGSDTYIVFGEAK.I | NAC domain-containing protein (24) | Brugia malayi | 136 | 12 | |
| K.NILFVINKPDVYK.S | |||||
| K.AGIVFTGK.G | PCNA (proliferating cell nuclear antigen) (29) | Caenorhabditis briggsae | 98 | 11 | |
| K.YMNQFTK.A | |||||
| K.LEVGLFDTYR.C | |||||
| 3 | R.YDDMAQSMK.K | 14-3-3 protein isoform 2 (28) | Ancylostoma caninum | 234 | 34 |
| K.DSTLIMQLLR.D | |||||
| R.DICQDVLNLLDK.F | |||||
| K.VTELGAELSNEER.N | |||||
| K.SQQSYQEAFDIAK.D | |||||
| K.MQPTHPIR.L | |||||
| K.SPGSDTYIVFGEAK.I | NAC domain containing protein (24) | Brugia malayi | 171 | 12 | |
| K.NILFVINKPDVYK.S | |||||
| K.EDQTEVLEER.R | Heat shock protein 90 (80) | Loa loa | 128 | 7 | |
| R.ELISNSSDALDK.I | |||||
| K.TLTIMDTGIGMTK.A | |||||
| R.YQALTEPAELESGK.E | |||||
| 4 | R.LAEYQNATDK.Q | Putative epsilon subunit of coatomer protein complex isoform 2 (33) | Angiostrongylus cantonensis | 289 | 30 |
| K.ASLVLNEISER.T | |||||
| K.AKENLFDELVAA | |||||
| K.DAEALLHEAQLR.D | |||||
| R.DINPNHPWVIDLK.A | |||||
| R.VISSIEQK.T | 14-3-3b protein (28) | Meloidogyne incognita | 277 | 29 | |
| K.DSTLIMQLLR.D | |||||
| K.VTELGAELSNEER.N | |||||
| K.SQQSYQEAFDIAK.D | |||||
| R.VGPGIGEYIFDK.E | Putative ferritin protein 2 (6.8) | Angiostrongylus cantonensis | 155 | 67 | |
| K.FLDEQVESIAEIAK.M | |||||
| K.ASAANDPHMSDFLESK.F | |||||
| K.LAQIISQFER.A | Putative uncoordinated protein 23 (11) | Angiostrongylus cantonensis | 150 | 39 | |
| R.ALTSVNSLIEGVVQK.M | |||||
| K.SPGSDTYIVFGEAK.I | NAC domain containing protein (24) | Brugia malayi | 92 | 6 |
The spots were excised from respective gels and subjected to trypsin digestion and then analyzed by mass spectrometry for protein identification.
Mascot score is −10×log (P), where P is the probability that the observed match is a random event. Mass is the molecular weight in kilodaltons.
Discussion
The identified peptides present in the 1DE band did not correspond to the peptides identified in the 2DE gel spots. This is likely a result of the better separation obtained during 2DE, which allowed for improved isolation of the antigenic protein. A comparison of the 2DE immunoblot and the total protein stain of the 2DE gel demonstrates that most of the proteins in the 31-kDa range are not immunogenic (Fig. 2). Thus, mass spectrometry analysis following 2DE gel separation allowed for more specific identification of those proteins with antigenic activity. One of the peptides obtained by 1DE showed the highest Mascot score match to the 33-kDa tropomyosin from Heligmosomoides polygyrus, a rodent nematode.
Tropomyosin is a highly conserved muscle protein with potent allergenic potential. This protein is known to induce IgE production in parasitic nematode infections such as anisakiasis and onchocerciasis (Sereda et al. 2008), but due to similarities between invertebrate tropomyosins, IgE antibodies cross-react with tropomyosins from other species, and therefore tropomyosins are not useful for diagnostic purposes (Sereda et al. 2008). However, specificity may be further tested by epitope mapping of this protein.
The 14-3-3 proteins are dimeric phosphoserine-binding proteins, which are members of a family of acidic regulatory molecules that participate in signal transduction, transport, and regulation, of several eukaryotic biochemical processes (Obsilova et al. 2008; Mrowiec and Schwappach 2006). In some parasites, such as Echinococcus multilocularis and Schistosoma mansoni, 14-3-3 proteins have been described to be immunogenic, and therefore have been promoted as potential vaccine targets (Schechtman et al. 2001; Siles-Lucas et al. 2008; Wang et al. 2009). In addition, the 14-3-3 protein has been identified as a prominent product in the S. mansoni female worm reproductive system (Schechtman et al. 2001). This may explain previous findings showing the female reproductive system as the main source of antigenic targets useful for the diagnosis of abdominal angiostrongyliasis caused by A. costarecensis (Bender, 2003). Moreover, these proteins might directly interact with immune system components, since these interactions have been modulated by 14-3-3 proteins secreted by Toxoplasma gondii and E. granulosus (Assossou et al. 2004; Siles-Lucas et al. 2008).
Coatomer proteins (COP) form a coat protein complex that mediates protein transport between the Golgi compartment (COPI), endoplasmic reticulum (COPII), and the plasma membrane (clathrin/adaptin; Lee and Goldberg, 2010). COPI from rat liver peroxisomes contains stoichiometric amounts of seven subunits, including alpha-COP (160 kDa), beta-COP (107 kDa), beta-prime-COP (102 kDa), delta-COP (57 kDa), epsilon-COP (36 kDa), gamma-COP (97 kDa), and zeta-COP (20 kDa; Lay et al. 2006). To date, there is no evidence that these proteins can induce immune responses. However, a crystallographic analysis showed that the epsilon-COP and alpha-COP complex were exposed on COPI vesicles, thereby facilitating their extracellular targeting (Hsia and Hoelz, 2010), suggesting that the complex might be attached to the Golgi membrane while transporting proteins that are eventually exposed to the host's immune system.
The nascent polypeptide-associated complex (NAC) is associated with ribosomes and involved in nascent polypeptide chain folding (Hayashi et al. 2011). NAC is also implicated in the targeting of ribosomes to the ER membrane (Wiedmann and Prehn, 1999).
Angiostrongylus 31-kDa antigen was first described as a glycoprotein, and its antigenicity was considered independent of carbohydrate moieties (Eamsobhana et al. 1998). In the present study m-periodate treatment eliminated the recognition by sera from infected individuals (Fig. 2f), demonstrating that carbohydrate moieties are essential for antibody recognition of the 31-kDa protein. The better separation of the proteins by 2DE allowed us to distinguish the specific antigenic spots corresponding to the previously described 31-kDa antigen, which was first detectable as a single band in 1DE preparations. Another explanation for this conflicting result is the amount of antigen loaded in the previous characterization of the 31-kDa protein, for which 10 μg were applied in the gel, while here only 3 μg in 2DE gels were loaded. The largest amount of antigen in 1DE gels could contain unspecific sugars being recognized by infected sera. This finding has strong implications for the choice of appropriate vectors to express such recombinant targets for the development of diagnostic tests.
In order to achieve the complete DNA sequence of each identified protein for further recombinant protein studies, we sequenced the DNA in a random way using the parallel sequencing approach (Morassutti et al. in preparation). The NAC domain was revealed to be composed of 185 amino acids, while 14-3-3 protein had 249 amino acids. The sequences were published in Genebank under the numbers GI: 341864443 for NAC domain-containing protein, and GI: 341864441 for 14-3-3.
Analysis of the data presented in this report raises the question of whether the reactivity observed with the native parasite 31-kDa molecules is due to reactivity with one or more of the putative proteins identified by MS/MS. Interestingly, the NAC domain-containing protein, epsilon-COPI, and 14-3-3 protein, all play putative biological roles in protein translocation. Therefore we hypothesize that they may form a cell membrane complex, which may have led to co-isolation of these proteins in the original TE preparation, and may ultimately explain how all three could be antigenic.
In conclusion, the set of proteins with an estimated molecular weight of 31 kDa identified by 2DE consisted of several potential antigens. Cloning of the corresponding cDNAs and expression of these proteins is the next critical step to further define their roles as diagnostic targets, and may represent a tool to better understand host-Angiostrongylus interactions.
Acknowledgments
Financial support was provided by CNPq, CAPES, FAPERGS, and APHL-USA. is the recipient of a CNPq PQ 1D fellowship, and of grants 300456/2007-7 and 477260/2007-1 (Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico do Brasil).
Author Disclosure Statement
No conflicting financial interests exist
References
- Assossou O. Besson F. Rouault JP, et al. Characterization of an excreted/secreted antigen form of 14-3-3 protein in Toxoplasma gondii tachyzoites. FEMS Microbiol Lett. 2004;234:19–25. doi: 10.1016/j.femsle.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Bender AL. Maurer RL. da Silva MC, et al. Eggs and reproductive organs of female Angiostrongylus costaricensis are more intensely recognized by human sera from acute phase in abdominal angiostrongyliasis. Rev Soc Bras Med Trop. 2003;36:449–454. doi: 10.1590/s0037-86822003000400003. [DOI] [PubMed] [Google Scholar]
- Ben R. Rodrigues R. Agostini AA, et al. Use of heterologous antigens for the immunodiagnosis of abdominal angiostrongyliasis by an enzyme-linked immunosorbent assay. Mem Inst Oswaldo Cruz. 2010;105:914–917. doi: 10.1590/s0074-02762010000700013. [DOI] [PubMed] [Google Scholar]
- Caldeira RL. Mendonça CL. Goveia CO, et al. First record of molluscs naturally infected with Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Metastrongylidae) in Brazil. Mem Inst Oswaldo Cruz. 2007;102:887–889. doi: 10.1590/s0074-02762007000700018. [DOI] [PubMed] [Google Scholar]
- Dekumyoy P. Komalamisra C. Nuamtanong S, et al. Angiostrongyliasis: analysis of antigens of Angiostrongylus costaricensis adult worms versus IgG from infected patients with Angiostrongylus cantonensis. Southeast Asian J Trop Med Public Health. 2000;1:48–53. [PubMed] [Google Scholar]
- Diaz JH. Helminthic eosinophilic meningitis: emerging zoonotic diseases in the South. J La State Med Soc. 2008;160:333–342. [PubMed] [Google Scholar]
- Eamsobhana P. Tungtrongchitr A. Wanachiwanawin D, et al. Characterization of 31kda specific antigen from Parastrongylus cantonensis (Nematoda: Metastrongylidae) Int Med Res J. 1998;2:9–12. [Google Scholar]
- Eamsobhana P. Yong HS. Immunological diagnosis of human angiostrongyliasis due to Angiostrongylus cantonensis (Nematoda: Angiostrongylidae) Int J Infect Dis. 2009;13:425–431. doi: 10.1016/j.ijid.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Eamsobhana P. Yoolek A. Suvouttho S, et al. Purification of a specific immunodiagnostic Parastrongylus cantonensis antigen by electroelution from SDS-polyacrylamide gels. Southeast Asian J Trop Med Public Health. 2001;32:308–313. [PubMed] [Google Scholar]
- Eamsobhana P. Yoolek A. Punthuprapasa P. Dot-blot ELISA for the immunological detection of specific antibody to Parastrongylus cantonensis. Trop Biomed. 2003;20:1–6. [Google Scholar]
- Graeff-Teixeira C. da Silva AC. Yoshimura K. Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev. 2009;22:322–348. doi: 10.1128/CMR.00044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S. Andoh T. Tani T. EGD1 (β-NAC) mRNA is localized in a novel cytoplasmic structure in Saccharomyces cerevisiae. Genes Cells. 2011;16:316–329. doi: 10.1111/j.1365-2443.2011.01489.x. [DOI] [PubMed] [Google Scholar]
- Hsia KC. Hoelz A. Crystal structure of alpha-COP in complex with epsilon-COP provides insight into the architecture of the COPI vesicular coat. Proc Natl Acad Sci USA. 2010;107:11271–11276. doi: 10.1073/pnas.1006297107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch S. Dekumyoy P. Löscher T, et al. A case of eosinophilic meningitis in Germany. J Neurol. 2008;255:1102–1103. doi: 10.1007/s00415-008-0846-2. [DOI] [PubMed] [Google Scholar]
- Lay D. Gorgas K. Just WW. Peroxisome biogenesis: Where Arf and coatomer might be involved. Biochimica et Biophysica Acta. 2006;1763:1678–1687. doi: 10.1016/j.bbamcr.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Lee C. Goldberg J. Structure of coatomer cage proteins and the relationship among COPI, COPII and clathrin vesicle coats. Cell. 2010;142:123–132. doi: 10.1016/j.cell.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado A., Jr Simões RO. Oliveira AP, et al. First report of Angiostrongylus cantonensis (Nematoda: Metastrongylidae) in Achatina fulica (Mollusca: Gastropoda) from Southeast and South Brazil. Mem Inst Oswaldo Cruz. 2010;105:938–941. doi: 10.1590/s0074-02762010000700019. [DOI] [PubMed] [Google Scholar]
- Mortz E. Krogh TN. Vorum H, et al. Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics. 2001;11:1359–1363. doi: 10.1002/1615-9861(200111)1:11<1359::AID-PROT1359>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Mrowiec T. Schwappach B. 14-3-3 proteins in membrane protein transport. Biol Chem. 2006;387:1227–1236. doi: 10.1515/BC.2006.152. [DOI] [PubMed] [Google Scholar]
- Nuamtanong S. The evaluation of the 29 and 31 kDa antigens in female Angiostrongylus cantonensis for serodiagnosis of human angiostrongyliasis. Southeast Asian J Trop Med Public Health. 1996;27:291–296. [PubMed] [Google Scholar]
- Obsilova V. Nedbalkova E. Silhan J, et al. The 14-3-3 protein affects the conformation of the regulatory domain of human tyrosine hydroxylase. Biochemistry. 2008;47:1768–1777. doi: 10.1021/bi7019468. [DOI] [PubMed] [Google Scholar]
- Pincay T. García L. Narváez E, et al. Angiostrongyliasis due to Parastrongylus (Angiostrongylus) cantonensis in Ecuador. First report in South America. Trop Med Int Health. 2009;14:37. [Google Scholar]
- Schechtman D. Winnen R. Tarrab-Hazdai R, et al. Expression and immunolocalization of the 14-3-3 protein of Schistosoma mansoni. Parasitology. 2001;123:573–582. doi: 10.1017/s0031182001008769. [DOI] [PubMed] [Google Scholar]
- Sereda MJ. Hartmann S. Lucius R. Helminths and allergy: the example of tropomyosin. Trends Parasitol. 2008;24:272–278. doi: 10.1016/j.pt.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Siles-Lucas M. Merli M. Gottstein B. 14-3-3 proteins in Echinococcus: their role and potential as protective antigens. Exp Parasitol. 2008;119:516–523. doi: 10.1016/j.exppara.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Wang QP. Lai DH. Zhu XQ, et al. Human angiostrongyliasis. Lancet Infect Dis. 2008;10:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- Wang Y. Cheng Z. Lu X, et al. Echinococcus multilocularis: Proteomic analysis of the protoscoleces by bi-dimensional electrophoresis and mass spectrometry. Exp Parasitol. 2009;123:162–167. doi: 10.1016/j.exppara.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Wiedmann B. Prehn S. The nascent polypeptide-associated complex (NAC) of yeast functions in the targeting process of ribosomes to the ER membrane. FEBS Lett. 1999;458:51–54. doi: 10.1016/s0014-5793(99)01118-7. [DOI] [PubMed] [Google Scholar]
- Yii CY. Clinical observations on eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis on Taiwan. Am J Trop Med Hyg. 1976;25:233–249. doi: 10.4269/ajtmh.1976.25.233. [DOI] [PubMed] [Google Scholar]