Abstract
Seroprevalence rates of selected arboviruses in animal populations in Trinidad were determined using serum samples collected between 2006 and 2009 from horses (n=506), cattle (n=163), sheep (n=198), goats (n=82), pigs (n=184), birds (n=140), rodents (n=116), and other vertebrates (n=23). The sera were screened for antibodies to West Nile virus (WNV), St. Louis encephalitis virus (SLEV), Ilheus virus (ILHV), Bussuquara virus (BSQV), Venezuelan equine encephalitis virus (VEEV), eastern equine encephalitis virus (EEEV), and western equine encephalitis virus (WEEV), using hemagglutination inhibition assay (HIA) and epitope-blocking enzyme-linked immunosorbent assays (ELISA). Antibodies to SLEV were detected in a total of 49 (9.7%) horses, 8 (4.9%) cattle, 1 (1.2%) goat, 2 (1.4%) wild birds, and 3 (2.2%) wild rodents by both methods. In contrast, antibodies to EEEV, VEEV, and WNV were detected only in horses, at rates of 4.3%, 0.8%, and 17.2%, respectively, by ELISA, and IgM capture ELISA was WNV-positive in 3 (0.6%) of these sera. Among locally bred unvaccinated horses that had never left Trinidad, seroprevalence rates against WNV were 12.1% and 17.2% by ELISA and HIA, respectively. The presence of WNV- and SLEV-specific antibodies in a representative sample of horse sera that were both ELISA- and HIA-seropositive was confirmed by plaque reduction neutralization testing (PRNT). Antibodies to ILHV and BSQV were not detected in any of the serum samples tested (i.e., sera from horses, other livestock, and wild birds in the case of ILHV, and wild mammals in the case of BSQV). The data indicate the presence of WNV in Trinidad, and continuing low-level circulation of SLEV, EEEV, and VEEV.
Key Words: Alphavirus, Arbovirus, Eastern equine encephalitis virus, Flavivirus, Seroprevalence, St. Louis encephalitis virus, Trinidad, Venezuelan equine encephalitis virus, West Nile virus
Introduction
Between 1959 and 1969, the Trinidad Regional Virus Laboratory (TRVL), in collaboration with the Rockefeller Foundation, demonstrated through viral isolation and serology the existence of several arboviruses in human and animal populations in the Caribbean region, including Trinidad. The work done in Trinidad provided valuable information on circulating viruses (Aitken et al. 1956, 1960, 1964, 1968a; Jonkers et al. 1968a; Price, 1978b), taxonomic classification of the vectors (Aitken et al. 1968c), and their associated ecological characteristics (Downs et al. 1968; Jonkers et al. 1966). These studies resulted in the identification and characterization of hundreds of arboviral isolates, primarily from the Bush Bush Forest, which is an approximately 0.85-sq km region within the Nariva swamp in southeastern Trinidad (Aitken et al. 1968c; Jonkers et al. 1968a, 1968b; Worth et al. 1968). With the exception of the aforementioned studies, there have been only a few serological studies in the 1970s and early 1980s (Price 1978a, 1978b; Tikasingh et al. 1983), and more recently, mosquito surveillance studies and opportunistic sampling of carcasses of Alouatta seniculus monkeys confirmed the continued circulation of VEE subytpe IIIA viruses (Mucambo virus; MUCV), several orthobunyaviruses, and Yellow fever virus (YFV) (Auguste et al. 2009, 2010a, 2010b).
Despite the extensive surveillance performed in Trinidad, the role of rodents, other mammals, and birds in the maintenance and spread of these viruses locally is unclear. It is also not clear to what extent the livestock and wildlife populations in Trinidad are affected or potentially contribute to amplification, as routine surveillance and virological assays on sick livestock are rarely performed and reported.
Given the significant changes in human demographics since the comprehensive studies in the 1950s and 1960s, we performed a serological survey for flaviviruses and alphaviruses of veterinary public health importance in the Americas. Sera from livestock (horses, cattle, sheep, goats, and pigs), birds, rodents, and other wildlife in Trinidad were collected over a 2-year period, and subsequently screened for antibodies to West Nile (WNV), St. Louis encephalitis (SLEV), Ilheus (ILHV), Bussuquara (BSQV), Venezuelan equine encephalitis (VEEV), eastern equine encephalitis (EEEV), and western equine encephalitis viruses (WEEV), using the hemagglutination inhibition assay (HIA), a traditional diagnostic method for detecting arboviral antibodies (Clarke and Casals, 1958), and a more recently developed epitope-blocking enzyme-linked immunosorbent assay (ELISA) (Blitvich et al. 2003a, 2003c; Hall et al. 1995).
Materials and Methods
Sampling strategies
Horses
Horses were selected from lists of registered horses from the Trinidad and Tobago Racing Authority (TTRA), and the Trinidad and Tobago Equestrian Society (TTES), mainly by stratified random sampling according to gender and age, in categories of age <1 year, age 1–5 years, age 6–10 years, and age >10 years. Blood samples were collected from the jugular veins of 506 horses (200 male and 306 female), from stud farms, equestrian facilities, and racetracks, at 25 locations within 20 geographic areas (Fig. 1a). Demographic data for the horses and stables were also noted. Of the 506 horses, 5.3% (n=27) were <1 year old, 40.7% (n=206) were 1–5 years of age, 31.0% (n=157) were 6–10 years of age, 15.3% (n=77) were older than 10 years, and the ages could not be determined for 7.7% (n=39). Also, 121 (23.9%) were imported (foreign-bred), 341 (67.4%) were locally-bred, and the origin of 44 (8.7%) was uncertain. Finally, 36 (7.1%) horses were from rural towns, while 470 (92.9%) originated from urban areas (i.e., regions with ≥200 persons per square kilometer; Central Statistical Office, 1998).
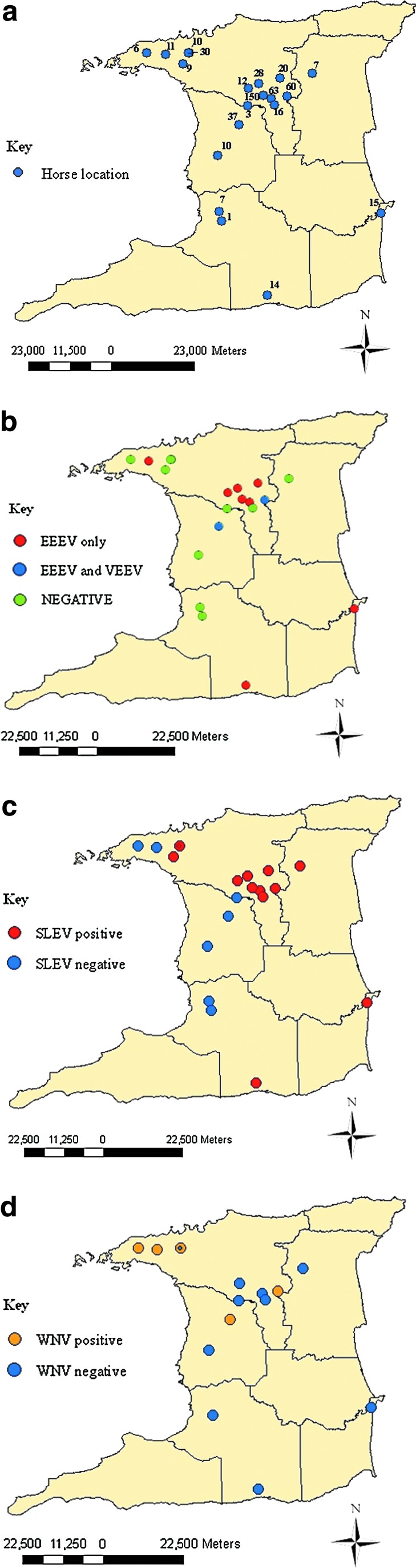
FIG. 1.
(a) Geographic distribution of horse samples in Trinidad, and (b) geographic distribution of alphavirus-positive horses in Trinidad. The VEEV-positive locations were also EEEV-seropositive. (c) Geographic distribution of SLEV-positive horses in Trinidad. (d) Geographic distribution of WNV-positive horses in Trinidad. Spatial distribution was documented and analyzed with Arcmap 9.2, ArcGIS 9 (ESRI, Redlands, CA; SLEV, St. Louis encephalitis virus; WNV, West Nile virus; VEEV, Venezuelan equine encephalitis virus; EEEV, eastern equine encephalitis). Color images available online at www.liebertpub.com/vbz
Livestock
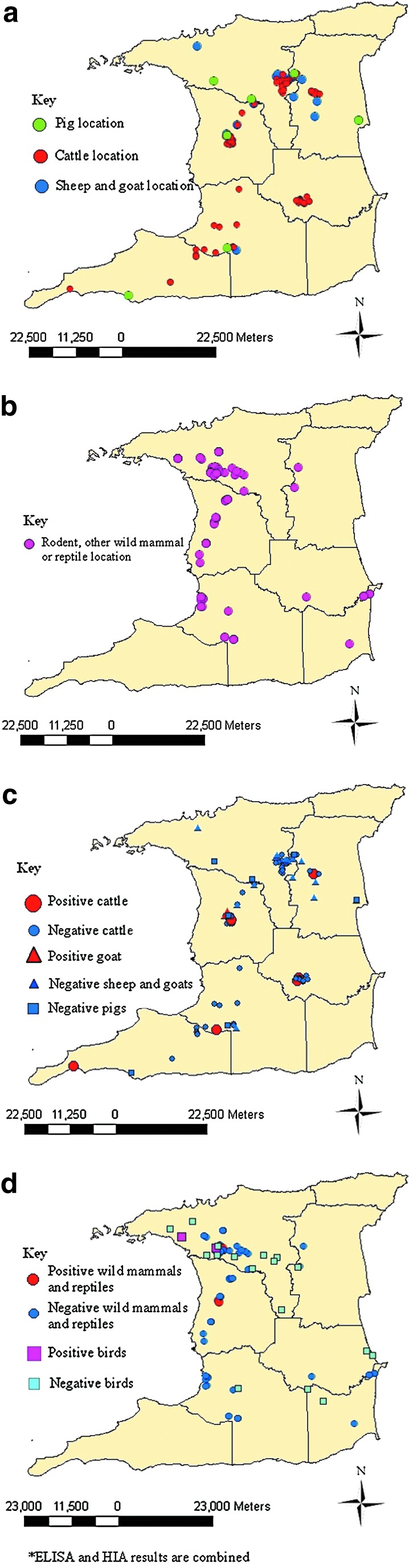
A list of farms was prepared using information from the Ministry of Agriculture, Land and Marine Resources (MALMR) and Animal Health Assistants (AHAs) that usually assist government veterinarians. Small ruminant and pig farms were grouped by size as follows: for small ruminants, ≤15 animals=small, 16–49 animals=medium, and ≥50 animals=large; for pigs, ≤25 animals=small, 26–50 animals=medium, and ≥50 animals=large. For cattle, small farms were those with fewer than 20 animals, medium farms had 21–39 animals, and large farms were those with 40 or more animals. A systematic random sampling protocol was then used to select the farms. Within each of the cattle and small ruminant farms selected, stratified random sampling according to age, gender, and species (in the case of small ruminant farms) was used. Where farmers from selected farms declined to participate in the study, the next scheduled farm was substituted and a restart of the sequence was done. Medium and large pig farms were sampled randomly; however, small pig farms were sampled conveniently, as the majority on the compiled list were inactive. Figure 2a illustrates the locations of the livestock sampling sites. Blood samples were collected from the jugular veins of small ruminants and cattle, and of the jugular vein or anterior vena cava of pigs.
FIG. 2.
(a) Geographic distribution of livestock samples in Trinidad. (b) Geographic distribution of rodent and wildlife samples in Trinidad. (c) Geographic distribution of SLEV-positive livestock in Trinidad. (d) Geographic distribution of St. Louis encephalitis virus-positive rodents and wildlife in Trinidad. Spatial distribution was documented and analyzed with Arcmap 9.2, ArcGIS 9. Color images available online at www.liebertpub.com/vbz
Wildlife
Wild animals were sampled from the Emperor Valley Zoo in Trinidad, from wildlife farms, or by trapping in forested areas. Supplementary Table S1 (see online supplementary material at http://www.liebertpub.com) shows the species and numbers of animals sampled. Systematic random sampling was used for the selection of wildlife farms where animals were then sampled according to gender and growth stage (i.e., juvenile or adult). Free-living rats (Rattus spp.) were also specifically targeted and trapped using humane metal cage traps at various locations in close proximity to homes, public buildings and markets.
Avian sera were obtained from confiscated birds at the Wildlife Section of the Ministry of Agriculture, Land and Marine Resources, from birds collected in the wild, and from captive birds at the Emperor Valley Zoo. Collection of wild birds was carried out using hand nets and mist nets (35×35 mm), after which the birds were bled by cutaneous ulnar or jugular venipuncture and then released. Supplementary Table S1 (see online supplementary material at http://www.liebertpub.com) shows the avian species sampled, including their migrant status in Trinidad.
Two forested areas, the Aripo Savannah Scientific Reserve and the Bush Bush Forest in Nariva, were chosen for wildlife trapping based on the results of previous and ongoing arbovirus surveillance studies (Aitken et al. 1968b, 1968c; Jonkers et al. 1968a; Auguste et al. 2009). In Aripo, trapping was done weekly from November to December 2007 and February to April 2008, whereas in the Bush Bush Forest, trapping was done daily between April and August 2008. A total of 11 traps (9 measuring 16″×5″×5″ and 2 being 32″×10″×12″) were set from 4 pm to 6 pm, and removed or re-set between 6 am and 9 am the following morning. Baits used were peanut butter, cheese, ripe bananas, bread, salt fish, smoked herring, coconut, and seasonal fruits, depending on the target species (Aitken et al. 1968b; Barrera et al. 2002).
Hemagglutination inhibition assay (HIA)
HIA was performed as described in Beaty and associates (Beaty et al. 1989), using sucrose acetone antigens that had been previously prepared and stored at −70°C. For VEEV, WEEV, and EEEV, the strains used were TC-83 (subtype I-AB), McMillan, and North American NJ/60, respectively. For the flaviviruses, SLEV (T-35573), WNV (T-36296), and ILHV (BeH 7445) were used for testing sera from all species except rodents and other non-avian wildlife. In addition, BSQV strain BeAn 4116 was used for rodents and other non-avian wildlife. Seroprevalence due to specific antibodies was determined by the demonstration of a fourfold or greater difference in antibody titers caused by the various antigens. A positive control serum titrated to its endpoint was included for each antigen.
Epitope-blocking enzyme-linked immunosorbent assay (ELISA)
An identification algorithm of murine monoclonal antibodies (MAbs) for subtyping EEEV (Roehrig et al. 1990) was modified and the following MAbs were used: MAb 5B4D-6, specific for detecting VEEV TC-83 vaccine strain, and MAb 1B1C-4 cross-reactive for North American (NJ/60) and South American (BeAn 5122) EEEV (both from Chemicon, Temecula, CA). Flaviviral antibodies included WNV MAb 3.1112G and SLEV MAb 6B5A-2 (both from Chemicon), and a generic Flavivirus MAb 6B6C-1 (InBios International, Inc., Seattle, WA). MAb 6B6C-1 was used to test horse sera only. Flavivirus antigens used were SLEV strain TBH-28 and WNV NY-99-35261 strain. ELISAs to detect flavivirus antibodies were performed as described in Blitvich and colleagues (Blitvich et al. 2003c). Alphavirus antigens used were TC-83 (subtype 1) and North American (NJ/60) for VEEV and EEEV, respectively. Optimal concentrations of viral antigens were first titrated according to Wang and associates (Wang et al. 2005). ELISAs to detect alphavirus antibodies were performed according to Wang and associates (Wang et al. 2005) with minor modifications.
Seropositivities were calculated using the formula: % inhibition=100 – {(TS – B)/(CS – B)}×100, where TS is the optical density of test sera, CS is the optical density of control sera, and B is the background optical density (Hall et al. 1995). Each sample was tested in duplicate so an average optical density was first calculated. An inhibition value of 30% or more was considered seropositive (Blitvich et al. 2003a, 2003b). Calculated percentage inhibition values that resulted in values below zero (negative) were recorded as zero. Due to the limited volume of serum collected from the small animals, particularly birds, some sera were tested only once by ELISA.
IgM capture ELISA for West Nile virus
Immunoglobulin M (IgM) West Nile Detect™ IgM capture ELISA (InBios International, Inc.) was used to detect antibodies to WNV in horse sera as stipulated by the manufacturer and using the test controls provided to determine the immune status ratio (ISR). ISR was calculated for the positive and negative controls by dividing the OD450nm of the WNRA by OD450mm of the normal cell antigen. An ISR for test serum ≤2 was considered negative, ≥3.0 as positive, and between 2.0 and 3.0 were deemed equivocal. All positive and equivocal samples were repeated.
Plaque reduction neutralization test (PRNT)
The ability of seropositive sera to neutralize WNV (NY-99 strain), SLEV (TBH-28 strain), VEEV (FSL190 strain), and EEEV (Trinidad 1959 strain), was determined by PRNT. WNV and SLEV PRNTs were performed as described by Blitvich and associates (Blitvich et al. 2003c), with the exception that plaques for SLEV were counted 8 days post-infection. VEEV and EEEV PRNTs were performed as previously described (Beaty et al. 1989).
Ethical approval and permits
This study was approved by the Ethics Committee of the Faculty of Medical Sciences at the University of the West Indies, St. Augustine Campus, prior to commencement. Permits were obtained from the Wildlife Section of the Ministry of Agriculture, Land and Marine Resources for capture of animals for the study.
Results
Horses
Seroprevalence rates of EEEV, VEEV, WNV, and SLEV, at each of the sampled locations are shown in Table 1. Figure 1 shows all of the horse sampling locations and the locations where VEEV-, EEEV-, SLEV-, and WNV-seropositive horses were detected. Based on the combined ELISA and HIA results there were 26 (5.1%) EEEV-seropositive horses, 4 (0.8%) VEEV-seropositive animals, and 7 (1.4%) samples that exhibited cross-reactivity within the Alphavirus genus (i.e., with at least a fourfold difference), such that their identities could not be determined. Of the 26 horses ELISA-seropositive for EEEV, 21 (80.8%) were from stud farms, and 5 (19.2%) were from race tracks, making the seropositive rates at these locations 6.7% (21 out of 315 horses), and 3.3% (5 out of 150), respectively. All 22 HIA-seropositive horses were detected with ELISA including 19 (6.0%) of 315 stud farm horses, and 3 (2.0%) of 150 racetrack horses. All four (1.3%) VEEV-seropositive horses originated from stud farms. Of the 26 EEEV-seropositive horses, 16 (61.2%), 5 (19.2%), and 4 (15.4%), were aged 6–10, 1–5, and >10 years, respectively. Twenty EEEV-seropositive horses were imported and 6 were locally bred. PRNT80 performed on EEEV- and VEEV-seropositive horses confirmed the presence of VEEV-neutralizing antibodies in 2 of 4 samples tested, and EEEV-neutralizing antibodies in 7 of 26 samples tested (antibody titers >20). Of the 58 horses that had not been vaccinated against WNV and were locally bred, a total of 11 (19.0%) were seropositive for WNV (Table 2), with 7 (12.1%) seropositive for WNV by ELISA, and 10 (17.2%) by HIA.
Table 1.
Seropositivity for Arboviral Antibodies in Horses by ELISA and HIA
| |
|
|
|
No. (%) positive for flaviviral antibodies |
No. (%) positive for alphaviral antibodies |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
No. of horses tested |
SLEV |
WNVb |
EEEV |
VEEV |
|||||
| Location type | Locationa | SLEV, EEEV and VEEV | WNV | ELISA | HIA | ELISA | HIA | ELISA | HIA | ELISA | HIA |
| Stud farm | Arima | 63 | 1 | 7 (11.1) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 9 (14.3) | 9 (14.3) | 0 (0.0) | 0 (0.0) |
| Santa Cruz | 30 | 3 | 5 (16.7) | 0 (0.0) | 3 (100) | 3 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| D'Abadie | 28 | 4 | 3 (10.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.6) | 2 (7.1) | 0 (0.0) | 0 (0.0) | |
| Freeport | 10 | 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Chaguanas | 37 | 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 1 (2.7) | 0 (0.0) | 2 (5.4) | 0 (0.0) | |
| Wallerfield | 60 | 13 | 0 (0.0) | 4 (6.7) | 1 (7.7) | 2 (15.4) | 6 (10.0) | 5 (8.3) | 2 (3.3) | 0 (0.0) | |
| Heights of Guanapo | 20 | 0 | 6 (30.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Arouca | 12 | 8 | 3 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | |
| Petit Valley | 11 | 2 | 0 (0.0) | 0 (0.0) | 2 (100) | 2 (100) | 1 (9.1) | 1 (9.1) | 0 (0.0) | 0 (0.0) | |
| Mayaro | 15 | 1 | 5 (33.3) | 1 (6.7) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Moruga | 14 | 2 | 1 (7.1) | 2 (14.3) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 1 (7.1) | 0 (0.0) | 0 (0.0) | |
| Gasparillo | 7 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Princes Town | 1 | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Valencia | 7 | 2 | 1 (14.3) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Subtotal | 315 | 43 | 31 (9.8) | 9 (2.9) | 6 (14.0) | 8 (18.6) | 21 (6.7) | 19 (6.0) | 4 (1.3) | 0 (0.0) | |
| Equestrian facility | Chaguaramas | 6 | 4 | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Santa Cruz | 10 | 0 | 3 (30.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Piarco | 3 | 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| St. Anns | 6 | 0 | 2 (22.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Guanapo | 16 | 3 | 3 (18.8) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Subtotal | 41 | 9 | 8 (19.5) | 1 (2.4) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Race track | Arima | 150 | 6 | 10 (6.7) | 3 (2.0) | 0 (0.0) | 2 (33.3) | 5 (3.3) | 3 (2.0) | 0 (0.0) | 0 (0.0) |
| Total | 506 | 58 | 49 (9.7) | 13 (2.6) | 7 (12.1) | 10 (17.2) | 26 (5.1) | 22 (4.3) | 4 (0.8) | 0 (0.0) | |
Results of 4 locations were merged due to overlap of global positioning system points.
Includes only unvaccinated and locally-bred horses.
ELISA, enzyme-linked immunosorbent assay; HIA, hemagglutination inhibition assay; SLEV, St. Louis encephalitis virus; EEEV, eastern equine encephalitis virus; VEEV, Venezuelan equine encephalitis virus; WNV, West Nile virus.
Table 2.
Unvaccinated and Locally-Bred Horses Positive for Antibodies to WNV, SLEV, ILHV, and FLAV
| |
|
|
|
HIA titer |
Epitope b-ELISA (%I)a |
|
|
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Locality by town | Gender | Age group (y) | WNV | SLEV | ILHV | FLAV | WNV | SLEV | WNV capture IgMb | WNV PRNT titerc | SLEV PRNT titerd |
| 1C11H | Chaguanas | F | 1–5 | 1:40 | 0 | 0 | 55.2 | 21.4 | 4.1 | 1.0 | 160 | <20 |
| 1CH3H | Chaguaramas | M | >10 | 0 | 0 | 0 | 39.0 | 36.4 | 1.6 | NT | NT | NT |
| 1O7H | Wallerfield | F | 6–10 | 1:320 | 1:20 | 0 | 87.7 | 0 | 26.3 | 1.4 | 320 | <20 |
| 1OR2H | Wallerfield | F | Unknown | 1:320 | 1:20 | 0 | 90.3 | 83 | 19.7 | 1.1 | >640 | <20 |
| 1P1H | Petit Valley | F | 1–5 | 1:320 | 1:40 | 1:20 | 62.1 | 47.9 | 10.6 | 2.6 | NT | NT |
| 1P3H | Petit Valley | F | 6–10 | 1:320 | 1:40 | 1:40 | 73.7 | 43.2 | 29.5 | 1.2 | NT | NT |
| 1S3H | Santa Cruz | M | 1–5 | 1:2560 | 1:160 | 1:160 | 94.9 | 80.7 | 23.3 | 16.3 | >640 | 160 |
| 1S4H | Santa Cruz | M | 1–5 | 1:2560 | 1:80 | 1:40 | 93.5 | 86.9 | 23.3 | 2.7 | >640 | 40 |
| 1S5H | Santa Cruz | M | 1–5 | 1:1280 | 1:160 | 1:80 | 81.3 | 75.2 | 2.7 | 3.0 | >640 | 40 |
| 3RT5H | Arima | F | 1–5 | 1:40 | 0 | 0 | 43.7 | 0 | 29.4 | 1.1 | 160 | <20 |
| 3RT19H | Arima | F | 1–5 | 1:160 | 1:20 | 0 | 41.4 | 0 | 21.5 | 1.3 | 320 | 20 |
Interpretation for epitope-blocking ELISA: <30% inhibition (%I) is antibody-negative, and >30% inhibition is antibody-positive.
West Nile immunoglobulin type M capture ELISA interpretation: <2.0 is antibody-negative, 2.0–3.0 is equivocal, and >3.0 is antibody-positive.
Plaque reduction neutralization test (PRNT) for WNV (titers are expressed as the reciprocal of the serum dilution yielding 90% reduction in the number of plaques).
PRNT for SLEV (titers are expressed as the reciprocal of the serum dilution yielding 90% reduction in the number of plaques).
ELISA, enzyme-linked immunosorbent assay; HIA, hemagglutination inhibition assay; SLEV, St. Louis encephalitis virus; EEEV, eastern equine encephalitis virus; VEEV, Venezuelan equine encephalitis virus; WNV, West Nile virus; ILHV, Ilheus virus; NT, not tested.
Of the 506 horses studied, 49 (9.7%) were seropositive for SLEV by epitope-blocking ELISA, compared with 13 (2.6%) that were positive by HIA (Table 3), with 12/13 HIA-positives also being ELISA-positive. The summary of location types, age groups, gender, and origin of SLEV-positive horses by ELISA is shown in Table 3. Of the 49 seropositive animals, 45 (91.8%) were from urban areas, and 4 (8.2%) were from rural areas.
Table 3.
Frequency of SLEV-Specific Antibodies by ELISA in Horses by Age, Gender, and Origin
| |
|
|
|
Frequency of SLEV antibodies |
Frequency of SLEV antibodies |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location type | CategoryAge (y) | No. tested | No. (%) positive by ELISA | Male (n=89) | Female (n=226) | Foreign bred (n=89) | Locally bred (n=207) | NA (n=19) | |||||
| Stud farm | <1 | 27 | 2 (7.4) | 12 | 1 (0.7) | 15 | 1 (6.7) | 1 | 0 (0.0) | 26 | 2 (7.7) | 0 | 0 (0.0) |
| 1–5 | 83 | 8 (9.6) | 37 | 4 (10.8) | 46 | 4 (8.7) | 23 | 4 (17.4) | 56 | 4 (7.1) | 4 | 0 (0.0) | |
| 6–10 | 128 | 17 (13.3) | 31 | 6 (19.4) | 97 | 11 (1.0) | 42 | 5 (11.9) | 82 | 12 (14.6) | 4 | 0 (0.0) | |
| >10 | 60 | 4 (6.7) | 5 | 0 (0.0) | 55 | 4 (7.3) | 21 | 1 (4.8) | 39 | 3 (7.7) | 0 | 0 (0.0) | |
| NA | 17 | 0 (0.0) | 4 | 0 (0.0) | 13 | 0 (0.0) | 2 | 0 (0.0) | 4 | 0 (0.0) | 11 | 0 (0.0) | |
| Subtotal | 315 | 31 (9.8) | 89 | 11 (12.4) | 226 | 20 (8.8) | 89 | 10 (11.2) | 207 | 21 (10.1) | 19 | 0 (0.0) | |
| |
|
|
|
Frequency of SLEV antibodies |
Frequency of SLEV antibodies |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equestrian facility | CategoryAge (y) | No. tested | No. (%) positive | Male (n=29) | Female (n=12) | Foreign bred (n=8) | Locally bred (n=32) | NA (n=1) | |||||
| <1 | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| 1–5 | 10 | 2 (20.0) | 7 | 2 (28.6) | 3 | 0 (0.0) | 1 | 0 (0.0) | 9 | 2 (22.2) | 0 | 0 (0.0) | |
| 6–10 | 14 | 2 (14.3) | 10 | 1 (10.0) | 4 | 1 (25.0) | 4 | 0 (0.0) | 10 | 2 (20.0) | 0 | 0 (0.0) | |
| >10 | 16 | 4 (23.5) | 11 | 4 (36.4) | 5 | 0 (0.0) | 3 | 2 (66.7) | 13 | 2 (15.4) | 0 | 0 (0.0) | |
| NA | 1 | 0 (0.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 0 (0.0) | |
| Subtotal | 41 | 8 (19.5) | 29 | 7 (24.1) | 12 | 1 (8.3) | 8 | 2 (25.0) | 32 | 6 (18.8) | 1 | 0 (0.0) | |
| |
|
|
|
Frequency of SLEV antibodies |
Frequency of SLEV antibodies |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race track | CategoryAge (y) | No. tested | No. (%) positive | Male (n=82) | Female (n=68) | Foreign bred (n=24) | Locally bred (n=102) | NA (n=24) | |||||
| <1 | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| 1–5 | 113 | 7 (6.2) | 70 | 5 (7.1) | 43 | 2 (4.7) | 19 | 3 (15.8) | 90 | 4 (4.4) | 4 | 0 (0.0) | |
| 6–10 | 15 | 0 (0.0) | 12 | 0 (0.0) | 3 | 0 (0.0) | 4 | 0 (0.0) | 11 | 0 (0.0) | 0 | 0 (0.0) | |
| >10 | 1 | 0 (0.0) | 0 | 0 (0.0) | 1 | 0 (0.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| NA | 21 | 3 (14.3) | 0 | 0 (0.0) | 21 | 3 (14.3) | 0 | 0 (0.0) | 1 | 0 (0.0) | 20 | 3 (15.0) | |
| Subtotal | 150 | 10 (6.7) | 82 | 5 (6.1) | 68 | 5 (7.4) | 24 | 3 (12.5) | 102 | 4 (3.9) | 24 | 3 (12.5) | |
| Total | 506 | 49 (9.7) | 200 | 23 (11.5) | 306 | 27 (8.8) | 121 | 15 (12.4) | 341 | 31 (9.0) | 44 | 4 (8.9) | |
SLEV, St. Louis encephalitis virus; ELISA, enzyme-linked immunosorbent assay; NA, not applicable due to unknown status.
A representative sample of 18 horse sera (8 WNV and 10 SLEV samples) was tested by PRNT for neutralization antibodies. WNV was confirmed in all 8 samples by PRNT90, with antibody titers >160 (>640 in 4/10 samples; titers are expressed as the reciprocal of the serum dilution yielding 90% reduction in the number of plaques), and SLEV was confirmed in 9 of 10 samples, with antibody titers >40 (using a 1:20 cut-off and/or a greater than fourfold difference in antibody titer between the viruses tested; Table 4). Cross-PRNTs were performed on these 18 samples for confirmation. PRNT80 (titers expressed as the reciprocal of the serum dilution yielding 80% reduction in the number of plaques) were also performed to confirm infection with EEEV and VEEV with antibody titers >20, but cross-PRNTs were not done as with WNV and SLEV. Six of the 22 EEEV samples tested were positive by PRNT, and none of the four VEEV samples tested had neutralizing antibodies to this virus.
Table 4.
Locally-Bred Horses Seropositive for Antibodies to St. Louis Encephalitis Virus
| |
|
|
|
|
HIA titer |
Epitope b-ELISA (%I)a |
|
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Sample ID | Locality by town | Gender | Age group (y) | WNV | SLEV | ILHV | FLAV | WNV | SLEV | SLEV PRNT90 titerb | WNV PRNT90 titerc |
| 1 | 1MG4H | Moruga | F | >5 | 0 | 1:40 | 0 | 60.8 | 0 | 31.0 | >640 | <20 |
| 2 | 1MG9H | Moruga | F | 1–5 | 0 | 1:20 | 0 | 31.7 | 0 | 32.0 | 80 | <20 |
| 3 | 1MY7H | Mayaro | F | 1–5 | 0 | 1:40 | 0 | 42.2 | 0 | 20.5 | 160 | <20 |
| 4 | 1OR10H | Oropune | F | >5 | 0 | 1:80 | 0 | 39.1 | 0 | 0 | 160 | <20 |
| 5 | 1OR17H | Oropune | F | >5 | 0 | 1:40 | 0 | 30.2 | 19.7 | 9.6 | 40 | 40 |
| 6 | 1V2H | Valencia | F | >5 | 1:40 | 1:160 | 1:40 | 46.1 | 0 | 37.5 | >640 | 20 |
| 7 | 4RT16H | Arima | M | 1–5 | 0 | 1:20 | 0 | 38.5 | 0 | 19.5 | 80 | 20 |
| 8 | 4RT7H | Arima | F | 1–5 | 0 | 1:20 | 0 | 33.2 | 0 | 36.1 | 40 | <20 |
| 9 | 5RT10H | Arima | M | 1–5 | 0 | 1:20 | 0 | 31.1 | 0 | 15.5 | 40 | <20 |
| 10 | 1GU4H | Guanapo | F | >5 | 0 | 1:40 | 0 | 33.3 | 0 | 13.0 | <20 | <20 |
Interpretation for epitope-blocking ELISA: <30% inhibition (%I) is antibody-negative, and >30% inhibition is antibody-positive.
Plaque reduction neutralization test (PRNT) for SLEV (titers are expressed as the reciprocal of the serum dilution yielding 90% reduction in the number of plaques).
Plaque reduction neutralization test for WNV (titers are expressed as the reciprocal of the serum dilution yielding 90% reduction in the number of plaques).
ELISA, enzyme-linked immunosorbent assay; HIA, hemagglutination inhibition assay; SLEV, St. Louis encephalitis virus; WNV, West Nile virus; ILHV, Ilheus virus.
Livestock and wildlife
Of the 906 sera representing livestock, birds, wild mammals, and reptiles, all were negative for antibodies to EEEV, VEEV, and WEEV. Figure 2 illustrates the locations of the rats, other wild mammals, and reptiles sampled. Eight (4.9%) of 163 cattle were seropositive for SLEV in HIAs performed with T-35573 antigen, but were all negative in ELISAs performed with the same antigen. Two animals seropositive for SLEV were aged 1–5 years, and 6 were older than 5 years.
Of 82 goat sera tested, 1 (1.2%) was seropositive for SLEV by HIA, but all were negative by ELISA. All sheep tested for antibodies to SLEV by both methods were negative. All titers determined in cattle and goat sera were 1:20 by HIA. Overall, the seroprevalence rate of SLEV in livestock was 2.0% (9 of 443) by HIA. With the exception of horses, no other livestock species were seropositive for WNV or ILHV by either serological method.
Two (1.4%) of 140 wild bird sera were seropositive for SLEV by ELISA. Of the 98 birds also tested by HIA only 1 (1.0%) was seropositive. One bird, a 20-year old captive turkey vulture, was seropositive by both methods. The ELISA inhibition value was 35.3%, while the HIA titer was 1:40 for this bird. The other positive bird was a fledgling rock pigeon with an ELISA inhibition value of 31.4%.
Three (2.2%) rodents of 139 tested by ELISA were positive for antibodies to SLEV TBH-28 antigen: a juvenile agouti (Dasyprocta leporina) and two rats (Rattus spp.) with inhibition values of 38.0%, 54.9%, and 55.9%, respectively. The ELISA-positive agouti was also positive by HIA, with a high specific titer of 1:1320. No wildlife species was seropositive for WNV, ILHV, or BSQV, and no reptile was seropositive for any of the viruses tested.
Discussion
We determined the current seroprevalence rates of four arboviruses in Trinidad that are of public health importance in the Americas. With the exception of WNV, ELISA detected more potential seropositives than HIA for both the flaviviruses and alphaviruses tested. Previous researchers (Fernandez and Vazquez 1990; Shi and Wong 2003) have shown that the blocking ELISA is more sensitive than the HIA, although blocking ELISAs may occasionally show lower sensitivity because there is reliance on only one epitope for antibody binding, and some individuals may not produce antibodies against that specific epitope (Wang et al. 2005). Nonetheless, the epitope-blocking ELISA has the major advantage of being species-independent for the reagents required. Our findings suggest the presence of all four viruses in horse populations across the island in both urban and rural areas. Importantly, while SLEV-specific antibodies were detected in a wide variety of species (domestic and wildlife), EEEV-, VEEV-, and WNV-specific antibodies were only detected in horses.
In the case of EEEV, the estimated seroprevalence rates among horses of 4.3% by HIA and 5.1% by ELISA were close to the rate of 4.5% determined in a 1954 survey of 22 horses in Trinidad (Anderson et al. 1956). However, as vaccination against EEEV is not a requirement in Trinidad and Tobago, we cannot exclude the possibility that some of the foreign-bred horses in our study may have been seropositive as a result of vaccination in their country of origin. Serological testing using South American strains (which were unavailable for the current study) could confirm local versus foreign exposure. Nonetheless, our results indicate that horses continue to be exposed to EEEV at low levels in Trinidad, since 5 (1.5%) of the 344 locally-bred animals were seropositive and had never left Trinidad.
Our data also indicate low-level circulation of VEEV, as the estimated seroprevalence rate for antibodies to the virus among locally-bred horses that have never left Trinidad was 0.8%. The serological approach used (TC-83 antigen for HIA and subtype I-specific MAb for ELISA) identifies all subtype I VEEV, but it was not possible to determine the corresponding strains within subtype I. Given the evidence for the exposure of local horses, investigations of clinically-ill horses with encephalitis should consider EEEV and VEEV in the differential, particularly since equine clinical cases were recently reported (OIE 2009) from Central America.
Both VEEV and EEEV have previously been detected serologically in non-equid species in Trinidad, so the failure to detect antibodies in the current study may indicate that the rodents and bird species sampled are not preferred reservoir species for these two viruses. For example, probable VEEV reservoir hosts such as Proechimys spp. (Jonkers et al. 1968b; Barrera et al. 2002), Zygodontomys spp., and Orzyomys spp. (Jonkers et al. 1968b; Deardorff et al. 2009), Cathartes aura (Aguirre et al. 1992), and other birds and small rodent species which were previously reported to be VEEV-seropositive (Scherer et al. 1966; Jonkers et al. 1968b; Aguirre et al. 1992; Estrada-Franco et al. 2004; Navarro et al. 2005), were not among the species trapped and tested in the current study. Given the short period of time during which the traps were set, it is possible that certain host or reservoir species may have been missed by this trapping system. With regard to EEEV, some avian species that were previously recorded to be EEEV-seropositive in Trinidad (Tikasingh, 1968, 1973, 2004) were negative in the current study. Considering reports that both birds and rodents become viremic after EEEV infection (Arrigo et al. 2010), a larger sample of avian and rodent sera, as well as a more focused survey in EEEV- and VEEV-positive locations, may prove useful to gain further insight into the local circulation and maintenance of these viruses.
Our results indicate that 12.1% and 17.2% of locally-bred unvaccinated horses (n=58) were WNV-seropositive by epitope-blocking ELISA and HIA, respectively. These rates are significantly higher than the 4.0% (8 of 200 unvaccinated horses) observed in a previously unpublished study (referred to in Komar and Clark, 2006) in 2004 in Trinidad. It is also higher than the rate reported for horses in Venezuela (4.5%; Bosch et al. 2007) and Cuba (9.0%; Pupo et al. 2006), but lower than in Mexico (29.5–62.5%; Blitvich et al. 2003b; Marlenee et al. 2004; Alonso-Padilla et al. 2009), Guatemala (42.3%; Morales-Betoulle et al. 2006), and Guadeloupe (61.6%; Quirin et al. 2004). Komar and associates (Komar et al. 2003) suggested that the differences in seroprevalence among locations in South and Central America may be a consequence of the difference in the length of time the virus has been in circulation. Four of the five geographic locations where WNV antibodies were detected in horses were in northeast of Trinidad. This may represent a developing focus of infection, so mosquito surveillance studies at the locations of the positive horse farms may be warranted.
Among the 8 samples tested by PRNT90, antibody titers were >160, clearly demonstrating WNV infection as opposed to infection with another closely-related flavivirus. Given the high seroprevalence rate for SLEV, WNV-seropositive sera were also tested by PRNT90 against SLEV to clearly determine the cause of seroprevalence. The majority (62.5%) of sera resulted in an antibody titer of <20 when tested against SLEV (TBH-28 strain), thereby further confirming WNV as the cause of seroprevalence. The highest SLEV antibody titer observed was 160, which was fourfold less than the corresponding WNV-antibody titer (sample 1S3H), and was therefore considered SLEV-negative. It should be noted that three of the PRNT-seropositive samples were negative by the epitope-blocking ELISA test, and this may simply reflect the differences in the antibody types detected in the two tests. Neutralization antibodies have greater longevity than non-neutralization antibodies (Casals 1973), and may be more sensitive for older infections. This is also supported by the IgM antibody-negative result for these three samples. Additionally, 3 of the 11 WNV-seropositive horses in this study tested IgM positive, suggesting recent infection. All seropositive horses were asymptomatic but this is not surprising, as a significant proportion of WNV cases present with fever only (Davidson et al. 2005), so it is easy to understand how some clinical cases might go unnoticed, misdiagnosed, or remain unreported. In addition to the detection of IgM antibodies, there was no apparent increase in seroprevalence with increased exposure age, also suggesting recent WNV activity in Trinidad. Seven of the 11 WNV-seropositive horses were aged 1–5 years, two were aged 6–10 years, one horse was >10 years old, and the age of one horse could not be determined.
Antibodies to WNV have been detected in various livestock, wild mammal, and reptile species (such as cow, goat, opossum, pig, mouse, raccoon, rat, sheep, white-tailed deer, green iguana, and crocodile), as reviewed by Blitvich (Blitvich, 2008), but in the current study none of these species were seropositive. This suggests that in Trinidad there is no or very low (and thus undetectable) transmission of WNV in these species. Alternatively, WNV may not yet be well established in Trinidad and perhaps remains focal. Surveys of vertebrates in locations where seropositive horses were detected are needed to confirm the status of WNV in Trinidad.
We detected antibodies to SLEV in horses, livestock, and wildlife. Of the 506 asymptomatic horses, 49 (9.7%) were seropositive by ELISA, while 13 (2.6%) were seropositive by HIA. Nine of the 10 samples tested by PRNT90 had antibody titers >40 (using a 1:20 cut-off and/or a greater than fourfold difference in antibody titer compared to the corresponding WNV antibody titer), confirming SLEV infection. There were insufficient sera from wildlife for testing by PRNT, but considering the results from the horse sera, it is likely that the SLEV-seropositives among wildlife are also a result of SLEV infection. However, the possibility of infection with another closely-related flavivirus cannot be excluded. This is because the data suggest the circulation of flaviviruses other than the ones we tested for (i.e., 21 horse sera were flavivirus-seropositive but were not SLEV-, WNV-, or ILHV-seropositive). For wild birds, 28 (1.9%) of 1505 Manacus spp. were previously reported as seropositive (Tikasingh et al. 1983), which is comparable to the 1.4% detected across all species in the present study. There are no previous reports of SLEV antibodies in livestock in Trinidad, so the seropositive findings of 4.9% in cattle and 1.2% in goats are considered the first documentation of the possible presence of this virus in both animal species in the country. However, given the low antibody titers determined, the low seroprevalence rates detected, and the inability to confirm these seropositives with other serological tests, the possibility that these seropositives may represent another closely-related flavivirus cannot be excluded.
The relatively high SLEV seroprevalence rate detected in this study, and the history of SLEV isolation and seroprevalence in Trinidad, is a reflection of the endemicity of this virus in the country. Although a higher number of SLEV-seropositives was observed among horses in urban stud farms (n=45) compared with rural stud farms (n=4), the seroprevalence rates were similar (0.096 for urban farms and 0.111 for rural farms). Further entomologic investigations are recommended to identify the vectors that are responsible for the transmission of the virus in these urban regions.
Seven (1.4%) of the 506 horse sera tested showed less than fourfold differences in antibody titers among the alphaviruses tested, and were therefore considered to have antibodies to undetermined alphaviruses or old infections. Similarly, cross-reactive flavivirus titers were detected in 21 (4.2%) horse sera. It is likely that a wider panel of known alphavirus and flavivirus antigens in the Americas, for example Mayaro virus, Rio Bravo virus, Yellow fever virus, and Rocio virus, may assist in ascertaining their identities.
Our study indicates that SLEV, VEEV, and EEEV continue to circulate at low levels in Trinidad, with SLEV being the most widespread among the species tested, and that WNV circulates among equids in Trinidad. Follow-up studies on vectors and viral isolation at locations where seropositive animals were detected in the current study would be useful to confirm these findings. Although WNV has yet to pose a serious disease threat to the Caribbean and wider Latin America, Komar and colleagues (Komar and Clark, 2006) speculated that the potential for an outbreak exists, and therefore vigilant surveillance must be conducted in mosquitoes and wild birds. It is also recommended that WNV be considered in the differential diagnosis in human patients with fever and flu-like symptoms, and meningitis and encephalitis of unknown causes.
Supplementary Material
Acknowledgments
This study was supported by grants from the University of the West Indies Campus Research and Publications Fund, the Trinidad and Tobago Government Research Fund, the Caribbean Health Research Council, and by National Institutes of Health grant AI057156. N.N.T. was supported by a University of the West Indies postgraduate scholarship. Special thanks go to Brandy Russell and Alva Stewart-Johnson for their laboratory support. The field assistance of regional health authorities, Kirk Williams, and Anthony Bastaldo is also greatly appreciated.
Author Disclosure Statement
The authors state that no competing financial interests exist.
References
- Aguirre AA. McLean RG. Cook RS, et al. Serologic survey for selected arboviruses and other potential pathogens in wildlife from Mexico. J Wildl Dis. 1992;28:435–442. doi: 10.7589/0090-3558-28.3.435. [DOI] [PubMed] [Google Scholar]
- Aitken TH. Anderson CR. Downs WG. The isolation of Ilheus virus from wild caught forest mosquitoes in Trinidad. Am J Trop Med Hyg. 1956;5:621–625. doi: 10.4269/ajtmh.1956.5.621. [DOI] [PubMed] [Google Scholar]
- Aitken TH. Downs WG. Anderson CR, et al. Mayaro virus isolated from a Trinidadian mosquito, Mansonia venezuelensis. Science. 1960;131:986. doi: 10.1126/science.131.3405.986. [DOI] [PubMed] [Google Scholar]
- Aitken TH. Downs WG. Spence L, et al. St. Louis encephalitis virus isolations in Trinidad, West Indies, 1953–1962. Am J Trop Med Hyg. 1964;1964;13:450–451. doi: 10.4269/ajtmh.1964.13.450. [DOI] [PubMed] [Google Scholar]
- Aitken TH. Spence L. Jonkers AH, et al. Wyeomyla-virus isolations in Trinidad, West Indies. Am J Trop Med Hyg. 1968a;17:886–888. doi: 10.4269/ajtmh.1968.17.886. [DOI] [PubMed] [Google Scholar]
- Aitken TH. Worth CB. Tikasingh ES. Arbovirus studies in Bush Bush Forest, Trinidad, W. I., September 1959–December 1964. 3. Entomologic studies. Am J Trop Med Hyg. 1968c;17:253–268. doi: 10.4269/ajtmh.1968.17.253. [DOI] [PubMed] [Google Scholar]
- Aitken TH. Worth CB. Jonkers AH, et al. Arbovirus studies in Bush Bush Forest, Trinidad, W. I., September 1959–December 1964. II. Field program and techniques. Am J Trop Med Hyg. 1968b;17:237–252. doi: 10.4269/ajtmh.1968.17.237. [DOI] [PubMed] [Google Scholar]
- Alonso-Padilla J. Loza-Rubio E. Escribano-Romero E, et al. The continuous spread of West Nile virus (WNV): seroprevalence in asymptomatic horses. Epidemiol Infect. 2009;137:1163–1168. doi: 10.1017/S0950268809002325. [DOI] [PubMed] [Google Scholar]
- Anderson CR. Downs WG. Theiler M. Neutralizing antibodies against certain viruses in the sera of residents of Trinidad, B.W.I. Am J Trop Med Hyg. 1956;5:626–641. doi: 10.4269/ajtmh.1956.5.626. [DOI] [PubMed] [Google Scholar]
- Arrigo NC. Adams AP. Watts DM, et al. Cotton rats and house sparrows as hosts for North and South American strains of eastern equine encephalitis virus. Emerg Infect Dis. 2010;16:1373–1380. doi: 10.3201/eid1609.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguste AJ. Adams AP. Arrigo NC, et al. Isolation and characterization of sylvatic mosquito-borne viruses in Trinidad: enzootic transmission and a new potential vector of Mucambo virus. Am J Trop Med Hyg. 2010a;83:1262–1265. doi: 10.4269/ajtmh.2010.10-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguste AJ. Lemey P. Pybus OG, et al. Yellow fever virus maintenance in Trinidad and its dispersal throughout the Americas. J Virol. 2010b;84:9967–9977. doi: 10.1128/JVI.00588-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguste AJ. Volk SM. Arrigo NC, et al. Isolation and phylogenetic analysis of Mucambo virus (Venezuelan equine encephalitis complex subtype IIIA) in Trinidad. Virology. 2009;392:123–130. doi: 10.1016/j.virol.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R. Ferro C. Navarro JC, et al. Contrasting sylvatic foci of Venezuelan equine encephalitis virus in northern South America. Am J Trop Med Hyg. 2002;67:324–334. doi: 10.4269/ajtmh.2002.67.324. [DOI] [PubMed] [Google Scholar]
- Beaty BJ. Calisher CH. Shope RE. Arboviruses. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. 6th. Washington: American Public Health Association; 1989. pp. 797–855. [Google Scholar]
- Blitvich BJ. Bowen RA. Marlenee NL, et al. Epitope-blocking enzyme-linked immunosorbent assays for detection of West Nile virus antibodies in domestic mammals. J Clin Microbiol. 2003a;41:2676–2679. doi: 10.1128/JCM.41.6.2676-2679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvich BJ. Fernandez-Salas I. Contreras-Cordero JF, et al. Serologic evidence of West Nile virus infection in horses, Coahuila State, Mexico. Emerg Infect Dis. 2003b;9:853–856. doi: 10.3201/eid0907.030166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvich BJ. Marlenee NL. Hall RA, et al. Epitope-blocking enzyme-linked immunosorbent assays for the detection of serum antibodies to West Nile virus in multiple avian species. J Clin Microbiol. 2003c;41:1041–1047. doi: 10.1128/JCM.41.3.1041-1047.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvich BJ. Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev. 2008;9:71–86. doi: 10.1017/S1466252307001430. [DOI] [PubMed] [Google Scholar]
- Bosch I. Herrera F. Navarro JC, et al. West Nile virus, Venezuela. Emerg Infect Dis. 2007;13:651–653. doi: 10.3201/eid1304.061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals J. Arbovirus infections. In: Paul JR, editor; White C, editor. Serological Epidemiology. New York: Academic Press; 1973. pp. 99–117. [Google Scholar]
- Central Statistical Office. CSO's household budget survey 1997–1998. Geographic Information Section, Central Statistical Office, Ministry of Finance; Trinidad and Tobago: 1998. [Google Scholar]
- Clarke DH. Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Davidson AH. Traub-Dargatz JL. Rodeheaver RM, et al. Immunologic responses to West Nile virus in vaccinated and clinically affected horses. J Am Vet Med Ass. 2005;226:240–245. doi: 10.2460/javma.2005.226.240. [DOI] [PubMed] [Google Scholar]
- Deardorff ER. Forrester NL. Travassos-da-Rosa AP, et al. Experimental infection of potential reservoir hosts with Venezuelan equine encephalitis virus, Mexico. Emerg Infect Dis. 2009;15:519–525. doi: 10.3201/eid1504.081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs W.G. Aitken T. H. Worth C. B., et al. Arbovirus studies in Bush Bush Forest, Trinidad, W. I., September 1959–December 1964. I. Description of the study area. Am J Trop Med Hyg. 1968;17:224–236. doi: 10.4269/ajtmh.1968.17.224. [DOI] [PubMed] [Google Scholar]
- Estrada-Franco JG. Navarro-Lopez R. Freier JE, et al. Venezuelan equine encephalitis virus, southern Mexico. Emerg Infect Dis. 2004;10:2113–2121. doi: 10.3201/eid1012.040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez RJ. Vazquez S. Serological diagnosis of dengue by an ELISA inhibition method (EIM) Mem Inst Oswaldo Cruz. 1990;85:347–351. doi: 10.1590/s0074-02761990000300012. [DOI] [PubMed] [Google Scholar]
- Hall RA. Broom AK. Hartnett AC, et al. Immunodominant epitopes on the NS1 protein of MVE and KUN viruses serve as targets for a blocking ELISA to detect virus-specific antibodies in sentinel animal serum. J Virol Methods. 1995;51:201–210. doi: 10.1016/0166-0934(94)00105-p. [DOI] [PubMed] [Google Scholar]
- Jonkers AH. Aitken TH. Spence L, et al. Ecological studies of Venezuelan equine encephalitis virus (VEEV) in the Bush Bush wilderness of Trinidad. Rev Venez Sanid Asist Soc Suppl. 1966;3:929. [PubMed] [Google Scholar]
- Jonkers AH. Spence L. Downs WG, et al. Arbovirus studies in Bush Bush forest, Trinidad, W. I., September 1959–December 1964. V. Virus isolations. Am J Trop Med Hyg. 1968a;17:276–284. doi: 10.4269/ajtmh.1968.17.276. [DOI] [PubMed] [Google Scholar]
- Jonkers AH. Spence L. Downs WG, et al. Arbovirus studies in Bush Bush Forest, Trinidad, W. I., September 1959–December 1964. VI. Rodent-associated viruses (VEE and agents of groups C and Guama): isolations and further studies. Am J Trop Med Hyg. 1968b;17:285–298. [PubMed] [Google Scholar]
- Komar N. Clark GG. West Nile virus activity in Latin America and the Caribbean. Rev Panam Salud Publica. 2006;19:112–117. doi: 10.1590/s1020-49892006000200006. [DOI] [PubMed] [Google Scholar]
- Komar N. Langevin S. Hinten S, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlenee NL. Lorono-Pino MA. Beaty BJ, et al. Detection of antibodies to West Nile and Saint Louis encephalitis viruses in horses. Salud Publica Mex. 2004;46:373–375. doi: 10.1590/s0036-36342004000500002. [DOI] [PubMed] [Google Scholar]
- Morales-Betoulle ME. Morales H. Blitvich BJ, et al. West Nile virus in horses, Guatemala. Emerg Infect Dis. 2006;12:1038–1039. doi: 10.3201/eid1206.051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro JC. Medina G. Vasquez C, et al. Postepizootic persistence of Venezuelan equine encephalitis virus, Venezuela. Emerg Infect Dis. 2005;11:1907–1915. doi: 10.3201/eid1112.050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE. Venezuelan equine encephalomyelitis, Costa Rica. World Animal Health Information Database Interface: Animal Health Information. 2009 [Google Scholar]
- Price JL. Isolation of Rio Bravo and a hitherto undescribed agent, Tamana bat virus, from insectivorous bats in Trinidad, with serological evidence of infection in bats and man. Am J Trop Med Hyg. 1978a;27(1 Pt 1):153–161. doi: 10.4269/ajtmh.1978.27.153. [DOI] [PubMed] [Google Scholar]
- Price JL. Serological evidence of infection of Tacaribe virus and arboviruses in Trinidadian bats. Am J Trop Med Hyg. 1978b;27(1 Pt 1):162–167. doi: 10.4269/ajtmh.1978.27.162. [DOI] [PubMed] [Google Scholar]
- Pupo M. Guzman MG. Fernandez R, et al. West Nile Virus infection in humans and horses, Cuba. Emerg Infect Dis. 2006;12:1022–1024. doi: 10.3201/eid1206.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin R. Salas M. Zientara S, et al. West Nile virus, Guadeloupe. Emerg Infect Dis. 2004;10:706–708. doi: 10.3201/eid1004.030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig JT. Hunt AR. Chang GJ, et al. Identification of monoclonal antibodies capable of differentiating antigenic varieties of eastern equine encephalitis viruses. Am J Trop Med Hyg. 1990;42:394–398. doi: 10.4269/ajtmh.1990.42.394. [DOI] [PubMed] [Google Scholar]
- Scherer WF. Sainz CC. De Mucha Macias J, et al. Serologic survey for neutralizing antibodies to eastern equine and western equine encephalitis viruses in man, wild birds and swine in southern Mexico during 1961. Am J Trop Med Hyg. 1966;15:211–218. doi: 10.4269/ajtmh.1966.15.211. [DOI] [PubMed] [Google Scholar]
- Shi PY. Wong SJ. Serologic diagnosis of West Nile virus infection. Expert Rev Mol Diagn. 2003;3:733–741. doi: 10.1586/14737159.3.6.733. [DOI] [PubMed] [Google Scholar]
- Tikasingh ES. Aitken TH. Butcher L, et al. Epidemiologic investigations relating to a case of eastern equine encephalitis in a Trinidadian horse. West Indian Med J. 1968;17:90–95. [PubMed] [Google Scholar]
- Tikasingh ES. Eastern equine encephalitis virus activity in Trinidad: A review. J Caribbean Veterinary Med Assn. 2004;4:18–23. [Google Scholar]
- Tikasingh ES. Jonkers AH. Spence L, et al. Arbovirus studies in an evergreen seasonal marsh forest in Trinidad, West Indies. West Indian Med J. 1983;32:223–231. [PubMed] [Google Scholar]
- Tikasingh ES. Worth CB. Jonkers AH, et al. A three-year surveillance of eastern equine encephalitis virus activity in Trinidad. West Indian Med J. 1973;22:24–31. [Google Scholar]
- Wang E. Paessler S. Aguilar PV, et al. A novel, rapid assay for detection and differentiation of serotype-specific antibodies to Venezuelan equine encephalitis complex alphaviruses. Am J Trop Med Hyg. 2005;72:805–810. [PubMed] [Google Scholar]
- Worth CB. Downs WG. Aitken TH, et al. Arbovirus studies in Bush Bush forest, Trinidad, W. I., September 1959–December 1964. IV. Vertebrate populations. Am J Trop Med Hyg. 1968;17:269–275. doi: 10.4269/ajtmh.1968.17.269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.