Abstract
Adipose tissue expansion involves enlargement of mature adipocytes and the formation of new adipocytes through the differentiation of locally resident preadipocytes. Factors released by the enlarged adipocytes are potential cues that induce the differentiation of the preadipocytes. Currently, there are limited options to investigate these cues in isolation from confounding systemic influences. A gradient generating microfluidic channel-based cell culture system was designed to enable solution patterning, while supporting long-term culture and differentiation of preadipocytes. Solution patterning was confirmed by selectively staining a fraction of uniformly seeded preadipocytes. An adipogenic cocktail gradient was used to induce the differentiation of a fraction of uniformly seeded preadipocytes and establish a spatially defined coculture of adipocytes and preadipocytes. Varying the adipogenic cocktail gradient generated cocultures of preadipocytes and adipocytes with different compositions. Transient application of the cocktail gradient, followed by basal medium treatment showed a biphasic induction of differentiation. The two phases of differentiation correlated with a spatial gradient in adipocyte size. Our results provide in vitro data supporting the size-dependent release of preadipocyte differentiation factors by enlarged adipocytes. Prospectively, the coculture system developed in this study could facilitate controlled, yet physiologically meaningful studies on paracrine interactions between adipocytes and preadipocytes during adipose tissue development.
Introduction
In larger adult mammals, body fat consists predominantly of white adipose tissue (WAT). The bulk of WAT consists of a loose association of white adipocytes held in a collagen (mostly type I) matrix. The tissue also contains a stromal-vascular fraction, which includes undifferentiated preadipocytes and endothelial cells.1 Adipose tissue expansion involves increases in both the size (hypertrophy) and number of adipocytes (hyperplasia). Hypertrophy results from the accumulation of intracellular triglycerides in existing adipocytes. Unlike preadipocytes, mature adipocytes cannot undergo mitotic division.2 Therefore, hyperplasia requires the proliferation and differentiation of preadipocytes into new adipocytes, or adipogenesis. It is hypothesized that the recruitment of preadipocytes for adipogenesis involves biochemical and, perhaps, biophysical cues derived from the mature adipocytes.2 In this regard, it is likely that interactions between adipocytes and locally resident preadipocytes play a key role in the tissue expansion process.3 Ideally, these adipose tissue intrinsic events are studied in isolation from confounding systemic influences.
To date, relatively little work has been done to investigate coculture models of adipocytes and preadipocytes. An extensive literature search found only a handful of published studies on the effects of adipocytes on the proliferation and/or differentiation of preadipocytes. Maumus and coworkers reported that treatment with a medium conditioned by adipocytes enhanced the proliferation of preadipocytes, but did not significantly affect adipogenic differentiation.4 Coculture experiments using trans-well inserts also found that mature adipocytes stimulated the proliferation of preadipocytes. This effect was significantly (four-fold) greater for adipocytes isolated from obese subjects (body mass index (BMI) >35) compared to adipocytes from lean subjects (BMI<25).5 Shillabeer and coworkers reported that coculture with mature adipocytes stimulated adipogenic conversion of preadipocytes without exogenous induction (using a chemical cocktail), although to a lesser extent than with exogenous induction.6 Another recent study involving trans-well inserts also found enhanced adipogenic conversion of preadipocytes in coculture with adipocytes compared to preadipocytes in monoculture.7 This result is, however, contradicted by an earlier study, which also utilized trans-well inserts, but found that the adipocytes inhibited the differentiation of preadipocytes in coculture.8
The above examples illustrate the limitations of current in vitro models, which involve either trans-well inserts or treatment with the conditioned medium. In vivo, cells respond to spatially and temporally organized signals in the surrounding microenvironment, while in vitro cocultures have generally been conducted in batch or fed-batch settings. In these settings, the cultured cells experience a well-mixed chemical environment lacking any spatial gradients in growth factors and other signaling molecules that could mediate the interactions between adipocytes and preadipocytes. Moreover, the batch or fed-batch culture environment is inherently unsteady with time-varying concentration profiles. In this regard, controlled studies on soluble factor-mediated interactions between adipocytes and preadipocytes would benefit from a flow-through coculture system capable of supporting steady chemical microgradients.
In this article, we describe a gradient generating microfluidic channel-based cell culture system that affords spatial control over in vitro adipogenesis. This system was used to characterize a coculture of preadipocytes and adipocytes that was established by solution patterning a differentiation inducing chemical cocktail. Our results show that mature adipocytes are indeed capable of locally stimulating the adipogenic conversion of preadipocytes in the absence of exogenously applied inducing chemicals. A critical determinant of this local recruitment was the adipocyte size, as small, newly formed adipocytes (with multiple small lipid droplets) did not elicit the differentiation of preadipocytes.
Materials and Methods
Materials
3T3-L1 preadipocytes were obtained from ATCC (Manassas, VA). Tissue culture reagents, including the Dulbecco's modified Eagle's medium (DMEM), calf serum (CS), fetal bovine serum (FBS), human insulin, and penicillin-streptomycin were purchased from Invitrogen (Carlsbad, CA). Rat tail type 1 collagen was purchased from BD Biosciences (Bedford, MA). Unless otherwise noted, all other chemicals were purchased from Sigma (Saint Louis, MO). Constant flow syringes were purchased from Warner Instruments (Hamden, CT). Calcein acetoxymethyl ester (calcein-AM) was purchased from Invitrogen (Eugene, OR).
Device design and fabrication
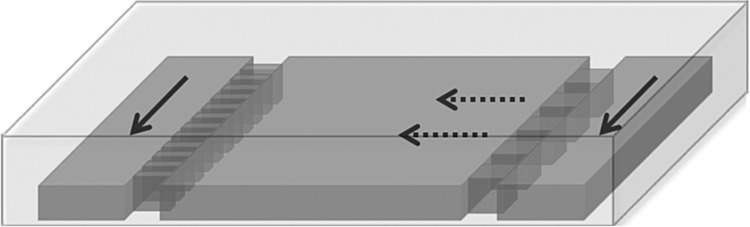
The microfluidic gradient culture device was fabricated in poly(dimethylsiloxane) (PDMS) using soft lithography and rapid prototyping.9 The device consisted of three microfluidic channel compartments partitioned by aligned posts (Fig. 1). The dimensions of the middle compartment were 3 mm (width) ×7 mm (length) ×100 μm (height). The dimensions of the two flanking channels were 1.5 mm (width) ×7 mm (length) ×100 μm (height). The postdimensions were 20 μm (width) ×100 μm (depth) ×100 μm (height) and 140 μm (width) ×100 μm (depth) ×100 μm (height). The spacing between the posts was 20 μm. A transparency mask with a minimum feature size of 20 μm was printed using a high-resolution printer (Page One, Irvine, CA) from a CAD file (Autodesk, San Rafael, CA). The mask was used in 1:1 contact photolithography of SU8-50 photoresist (MicroChem, Newton, MA) to generate a negative master consisting of 100-μm high-patterned photoresist on a Si wafer (Silicon, Inc., Boise, ID). Positive replicas with embossed channels were fabricated by molding PDMS (Sylgard 184; Dow Corning, Midland, MI) against the master. The cured PDMS was peeled off the master, and holes were punched with a sharpened 18-gauge blunt-end needle for fluidic interconnects. The PDMS was sealed against a glass slide by treating both surfaces with oxygen plasma for 50 s (Plasma cleaner Model PDC-001; Harrick Plasma, Ithaca, NY). The sealed devices were then heated overnight at 125°C to complete the bonding. Before the experiment, the glass surface inside the middle channel was coated with a dilute solution of type I collagen in PBS (0.36 mg/mL) for 1 h at room temperature.
FIG. 1.
Schematic of gradient generating cell culture device with nonuniform post sizes. Solid arrows indicate direction of bulk flow in the medium channels. Dashed arrows indicate the direction of net diffusion across the culture compartment.
Microfluidic cell culture
Undifferentiated 3T3-L1 preadipocytes were expanded in a T-flask with a growth medium consisting of the DMEM supplemented with 10% CS (v/v), 200 U/mL penicillin, and 200 mg/mL streptomycin. The preadipocytes were detached from the culture flask, washed, and resuspended in the growth medium. The suspension density was 6×106 cells/mL. The cell suspension was loaded into the culture compartment using a micropipette tip. The cells were allowed to settle for 1 h at 37°C. The two side channels were then filled with the prewarmed growth medium. The device was placed in an incubator with humidified atmosphere. After overnight incubation, each inlet of the medium channels was connected with 1.09-mm OD polyethylene (PET) tubing to a constant flow syringe filled with the prewarmed growth medium. The constant flow syringe consists of two nested containers. Medium drips from the inner container to the outer container through a side port, resulting in a nearly constant flow rate from the bottom of the outer container. Average flow velocities in the medium channels were estimated to be approximately 3–4 μL/min based on the volume discharged. Uniform elevation and head pressures maintained the same flow rate for both medium channels. The medium channel outlets were connected with PET tubing to a waste reservoir at a lower elevation. The microfluidic device, tubing, syringes, and waste reservoir were placed in an incubator to maintain control over temperature and medium pH. On day 3, both medium channels were connected to an induction medium (DM1) consisting of a basal medium (DMEM with 10% FBS and penicillin/streptomycin) supplemented with an adipogenic cocktail (1 mg/mL insulin, 0.5 mM isobutylmethylxanthine and 1 mM dexamethasone). On day 6, both medium channels were connected to a second induction medium (DM2) consisting of the basal medium supplemented with only insulin. On day 9, both medium channels were connected to the basal medium. The basal medium was applied for the remainder of the culture experiment.
Microfluidic gradient culture
Undifferentiated 3T3-L1 preadipocytes were loaded into collagen coated culture chambers as described before. In these experiments, cells were seeded near confluence (105 cells/cm2) using a higher density suspension (25×106 cells/mL) to reduce the proliferation time in the microfluidic device. After overnight incubation, one of the medium channels was connected to a syringe containing varying dilutions of DM1, while the other channel was connected to a syringe containing the growth medium. In one set of experiments, the DM1 gradients remained in place for 14 days. In another set of experiments, the differentiation cocktail gradient was removed after 6 days. On day 7, both medium channels were connected to syringes holding the basal medium to study the effects of adipocytes secreted factors on preadipocyte recruitment.
Characterization of the solution gradient
A suspension of undifferentiated preadipocytes at a density of 6×106 cells/mL was loaded into the culture compartment as described above. After overnight incubation, one medium channel was connected to a syringe holding a 1.6 μM solution of calcein-AM in the growth medium (Invitrogen). The other channel was connected to a syringe holding the growth medium. After 4 h, the calcein-AM gradient in the culture compartment was recorded using fluorescence microscopy. In a separate experiment, the calcein fluorescence across the culture chamber was recorded at 24 and 48 h to assess the long-term stability of the gradient.
Characterization of adipocyte differentiation with diluted induction cocktail
Undifferentiated 3T3-L1 preadipocytes were seeded in 12-well plates and induced to differentiate using the DM1-DM2 cycle as described above. To assess the effect of reducing the levels of the induction cocktail components, differentiation experiments were also performed using an induction medium (DM1) diluted 10-fold with the adipocyte basal medium (to keep the nutrient concentrations the same). Phase-contrast microscopy images were recorded on days 10 and 14 to monitor the progress of differentiation.
Image analysis
Preadipocyte differentiation (i.e., intracellular lipid accumulation, morphological changes) was monitored by taking images using phase-contrast microscopy (TE300; Nikon-US, Melville, NY). The images were analyzed using the Simple PCI (Compix, Inc., Cranberry Township, PA) and Image J10 software packages. Adipocyte sizes were estimated by averaging the volumes of randomly selected cells containing visible lipid droplets. The analysis selected ten cells from each of four images recorded per experiment.
Statistics
All experiments were performed at least twice with different batches of preadipocytes or adipocytes. Comparisons between two experimental groups were performed using the analysis of variance. Group means were deemed to be statistically significantly different when p<0.05.
Results
Stability of gradient
Figure 1 shows the basic device design. Aligned posts divide the device into a middle cell chamber and flanking medium channels. The cell chamber and medium channels each connect to a separate pair of inlet and outlet ports. When a seed suspension is loaded into the device through the middle inlet, the small inter-post distance and surface tension effectively localize the suspension to the middle chamber. Connected to medium reservoirs, the two flanking channels act as a source and a sink that are continuously replenished by flows. At steady state, diffusion from the source to the sink channel establishes a stable solution gradient across the middle compartment. The aligned posts present a convection barrier, preventing bulk motion of the medium across this compartment. The shape of the solution gradient depends on the dimensions and placement of the aligned posts.
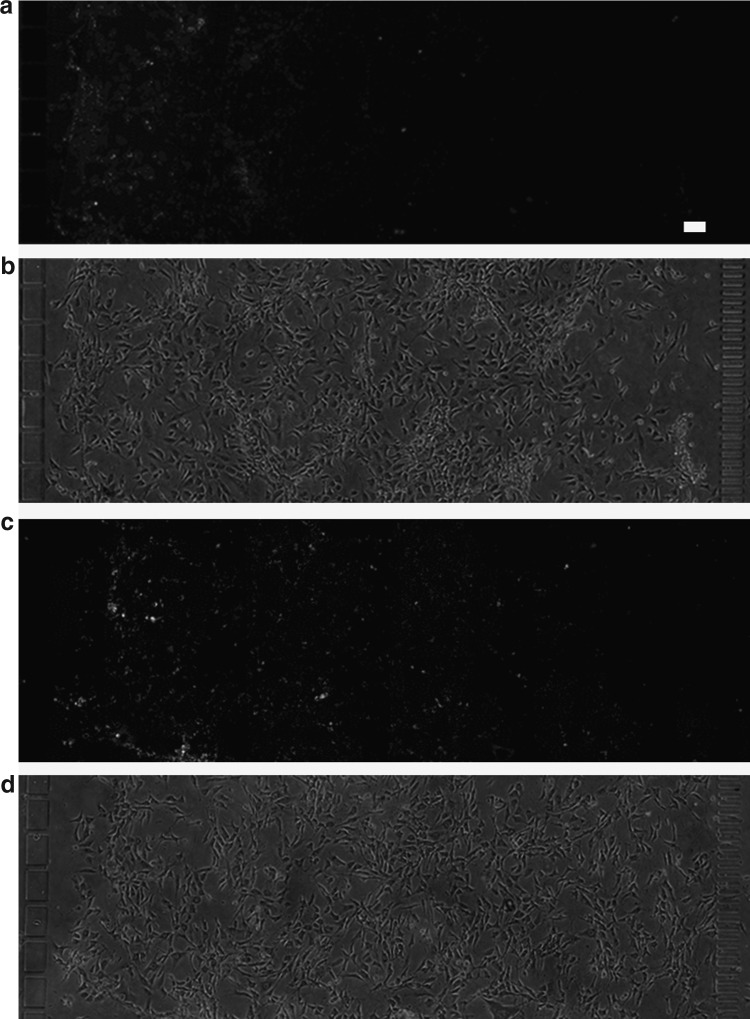
The stability of the solution gradient in the presence of a cell layer was experimentally evaluated by steadily infusing a dye for live cells (calcein-AM) through one of the two medium channels. Fluorescent microscopy images recorded after 24 h of infusion showed a gradient of stained cells in the middle culture compartment (Fig. 2a). Phase-contrast microscopy images taken at the same time showed an evenly distributed layer of preadipocytes in the culture compartment (Fig. 2b). Once established, a chemical gradient could be maintained for at least 48 h (Fig. 2c, d).
FIG. 2.
Solution gradient profiles in the culture compartment over the cell layer. The profiles were visualized with a stain for intracellular esterase activity (1.6 μM calcein-AM, MW 995) infused through the left medium channel. Fluorescence microscopy images were recorded 24 h (a) and 48 h (c) after the gradient was introduced. Corresponding phase contrast images (b, d) show uniformly seeded cell layers. Scale bar: 100 μm.
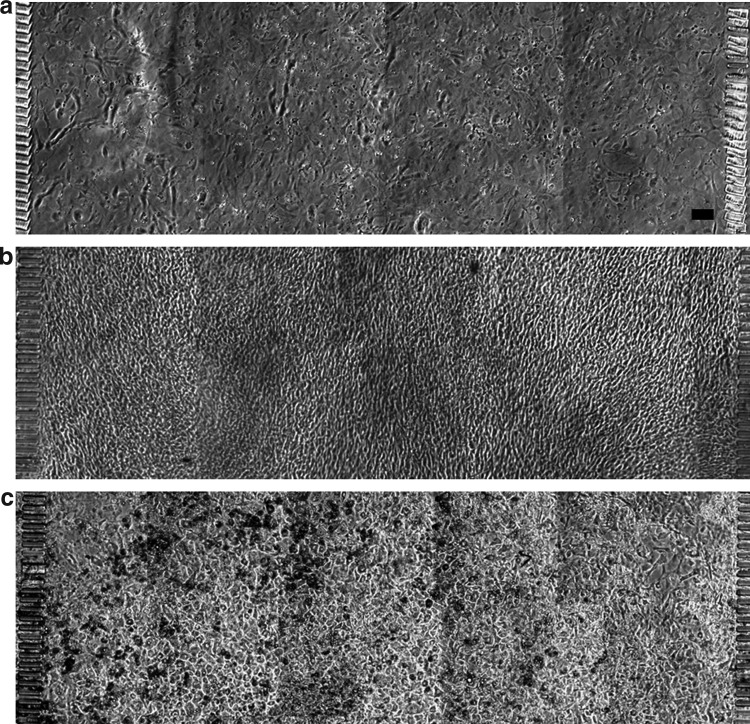
Long-term perfusion
At a seed suspension density of 6×106 cells/mL, an evenly distributed monolayer of preadipocytes was observed after an overnight incubation (Fig. 3a). Confluence was reached on day 3 of perfusion culture with the growth medium (Fig. 3b). Following DM1 and DM2 perfusion through both medium channels, the cells exhibited morphological changes characteristic of adipogenic differentiation, assuming a rounded shape and forming lipid inclusion bodies visible as microscopic droplets. By day 14, more than 90% of the cells in the culture compartment contained visible lipid droplets.
FIG. 3.
Proliferation and differentiation of 3T3-L1 preadipocytres in the gradient culture chamber. Phase contrast images show cells on day 1 (a), day 4 (b), and day 14 (c) after seeding. Scale bar: 100 μm.
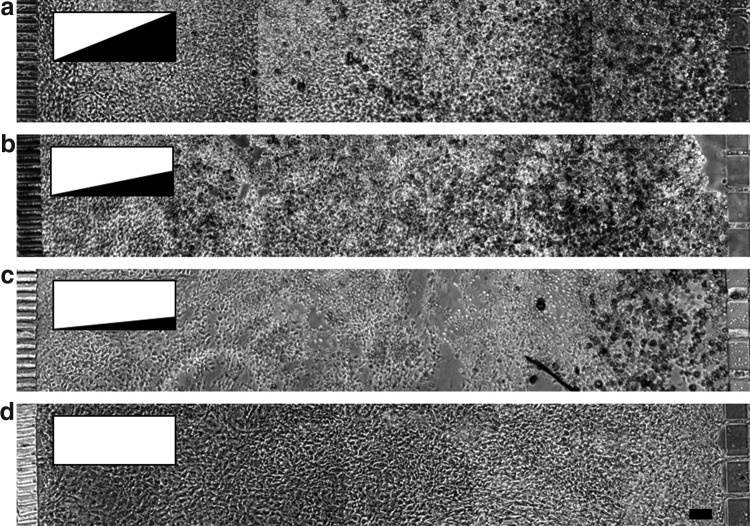
Differentiation cocktail gradients
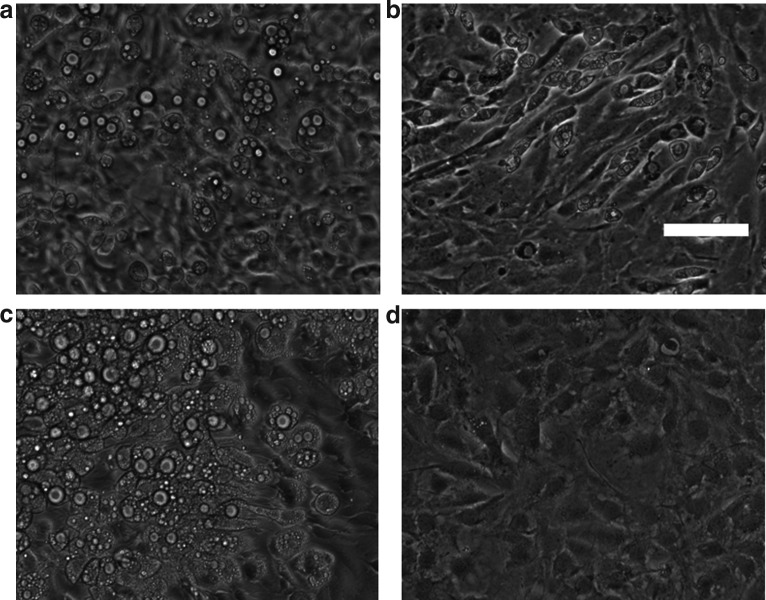
In conventional T-flask or multiwell culture, adipogenic differentiation of 3T3-L1 cells is initiated using a chemical cocktail, usually consisting of insulin, dexamethasone, and 3-isobutyl-1-methylxanthine. In these settings, the chemical cocktail is presented through a well-mixed medium environment, resulting in uniformly random differentiation throughout the culture area. The present study utilized the gradient generating feature of the microfluidic device to induce the differentiation of cells in a selected region within the culture area. Phase-contrast microscopy images recorded on day 14 showed that the selective induction led to a differentiation gradient across the culture compartment. Cells near the source channel (carrying DM1) exhibited the greatest extent of morphological changes, while the cells near the sink channel (carrying the growth medium) retained the morphology of undifferentiated 3T3-L1 preadipocytes (Fig. 4a). Decreasing the cocktail gradient by diluting the cocktail concentration in source channel reservoir with the maintenance medium attenuated the resulting differentiation gradient (Fig. 4b, c). No differentiation was observed when both medium channels carried the growth medium (Fig. 4d).
FIG. 4.
Differentiation of 3T3-L1 preadipocytes under varying gradients of the adipogenic cocktail (insulin, dexamethasone and isobutyl-methylxanthine). Inserts illustrate cocktail gradient profiles. Phase contrast images show cells on day 14 following induction. The left medium channel carried the preadipocyte growth medium (GM), whereas the right medium channel carried (a) undiluted DM1, (b) induction medium (DM1) diluted twofold with adipocyte basal medium, (c) DM1 diluted fourfold or (d) GM. Scale bar: 100 μm.
Adipocyte recruitment of preadipocytes
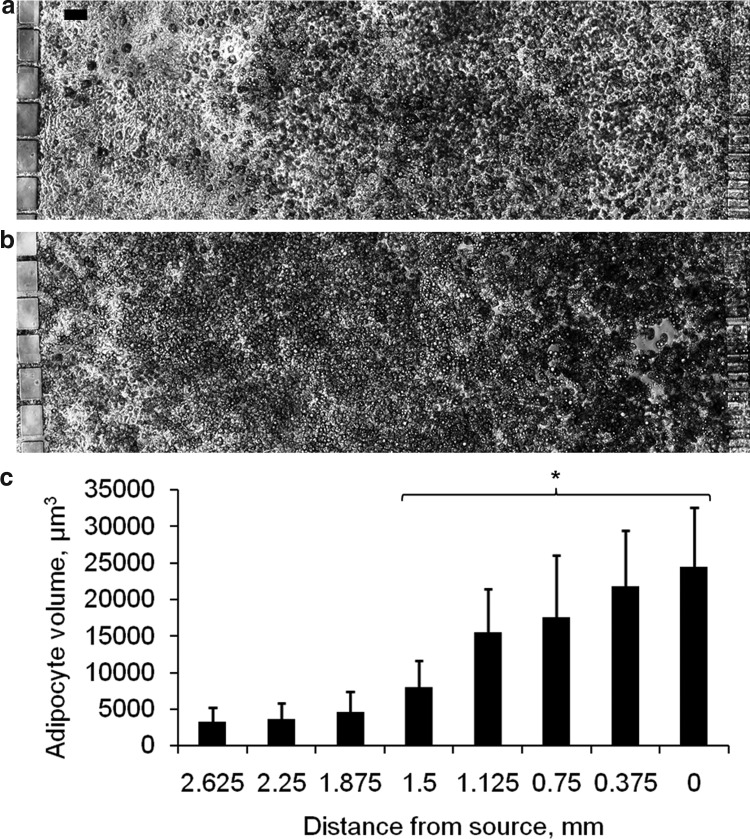
In vivo, adipogenesis occurs through the recruitment and differentiation of locally resident preadipocytes. It has been hypothesized that this recruitment involves growth factors secreted by mature adipocytes.4 The present study utilized the spatial pattern of differentiation generated by the induction cocktail gradient to determine whether mature adipocytes can trigger de novo adipogenesis in neighboring preadipocytes. To observe the preadipocytes in the absence of exogenously added chemical inducers, the cocktail gradient was removed after 6 days. From day 7, both medium channels carried the basal medium. By day 10, the cells on the source side of the cocktail gradient had assumed a round shape and contained microscopically visible lipid droplets, whereas the cells on the sink side retained the morphology of undifferentiated preadipocytes (Fig. 5a). At this time, the region of differentiated adipocytes covered about 67% of the culture area. By day 14, the region of differentiated cells had expanded further into the region of previously undifferentiated cells. At this time about 92% of the cells in the culture compartment exhibited the characteristic morphology of adipocytes (Fig. 5b). The size of the adipocytes varied across the cell culture compartment. The average volume decreased nearly eightfold from 24,500 μm3 in the region adjacent to the source side to 3200 μm3 in the region adjacent to the sink side (Fig. 5c). As adipocyte size correlates with the extent of differentiation,2 the size gradient suggested that the time of induction varied across the culture compartment due to factors secreted by larger adipocytes. To confirm this observation, we conducted separate induction experiments in multi-well plates using various dilutions of the cocktail. At 10-fold dilution, the cultures showed nearly no differentiation as assessed by morphological changes (Fig. 6), indicating that the delay in the differentiation of cells near the sink side was not due to a low-level exposure to the induction cocktail.
FIG. 5.
Induction of preadipocyte differentiation by mature adipocytes. The adipogenic cocktail gradient was removed after 6 days. From day 7, both medium channels carried the adipocyte basal medium. Phase contrast images show cells on (a) day 10 and (b) day 14 after seeding. (c) Distribution of adipocyte size across the culture compartment on day 14. Data shown are mean volumes of 10 mature adipocytes randomly selected from each region of the culture compartment. The culture compartment was binned into 8 equally spaced regions of width 0.375 mm. Error bars represent SD. *significantly different from the mean adipocyte volume of the region adjacent to the sink channel (2.625-3 mm) at p<0.001.
FIG. 6.
Effect of diluted induction cocktail. Images show cells on day 10 and 14 after treatment with normal (undiluted) induction cocktail (a, c) and 10-fold diluted induction cocktail (b, d). Scale bar: 100 μm.
Discussion
This study characterized the growth and differentiation of preadipocytes in a novel gradient generating microfluidic culture device. The results of this study demonstrate, for the first time, that a solution gradient of inducing chemicals, applied to evenly distributed preadipocytes, generates a gradient of adipocyte differentiation within a contiguous culture compartment. The differentiation gradient could be manipulated by varying the solution gradient, leading to cocultures of preadipocytes and adipocytes of different compositions. Over time, the region of differentiated adipocyte expanded into the region previously consisting of preadipocytes. This expansion occurred several (at least 4) days after the chemical cocktail gradient had been removed, suggesting that endogenous factors secreted by the adipocytes triggered a new round of differentiation. Our results also provide strong evidence that adipocytes cell size plays an important role in the formation of new adipocytes from preadipocytes.
In a recent study, Maumus and coworkers compared the size and number of adipocytes in subcutaneous fat pads isolated from obese (BMI>30) and lean/overweight (BMI<30) subjects. The fraction of very small adipocytes (with diameter 20–40 μm) was significantly greater (by a factor of about three) in the fat pad of obese subjects.4 Increased adiposity in lean/overweight subjects correlated negatively with the fraction of small adipocytes (<60 μm), but positively with the fraction of large adipocytes (>100 μm). In obese subjects, the fraction of large adipocytes remained stable, while the fraction of smaller adipocytes correlated positively with the increase in adipose tissue mass.4 These observations as well as other previous findings suggest that hypertrophy preceded hyperplasia in several forms of obesity.11,12 Mature adipocytes do not undergo mitosis.2 Therefore, the increase in adipocyte number reflects the formation of new adipocytes from preadipocytes, or adipogenesis. It has been suspected for some time4,5,13 that the microenvironment established by the hypertrophic adipocytes promotes the proliferation and/or differentiation of locally resident precursor cells. However, direct experimental evidence of this phenomenon has been lacking due to difficulties in isolating the specific contributions of the adipocytes in vivo. A limited number of studies have examined the effect of adipocyte-derived factors on preadipocytes using either transwell inserts or conditional medium treatment. These approaches provided segregation between adipocytes and preadipocytes, but sacrificed either cell-to-cell contact or reciprocal interaction.
In this study, we used a solution gradient of chemical inducers to pattern the differentiation of preadipocytes within a contiguous culture compartment. Establishing an initial pattern of differentiated and undifferentiated cells and then removing the exogenously added inducers produced a spatially defined, yet contacting coculture of adipocytes and preadipocytes. Replacing the medium in both medium channels with the basal medium decreased the likelihood that further differentiation of the remaining preadipocytes was caused by the exogenously added chemicals, and this is confirmed by dilution studies. On day 10, 4 days after removing the cocktail gradient, the fraction of undifferentiated preadipocytes in the culture compartment remained unchanged. On the other hand, differentiation steadily progressed in the cells exposed to the inducing chemicals, as evidenced by microscopically visible lipid droplets. On day 14, lipid filled, round cells were observed in the region where only undifferentiated preadipocytes were previously visible, indicating that a second round of differentiation had occurred.
It is unlikely that this later round of differentiation was triggered by residual amounts of exogenously added medium components that remained in the medium channels or culture compartment. Insulin is rapidly internalized and degraded by adipocytes. Goldstein and coworkers found that freshly isolated, intact mouse fat pads degraded ∼10% of exogenously added insulin after only 90 min of incubation at 37°C.14 Jochen and coworkers reported that the amount of insulin internalized by a suspension of freshly isolated human adipocytes peaked after 30 min of incubation at 37°C.15 By this time, 40% of the total insulin associated with the cells was internalized. After 60 min, the percentage of total cell-associated insulin within the cells dropped to 1.2%.15 It is also doubtful that components in the basal medium of the sink channel triggered the second round of differentiation. In control experiments, differentiation did not occur when both flow channels carried the basal medium. Furthermore, separate well-plate culture experiments confirmed that exposure to diluted concentrations of the cocktail components is not sufficient to induce even delayed differentiation, through day 18 postinduction.
Another unlikely explanation for the delayed induction is nutrient limitation near the sink channel. Preadipocyte differentiation proceeded from the source channel to the sink channel. Adipocyte metabolism increases dramatically with differentiation.16 Therefore, the medium concentrations of major nutrients, such as glucose, are expected to be lower near the source channel, rather than the sink channel. One last possible explanation is migration of differentiated adipocytes from the source channel to the sink channel. However, this is unlikely, because mature adipocytes lack motility and are not able to migrate in a directed fashion.17 For these reasons, the most likely explanation for the second round of differentiation involves endogenous factors derived from the adipocytes formed during the first round of differentiation.
The identities of these inducing factors and their mechanisms of action remain to be elucidated. The progression of differentiation (from the region adjacent to the source channel to the sink channel), points to soluble factors that diffused from the region of the largest, and hence, oldest adipocytes. A mechanism involving the diffusion of soluble factors is consistent with the findings of a previous study, suggesting that physical contact may not be required for the stimulatory effect of mature adipocytes on the adipogenic conversion of preadipocytes.6 Adipocytes are increasingly recognized to perform signaling functions by releasing a variety of factors, including cytokines, chemokines, and many other biologically active molecules collectively termed adipokines. Several of these adipokines enhance the differentiation of preadipocytes, including fibroblast growth factor 1,18 fibroblast growth factor 2,19 and insulin-like growth factor 1.20 Moreover, preadipocytes express corresponding receptors for each of these peptides.21,22
Possible nonpeptide factors include free fatty acids. Long chain poly-unsaturated fatty acids could stimulate preadipocyte differentiation by activating the peroxisome proliferator-activated receptor γ, providing substrates for triacylglycerol synthesis, or both.23,24 For example, linoleic acid has been shown to increase lipid accumulation in human preadipocytes.25 Likewise, arachidonic acid has been found to enhance the differentiation of Ob1771 preadipocytes.26
Questions also remain, regarding the biochemical and/or biophysical cues leading to the release of the differentiation factors by the adipocytes. Possible cues include the size or lipid content of the cell. In cultures of adipocytes isolated from individuals undergoing elective surgery, the cell size correlated positively with the secretion of several adipokines, including leptin, interleukin-6 (IL-6), IL-8, tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1, interferon γ-inducible protein 10, macrophage inflammatory protein-1β, granulocyte colony stimulating factor, IL-1 receptor antagonist, and adiponectin.27 An earlier study found that adipocytes isolated from obese subjects stimulated the proliferation of preadipocytes to a greater degree that adipocytes from lean subjects.5 Taken together, these findings suggest that beyond some threshold, the expansion of adipose tissue mass in obesity occurs primarily through an increase in adipocyte number.28
An upper cell size limit implies a limit for hypertrophic tissue growth as a mechanism to accommodate lipid storage in obesity, when caloric intake chronically exceeds utilization. As adipose tissue depots vary in their hyperplastic potential, the hypertrophic growth limit could explain the redistribution of fat, including the ectopic storage of triglycerides outside of adipose tissue depots observed in obese subjects.29 Ectopic accumulation of lipid is a major risk factor for obesity-related diseases, for example, nonalcoholic fatty liver disease.30 A hypertrophic growth limit could also explain the finding that adipocyte size by itself poorly predicts the metabolic characteristics of obese human subjects such as hyperinsulinemia, hypertriglyceridemia, and elevated fasting plasma glucose.28 It should be noted, however, that the correlation between cell size, number, and obesity-related metabolic alterations also depends on the type and location of the adipose tissue depot.31,32 Fortunately, depot-specific metabolic characteristics of adipocytes have been observed to persist in vitro, suggesting that it may be possible to investigate the impact of such characteristics on adipocyte recruitment of preadipocytes by using primary cells isolated from different depots.
More than three decades ago, Hirsch and Batchelor33 noted an apparent upper limit to the size of an adipocyte in individuals with only moderate obesity.28 This observation was subsequently corroborated in other in vivo studies.34,35 However, obtaining direct evidence for a critical cell size has been difficult due to the lack of an appropriate experimental system supporting controlled colocalization of preadipocytes and adipocytes of varying sizes. In this study, we used the cell volume analysis of adipocytes formed during the first and second rounds of differentiation in the gradient chamber to demonstrate a clear size gradient (Fig. 5c) that correlated with the timing of the phased induction. To the best of our knowledge, the results of this study provide the first in vitro data supporting the hypothesis that adipocyte enlargement could trigger the release of recruitment factors promoting the differentiation of preadipocytes. The mechanism through which the enlarged adipocyte senses its size and releases the differentiation factors remains to be elucidated and warrants further studies. Prospectively, the microfluidic gradient culture system described in this study could be used to screen for adipose tissue intrinsic paracrine factors that promote the differentiation of locally resident preadipocytes and other progenitor cells in the stromal-vascular fraction. Furthermore, the coculture system could be used to conduct controlled studies on the effects genetics, diet, and other environmental factors on the recruitment of preadipocytes by adipocyte-derived factors. Recently, Jo and coworkers reported a mathematical model of adipose tissue mass and cell size distribution, which pointed to diet as a major factor in accelerating hypertrophy and fat pad mass increase, which in turn enhances new adipocyte recruitment.36
Acknowledgments
We thank Dr. Ramin Haghgooie and Octavio Hurtado (BioMEMS Resource Center) for assistance in master mold fabrication. We gratefully acknowledge financial support from the National Science Foundation (0651359) and the National Institutes of Health (DK081768).
Disclosure Statement
No competing financial interests exist.
References
- 1.Crandall D.L. Hausman G.J. Kral J.G. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4:211. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 2.Gregoire F.M. Smas C.M. Sul H.S. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 3.Hausman D.B. DiGirolamo M. Bartness T.J. Hausman G.J. Martin R.J. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 4.Maumus M. Sengenes C. Decaunes P. Zakaroff-Girard A. Bourlier V. Lafontan M., et al. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab. 2008;93:4098. doi: 10.1210/jc.2008-0044. [DOI] [PubMed] [Google Scholar]
- 5.Considine R.V. Nyce M.R. Morales L.M. Magosin S.A. Sinha M.K. Bauer T.L., et al. Paracrine stimulation of preadipocyte-enriched cell cultures by mature adipocytes. Am J Physiol. 1996;270:E895. doi: 10.1152/ajpendo.1996.270.5.E895. [DOI] [PubMed] [Google Scholar]
- 6.Shillabeer G. Forden J.M. Lau D.C. Induction of preadipocyte differentiation by mature fat cells in the rat. J Clin Invest. 1989;84:381. doi: 10.1172/JCI114177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stacey D.H. Hanson S.E. Lahvis G. Gutowski K.A. Masters K.S. In vitro adipogenic differentiation of preadipocytes varies with differentiation stimulus, culture dimensionality, and scaffold composition. Tissue Eng Part A. 2009;15:3389. doi: 10.1089/ten.TEA.2008.0293. [DOI] [PubMed] [Google Scholar]
- 8.Janke J. Engeli S. Gorzelniak K. Luft F.C. Sharma A.M. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- 9.Whitesides G.M. Ostuni E. Takayama S. Jiang X. Ingber D.E. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 10.Rasband W.S. Image J. Bethesda MD. U. S. National Institutes of Health. pp. 1997–2007.
- 11.Hirsch J. Fried S.K. Edens N.K. Leibel R.L. The fat cell. Med Clin North Am. 1989;73:83. doi: 10.1016/s0025-7125(16)30693-9. [DOI] [PubMed] [Google Scholar]
- 12.Sjostrom L. Bjorntorp P. Body composition and adipose cellularity in human obesity. Acta Med Scand. 1974;195:201. doi: 10.1111/j.0954-6820.1974.tb08123.x. [DOI] [PubMed] [Google Scholar]
- 13.Faust I.M. Johnson P.R. Stern J.S. Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978;235:E279. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein B.J. Livingston J.N. Insulin degradation by adipose tissue. Studies at several levels of cellular organization. Biochem J. 1980;186:351. doi: 10.1042/bj1860351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jochen A.L. Berhanu P. Olefsky J.M. Insulin internalization and degradation in adipocytes from normal and type II diabetic subjects. J Clin Endocrinol Metab. 1986;62:268. doi: 10.1210/jcem-62-2-268. [DOI] [PubMed] [Google Scholar]
- 16.Si Y. Yoon J. Lee K. Flux profile and modularity analysis of time-dependent metabolic changes of de novo adipocyte formation. Am J Physiol Endocrinol Metab. 2007;292:E1637. doi: 10.1152/ajpendo.00670.2006. [DOI] [PubMed] [Google Scholar]
- 17.Li J.J. Xie D. Cleavage of focal adhesion kinase (FAK) is essential in adipocyte differentiation. Biochem Biophys Res Commun. 2007;357:648. doi: 10.1016/j.bbrc.2007.03.184. [DOI] [PubMed] [Google Scholar]
- 18.Hutley L. Shurety W. Newell F. McGeary R. Pelton N. Grant J., et al. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes. 2004;53:3097. doi: 10.2337/diabetes.53.12.3097. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi N. Toriyama K. Nicodemou-Lena E. Inou K. Torii S. Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1998;95:1062. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith P.J. Wise L.S. Berkowitz R. Wan C. Rubin C.S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J Biol Chem. 1988;263:9402. [PubMed] [Google Scholar]
- 21.Patel N.G. Kumar S. Eggo M.C. Essential role of fibroblast growth factor signaling in preadipoctye differentiation. J Clin Endocrinol Metab. 2005;90:1226. doi: 10.1210/jc.2004-1309. [DOI] [PubMed] [Google Scholar]
- 22.Modan-Moses D. Janicot M. McLenithan J.C. Lane M.D. Casella S.J. Expression and function of insulin/insulin-like growth factor I hybrid receptors during differentiation of 3T3-L1 preadipocytes. Biochem J. 1998;333(Pt 3):825. doi: 10.1042/bj3330825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kliewer S.A. Sundseth S.S. Jones S.A. Brown P.J. Wisely G.B. Koble C.S., et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krey G. Braissant O. L'Horset F. Kalkhoven E. Perroud M. Parker M.G., et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 25.Hutley L.J. Newell F.M. Joyner J.M. Suchting S.J. Herington A.C. Cameron D.P., et al. Effects of rosiglitazone and linoleic acid on human preadipocyte differentiation. Eur J Clin Invest. 2003;33:574. doi: 10.1046/j.1365-2362.2003.01178.x. [DOI] [PubMed] [Google Scholar]
- 26.Massiera F. Saint-Marc P. Seydoux J. Murata T. Kobayashi T. Narumiya S., et al. Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J Lipid Res. 2003;44:271. doi: 10.1194/jlr.M200346-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Skurk T. Alberti-Huber C. Herder C. Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 28.Mundi M.S. Karpyak M.V. Koutsari C. Votruba S.B. O'Brien P.C. Jensen M.D. Body fat distribution, adipocyte size, and metabolic characteristics of nondiabetic adults. J Clin Endocrinol Metab. 2010;95:67. doi: 10.1210/jc.2009-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi A.P. Fantin F. Zamboni G.A. Mazzali G. Rinaldi C.A. Del Giglio M., et al. Predictors of ectopic fat accumulation in liver and pancreas in obese men and women. Obesity. 2011;19:1747. doi: 10.1038/oby.2011.114. [DOI] [PubMed] [Google Scholar]
- 30.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Tchoukalova Y.D. Votruba S.B. Tchkonia T. Giorgadze N. Kirkland J.L. Jensen M.D. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107:18226. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veilleux A. Caron-Jobin M. Noel S. Laberge P.Y. Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60:1504. doi: 10.2337/db10-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsch J. Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5:299. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 34.Weyer C. Foley J.E. Bogardus C. Tataranni P.A. Pratley R.E. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 35.Tchoukalova Y.D. Koutsari C. Karpyak M.V. Votruba S.B. Wendland E. Jensen M.D. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr. 2008;87:56. doi: 10.1093/ajcn/87.1.56. [DOI] [PubMed] [Google Scholar]
- 36.Jo J. Gavrilova O. Pack S. Jou W. Mullen S. Sumner A.E., et al. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]