Abstract
The behavior of the mitochondrial permeability transition pore has been linked to mitochondrial maturation underlying cardiomyocyte differentiation in the embryo. Mitochondrial signaling in heart development has direct implications for cardiogenesis and stem cell lineage specification.
Heart formation requires maturation and integration of multiple systems to support development of the contractile apparatus in the nascent cardiomyocyte. Although a number of transcriptional networks that facilitate cardiogenesis have been mapped, master regulators of heart development remain elusive. A recent report highlights mitochondria, and more specifically the mitochondrial permeability transition pore (mPTP), as a gating mechanism underlying differentiation in the developing heart,1 implicating cross-talk between genetic and metabolic signaling.
Immature mitochondria of early embryonic hearts must transition into more complex structures to ensure proficient and energetically competent cardiac development.2–4 Developmental restructuring is recapitulated during spontaneous differentiation of stem cells, where pluripotent gene downregulation accelerates mitochondria DNA replication to promote mitochondrial biogenesis associated with lineage specification.2–6 Cardiomyocytes isolated from day 9.5 embryos (e9.5) harbor few fragmented mitochondria, with poorly defined and unorganized cristae, which undergo elaborate maturation and by day e13.5 evolve into filamentous networks of elongated and branched mitochondria with abundant and organized cristae.1 Moreover, mitochondria expansion from a predominately perinuclear localization to an extensive configuration across the cell facilitates energy supply and transfer between cellular compartments.1, 2, 4, 7–9 Mitochondrial structure and function are markers of differentiation capacity. Cell with less perinuclear mitochondria have greater spontaneous differentiation,10 and those with low mitochondrial membrane potential show greater propensity for mesodermal differentiation.11 Remodeling of the mitochondrial infrastructure matches the evolving bioenergetic demands, with contractile function driving the requirement for efficient oxidative ATP generation in the developing heart.2, 7, 12
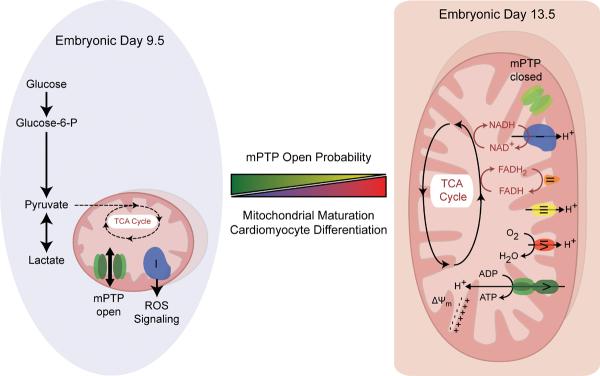
Hom et al. now demonstrate that modulators of mPTP closure promote mitochondrial maturation and cardiomyocyte differentiation in the embryo (Figure).1 A non-selective conduit with a molecular cut-off of 1.5 kDa, mPTP resides within the inner mitochondrial membrane.13 Transitional pore opening promotes mitochondrial permeability, dissipating ion and metabolite gradients and uncoupling oxidative metabolism from ATP generation.13 Excessive mitochondrial swelling triggers release of pro-apoptotic proteins, including cytochrome c, precipitating cell death via necrosis or apoptosis during extreme stress, such as ischemia/reperfusion injury.13 A physiological role for the mPTP opening-closing dynamics has however remained unclear as knockout of an essential pore component (cyclophilin-D) produces apparently healthy offspring,14, 15 albeit with abnormal cardiac response to stress.16 In wild-type embryos, developing e9.5 cardiogenic fields display lower mitochondrial membrane potential and greater reactive oxygen species (ROS) generation compared with older e13.5 cardiomyocytes, consistent with early mPTP involvement.1 Genetic or pharmacologic closure of the mPTP at e9.5 induces structural and functional mitochondrial maturation and cardiomyocyte differentiation, while increased mPTP open probability would impair development.1 Consistent with the proposed role of mPTP in heart development is an increased level of mitofusin-2, a dynamin-like protein involved in the rearrangement of mitochondrial membranes and permeability transition during stem cell cardiac differentiation.2, 17 Through influences on vital downstream processes, including ROS production, energy metabolism and calcium signaling, mPTP behavior could profoundly impact cardiac differentiation.
Figure.
Mitochondrial structure and energy metabolism supports the differentiation potential of pluripotent stem cells and early cardiac progenitors. Frequent opening of the mPTP maintains immature mitochondrial morphology and low oxidative capacity, requiring a high glycolytic capacity in early embryonic cardiomyocytes. Subsequent differentiation requires mPTP closure supporting the maturation of mitochondrial oxidative metabolism and reducing the reliance on glycolysis. ADP: adenosine diphosphate, ATP: adenosine triphosphate, FADH: flavin adenine dinucleotide, FADH2: reduced flavin adenine dinucleotide,NAD+: nicotinamide adenine dinucleotide, NADH: reduced nicotinamide adenine dinucleotide, NADP: nicotinamide adenine dinucleotide phosphate, NADPH: reduced nicotinamide adenine dinucleotide phosphate, TCA: tricarboxylic acid, Δψm: mitochondrial membrane potential.
Immature cardiomyocytes exhibit underdeveloped electron transport chain and elevated ROS load, reduced with natural or stimulated mPTP closure promoting differentiation.1 Mitochondrial ROS is positively correlated with mitochondrial membrane potential; yet permeability transition via mPTP can further promote ROS production by depleting mitochondrial antioxidants and impairing physiological electron transfer due to loss of electron transport chain components and conformational rearrangement of complex I.18, 19 Antioxidant-induced reduction of ROS at e9.5 promotes cardiomyocyte differentiation, while addition of stable oxidants impairs differentiation.1 Indeed, low levels of ROS have been implicated in stimulating expression of cardiac genes and transcription factors, and promoting stem cell cardiac differentiation.20–22 Such cardiogenic effects appear to be concentration-dependent, as high levels of ROS can delay cardiac differentiation.23, 24 The work of Hom et al. indicates that the redox status might control cardiogenesis in a temporal fashion, with early commitment of cardiac progenitors occurring in a highly oxidized environment, while subsequent cardiomyocyte differentiation proceeding under reduced ROS load.1, 25 The intimate effects of permeability transition on mitochondrial function and downstream pathways involved in differentiation, including ROS as well as associated energy metabolism and calcium signaling, would require further examination.
Transient mPTP openings may control mitochondrial ionic status to match oxidative metabolism with myocardial workload. In fact, mitochondria-dependent energetic circuits are critical regulators of de novo cardiogenesis.2 Transient mPTP opening directly regulates cellular energy metabolism as it uncouples oxidative metabolism from ATP synthesis, a mechanism that operates in concert with ROS flashes to promote cardiomyocyte differentiation.12, 13 Knockout of the mPTP component cyclophilin D results in elevated mitochondrial matrix calcium, which enhances the activation of Ca2+-dependent dehydrogenases reducing metabolic flexibility.16 The early embryonic heart is primarily dependent upon anaerobic glycolysis for ATP generation, as a potential consequence of low substrate supply and oxygen availability.3, 26 With growing oxygen supply following the e10 stage, oxidation of substrates, in particular lactate, increases as the heart requires more oxygen to maintain contraction.27, 28 Hom et al. demonstrate a reliance on complex II for electron entry at day e9.5 with increasing importance of complex I at e13.5, supporting bioenergetic remodeling during differentiation.1 Much of the evidence for metabolic remodeling during cardiogenesis is derived from in vitro differentiation of stem cells.2, 4, 5, 7–9 Like the early embryonic heart, pluripotent stem cells rely on glycolytic metabolism, with increased oxygen consumption and cellular respiration concomitant to the upregulation of tricarboxylic acid cycle and electron transport chain components associated with mitochondrial maturation during differentiation.2, 5–7, 12, 29, 30 Disruption of mitochondrial respiration impairs the ability of pluripotent stem cells to differentiate into cardiomyocytes and maintain stemness.2, 31 A growing body of literature has implicated a deterministic role for energy metabolism in driving cellular fate.6, 12, 32 Indeed, the differentiation potential of cardiac progenitors into cardiomyocytes relies upon mitochondrial content and the capacity for oxidative metabolism.33 The necessary shift from glycolytic to oxidative metabolism during differentiation of pluripotent stem cells is dependent upon the regulation of mitochondrial substrate entry, including by downregulation of uncoupling protein 2 and changes in hexokinase isoforms.6, 34–36 The reverse of this process, dedifferentiation of somatic cells back to the pluripotent state also requires metabolic remodeling, which precedes the expression of pluripotency markers.6, 12 Thus, beyond matching bioenergetic supply and demand, the regulation of energy metabolism is central to fueling specification of cell fate.6, 12, 32–36
The interconnectivity of mPTP, mitochondria maturation and embryonic development has significant implications for the understanding of normal cardiac differentiation, and heart vulnerability to injury. Opening of the mPTP is largely associated with dissipation of mitochondrial membrane potential, release of pro-apoptotic stimuli and induction of cell death, associated with pathologic conditions.37 Inhibition of pore opening using a variety of techniques, including pre-and post-conditioning, promotes cardioprotection.13, 37 Therefore, future studies to identify mechanisms regulating mPTP behavior during embryogenesis may provide novel targets against cardiac injury. In addition, embryonic hearts tolerate transient mPTP openings, suggesting the presence of pro-survival pathways early in development, offering potential avenues for targeted protection. In contrast, sustained mPTP opening and the associated impairment in mitochondrial and cardiomyocyte maturation may impede the developing heart from matching the energetic demands of the mature embryo and could ultimately lead to congenital defects or embryonic lethality. Beyond disease pathogenesis, regulation of mitochondrial maturation and myocyte differentiation by permeability transition has the potential to impact lineage specification. Indeed, targeting master regulators of cell fate plasticity offers an innovative technology for purposes of tissue regeneration.38 Case in point, cyclosporine A, a mPTP inhibitor, can augment the in vitro production of cardiac progenitor cells capable of integrating into the infarcted heart.39 Moreover, the mPTP associated peripheral benzodiazepine receptor (PBR) has been implicated in cell proliferation and differentiation with PBR ligands affecting stem cell fate.40 Manipulation of mPTP and its downstream signaling pathways may thus be considered to promote differentiation of resident cardiac stem cells for facilitated heart repair. Deciphering mechanisms underlying the role for mPTP and mitochondrial signaling in heart development offers implications for cardiac embryology and pathology, and more broadly may refine stem cell specification for regenerative applications.
Acknowledgments
Sources of Funding Authors are supported by National Institutes of Health, Canadian Institutes of Health Research, Marriott Foundation, and Mayo Clinic.
Footnotes
Disclosures None.
References
- 1.Hom JR, Quintanilla RA, Hoffman DL, de Mesy Bentley KL, Molkentin JD, Sheu SS, Porter GA., Jr. The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev. Cell. 2011;21:469–478. doi: 10.1016/j.devcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 2007;4(Suppl 1):S60–67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter GA, Jr., Hom J, Hoffman D, Quintanilla R, de Mesy Bentley K, Sheu SS. Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog Pediatr Cardiol. 2011;31:75–81. doi: 10.1016/j.ppedcard.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St John JC, Ramalho-Santos J, Gray HL, Petrosko P, Rawe VY, Navara CS, Simerly CR, Schatten GP. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells. 2005;7:141–153. doi: 10.1089/clo.2005.7.141. [DOI] [PubMed] [Google Scholar]
- 5.Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, St John JC. Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J. Cell Sci. 2007;120:4025–4034. doi: 10.1242/jcs.016972. [DOI] [PubMed] [Google Scholar]
- 6.Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung S, Arrell DK, Faustino RS, Terzic A, Dzeja PP. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J. Mol. Cell. Cardiol. 2010;48:725–734. doi: 10.1016/j.yjmcc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung S, Dzeja PP, Faustino RS, Terzic A. Developmental restructuring of the creatine kinase system integrates mitochondrial energetics with stem cell cardiogenesis. Ann. N. Y. Acad. Sci. 2008;1147:254–263. doi: 10.1196/annals.1427.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dzeja PP, Chung S, Faustino RS, Behfar A, Terzic A. Developmental enhancement of adenylate kinase-ampk metabolic signaling axis supports stem cell cardiac differentiation. PLoS One. 2011;6:e19300. doi: 10.1371/journal.pone.0019300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonergan T, Brenner C, Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J. Cell. Physiol. 2006;208:149–153. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- 11.Schieke SM, Ma M, Cao L, McCoy JP, Jr., Liu C, Hensel NF, Barrett AJ, Boehm M, Finkel T. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J. Biol. Chem. 2008;283:28506–28512. doi: 10.1074/jbc.M802763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folmes CD, Nelson TJ, Terzic A. Energy metabolism in nuclear reprogramming. Biomark Med. 2011;5:715–729. doi: 10.2217/bmm.11.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim. Biophys. Acta. 2009;1787:1402–1415. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin d-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 15.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin d reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 16.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD. Cyclophilin d controls mitochondrial pore-dependent ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol. Cell. Biol. 31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luetjens CM, Bui NT, Sengpiel B, Munstermann G, Poppe M, Krohn AJ, Bauerbach E, Krieglstein J, Prehn JH. Delayed mitochondrial dysfunction in excitotoxic neuron death: Cytochrome c release and a secondary increase in superoxide production. J. Neurosci. 2000;20:5715–5723. doi: 10.1523/JNEUROSCI.20-15-05715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puceat M. Role of rac-gtpase and reactive oxygen species in cardiac differentiation of stem cells. Antioxid. Redox. Signal. 2005;7:1435–1439. doi: 10.1089/ars.2005.7.1435. [DOI] [PubMed] [Google Scholar]
- 21.Sauer H, Rahimi G, Hescheler J, Wartenberg M. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. J. Cell. Biochem. 1999;75:710–723. doi: 10.1002/(sici)1097-4644(19991215)75:4<710::aid-jcb16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Buggisch M, Ateghang B, Ruhe C, Strobel C, Lange S, Wartenberg M, Sauer H. Stimulation of es-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and nadph oxidase. J. Cell Sci. 2007;120:885–894. doi: 10.1242/jcs.03386. [DOI] [PubMed] [Google Scholar]
- 23.Puceat M, Travo P, Quinn MT, Fort P. A dual role of the gtpase rac in cardiac differentiation of stem cells. Mol. Biol. Cell. 2003;14:2781–2792. doi: 10.1091/mbc.E02-09-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Na L, Wartenberg M, Nau H, Hescheler J, Sauer H. Anticonvulsant valproic acid inhibits cardiomyocyte differentiation of embryonic stem cells by increasing intracellular levels of reactive oxygen species. Birt. Defects Res. A. Clin. Mol. Teratol. 2003;67:174–180. doi: 10.1002/bdra.10030. [DOI] [PubMed] [Google Scholar]
- 25.Drenckhahn JD. Heart development: Mitochondria in command of cardiomyocyte differentiation. Dev. Cell. 2011;21:392–393. doi: 10.1016/j.devcel.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 2010;56:130–140. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 27.Cox SJ, Gunberg DL. Metabolite utilization by isolated embryonic rat hearts in vitro. J. Embryol. Exp. Morphol. 1972;28:235–245. [PubMed] [Google Scholar]
- 28.Fisher DJ, Heymann MA, Rudolph AM. Myocardial oxygen and carbohydrate consumption in fetal lambs in utero and in adult sheep. Am. J. Physiol. 1980;238:H399–405. doi: 10.1152/ajpheart.1980.238.3.H399. [DOI] [PubMed] [Google Scholar]
- 29.Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, Martinez D, Carnero A, Beach D. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–185. [PubMed] [Google Scholar]
- 30.Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, Bernard D, Gil J, Beach D. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid. Redox. Signal. 2007;9:293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- 31.Varum S, Momcilovic O, Castro C, Ben-Yehudah A, Ramalho-Santos J, Navara CS. Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Stem Cell Res. 2009;3:142–156. doi: 10.1016/j.scr.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Science breakthrough of the year, areas to watch. [Assessed January 9, 2012]. www.sciencemag.org/content/334/6063/1630.full#named-content-3. [Google Scholar]
- 33.San Martin N, Cervera AM, Cordova C, Covarello D, McCreath KJ, Galvez BG. Mitochondria determine the differentiation potential of cardiac mesoangioblasts. Stem Cells. 2011;29:1064–1074. doi: 10.1002/stem.654. [DOI] [PubMed] [Google Scholar]
- 34.Panopoulos AD, Izpisua Belmonte JC. Anaerobicizing into pluripotency. Cell Metab. 2011;14:143–144. doi: 10.1016/j.cmet.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, Wahjudi PN, Setoguchi K, Wang G, Do A, Jung HJ, McCaffery JM, Kurland IJ, Reue K, Lee WN, Koehler CM, Teitell MA. Ucp2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30:4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panopoulos AD, Yanes O, Ruiz S, Kida YS, Diep D, Tautenhahn R, Herrerias A, Batchelder EM, Plongthongkum N, Lutz M, Berggren WT, Zhang K, Evans RM, Siuzdak G, Belmonte JC. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2011 doi: 10.1038/cr.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dzeja PP, Holmuhamedov EL, Ozcan C, Pucar D, Jahangir A, Terzic A. Mitochondria: Gateway for cytoprotection. Circ. Res. 2001;89:744–746. [PubMed] [Google Scholar]
- 38.Terzic A, Folmes CD. Martinez-Fernandez A, Behfar A. Regenerative medicine: On the vanguard of health care. Mayo Clin. Proc. 2011;86:600–602. doi: 10.4065/mcp.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan P, Nagasawa A, Uosaki H, Sugimoto A, Yamamizu K, Teranishi M, Matsuda H, Matsuoka S, Ikeda T, Komeda M, Sakata R, Yamashita JK. Cyclosporin-a potently induces highly cardiogenic progenitors from embryonic stem cells. Biochem. Biophys. Res. Commun. 2009;379:115–120. doi: 10.1016/j.bbrc.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Lee DH, Kang SK, Lee RH, Ryu JM, Park HY, Choi HS, Bae YC, Suh KT, Kim YK, Jung JS. Effects of peripheral benzodiazepine receptor ligands on proliferation and differentiation of human mesenchymal stem cells. J. Cell. Physiol. 2004;198:91–99. doi: 10.1002/jcp.10391. [DOI] [PubMed] [Google Scholar]