Abstract
Objective
The aim of this study was to compare dual-energy computed tomography (DECT) and magnetic resonance imaging (MRI) for fat quantification using tissue triglyceride concentration and histology as references in an animal model of hepatic steatosis.
Materials and Methods
This animal study was approved by our institution's Research Animal Resource Center. After validation of DECT and MRI using a phantom consisting of different triglyceride concentrations, a leptin-deficient obese mouse model (ob/ob) was used for this study. Twenty mice were divided into 3 groups based on expected levels of hepatic steatosis: low (n = 6), medium (n = 7), and high (n = 7) fat. After MRI at 3 T, a DECT scan was immediately performed. The caudate lobe of the liver was harvested and analyzed for triglyceride concentration using a colorimetric assay. The left lateral lobe was also extracted for histology. Magnetic resonance imaging fat-fraction (FF) and DECT measurements (attenuation, fat density, and effective atomic number) were compared with triglycerides and histology.
Results
Phantom results demonstrated excellent correlation between triglyceride content and each of the MRI and DECT measurements (r2 ≥ 0.96, P ≤ 0.003). In vivo, however, excellent triglyceride correlation was observed only with attenuation (r2 = 0.89, P < 0.001) and MRI-FF (r2 = 0.92, P < 0.001). Strong correlation existed between attenuation and MRI-FF (r2 = 0.86, P < 0.001). Nonlinear correlation with histology was also excellent for attenuation and MRI-FF.
Conclusions
Dual-energy computed tomography (CT) data generated by the current Gemstone Spectral Imaging analysis tool do not improve the accuracy of fat quantification in the liver beyond what CT attenuation can already provide. Furthermore, MRI may provide an excellent reference standard for liver fat quantification when validating new CT or DECT methods in human subjects.
Keywords: dual-energy CT, hepatic steatosis, chemical shift, magnetic resonance imaging, proton density fat-fraction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease of both adults and children in the United States. The prevalence of hepatic steatosis likely exceeds 20% of the US population and is increasing, paralleling the current epidemic of obesity and its association with metabolic syndrome and diabetes.1,2 Early detection and treatment may halt or reverse progression to liver injury, inflammation, fibrosis, and ultimately, cirrhosis, which has dreaded complications including liver failure, portal hypertension, and hepatocellular carcinoma.3 Liver biopsy, the current clinical gold standard to assess liver fat and diagnose NAFLD, is not conducive to the repeated measurements necessary to monitor liver disease and/or treatment because of the risk of bleeding, expense, and most importantly, poor sampling variability.4,5 Hence, a valid noninvasive biomarker of liver fat content is needed to advance NAFLD research and improve patient care.
In recent years, there has been extensive development and validation of noninvasive magnetic resonance imaging (MRI) methods that provide quantitative fat-fraction (FF) measurements. These newer chemical shift–based methods6–8 have advanced upon traditional Dixon techniques9,10 and also account for confounding factors such as T1-related bias, noise bias, T2* correction, accurate spectral modeling, as well as compensation for the effects of eddy currents, all of which can corrupt accurate FF measurements. Such methods are well validated in phantoms, animal models, and patients.11–15 Quantitative MRI methods are very promising, and multiple vendors have implemented prototypes, some of which are expected to become commercially available products.
Although single-energy computed tomography (CT) has demonstrated sensitivity to fat,16,17 only the net attenuation of all materials within a voxel can be measured. In some cases, when materials such as iron and glycogen are present in the same voxel,18,19 the sensitivity to fat content is reduced. The recent advent of multidetector dual-energy CT (DECT) has led to interesting and important opportunities for improved tissue characterization through the use of different attenuation characteristics of materials at different x-ray energies. These techniques have shown great promise in distinguishing the attenuation of substances such as iodine, calcium, and uric acid crystals from soft tissues.20 Differentiation of water and triglycerides (TGs) also offers opportunities to quantify tissue fat concentration using DECT. Early experience is limited, but results from phantoms, animals, and patients appear promising.21–23
Although quantitative MRI techniques would be preferable for detecting and quantifying hepatic steatosis using a noninvasive technique, CT plays a central role in the imaging of abdominal pathology. Given the high prevalence of fatty liver disease and the widespread use of CT in abdominal imaging, DECT may provide an excellent opportunity to screen for hepatic steatosis in patients undergoing scans for unrelated reasons. Therefore, understanding the ability of DECT to quantify fat within the abdomen is of great interest. The main purpose of this work was to evaluate the ability of DECT to quantify liver fat concentration using tissue TG concentration and histological evaluation as references in an animal model of fatty liver disease. A secondary goal of this work was to compare the performance of DECTwith previously validated advanced quantitative MRI techniques.
MATERIALS AND METHODS
Water/Fat Phantom
A phantom correlation experiment was performed using DECT and MRI. Five gel phantoms with different TG contents (0%, 10%, 20%, 30%, and 50% volume) were constructed using water, peanut oil, agar, sodium dodecyl sulfate, sodium azide, and sodium chloride, according to the method described by Hines et al.24
Mouse Model of Fatty Liver
All animal studies were approved by our institution's Research Animal Resource Center. The ob/ob mouse was used for this study. The ob/ob mouse is a well-established model of obesity, metabolic syndrome, and fatty liver disease.25 Ob/ob mice are leptin deficient, leading to hyperphagia and subsequent obesity, diabetes, and hepatic steatosis that progresses with age.26 This model has proven to be excellent for hepatic fat quantification in MRI validation studies.11,27
Twenty male mice fed standard chow ad libitum were used for this study, which has 14% of calories from fat (diet 8604; Harlan Teklad, Madison, WI). Mice were divided into 3 groups to create different levels of steatosis: low, medium, and high fat. Low-fat mice (n = 6) were wild type (C57BL6/J; Harlan Sprague Dawley, Madison, WI) and served as controls, as wild type mice do not develop hepatic steatosis when fed standard chow. Medium-fat mice (n = 7) were 4-week-old ob/ob mice, and high-fat mice (n = 7) were 8-week-old ob/ob mice.
Prior to MRI, mice were sedated with an intraperitoneal injection of 40 mg/kg sodium pentobarbital (Nembutal; Ovation Pharmaceuticals, Deerfield, IL). After MRI, mice were euthanized with 100 mg/kg of sodium pentobarbital and immediately transferred to our DECT scanner (Discovery CT750 HD; GE Healthcare, Waukesha, WI), which is directly adjacent to the MRI scanner (MR750 v22.0; GE Healthcare). Immediately after DECT, tissue was perfused with saline, the caudate lobe was harvested and frozen at −80°C, and the left lateral lobe was placed in formalin for histology grading.
TG Quantification
The frozen samples of the caudate lobe were thawed on-site and processed at AniLytics, Inc (Gaithersburg, MD) for quantification of TGs using a colorimetric assay. Triglyceride mass-fraction results are expressed as a percentage, where the ratio of the mass of extracted TGs to the mass of the tissue subject for analysis was multiplied by 100.
Histology Analysis
The left lateral lobe was submitted for hematoxylin and eosin and trichrome staining and analysis. According to the scoring system used by Kleiner et al,28 a surgical pathologist with expertise in liver disease graded the slides for ballooning degeneration, steatosis, inflammation, and fibrosis, where the percentage of cells containing fat vacuoles was evaluated and also separated by grade: grade 0, less than 5%; grade 1, 5% to 33%; grade 2, 33% to 66%; and grade 3, greater than 66%. The total percentage of cells affected by steatosis was further separated into cells affected by microvesicular and macro-vesicular steatosis.
Dual-Energy CT
A GE Discovery CT750 HD (GE Healthcare) scanner was operated in Gemstone Spectral Imaging 21 mode (GE's trademarked commercial version of DECT) to perform a dual-energy scan using fast kilovoltage peak (kVp) switching (80/140 kVp) and the following parameters: 630 mA, 0.5-second gantry rotation time, small head scan field of view (FOV), 32 contiguous 0.6-mm slices (20 mm total coverage), and a volume CT dose index (CTDIvol) of 38.42 mGy. The same DECT acquisition was performed on both the phantom and the mice. Note that the parameters chosen are acceptable under clinical protocols. Because this CT system is not quantum limited, CTDIvol remains constant regardless of the imaged object. Therefore, the radiation doses listed above would be similar for a human. The voxel size and, hence, the signal-to-noise ratio, would be similar for a human as well. To compare attenuation measurements in the mice, a single-energy CT scan was also performed in high-resolution mode with only the following differences in parameters: 100 kVp, 100 mA, and a CTDIvol of 6.53 mGy.
Gemstone Spectral Imaging was used to reconstruct and analyze DECT images to generate synthesized monochromatic attenuation images at 65 keV (Hounsfield units [HU]), water and fat density images (mg/mL), and effective atomic number (Zeff) images on a 512 × 512 matrix with a display FOV of 6 cm.
Magnetic Resonance Imaging
Magnetic resonance imaging was performed at 3 T (MR750 v22.0' GE Healthcare) using a complex chemical shift–based multiecho, 3-dimensional, spoiled gradient-echo MRI method based on IDEAL (iterative decomposition of water and fat with echo asymmetry and least squares estimation)29 using a commercial 8-channel wrist coil. Standard imaging gradients (slew rate, 200 T m−1 s−1; maximum strength, 50 mT/m) were used for all imaging.
All 5 phantoms with different fat concentrations were imaged simultaneously with MRI using the following parameters: minimum echo time, 2.2 milliseconds; repetition time (TR), 300 milliseconds; flip angle, 3 degrees, to minimize T1 bias6; bandwidth, +100 kHz; 1 signal average; 256 × 128 matrix; FOV, 16 × 8 cm; 12 axial slices (4 mm thick) for a total scan time of 23 minutes; and true spatial resolution, 0.6 × 0.6 × 4 mm3. A total of 15 echoes were acquired with 1.2-millisecond echo spacing by using 3 interleaved echo trains with 5 echoes per TR. Previous work has demonstrated that 6 echo and 15 echo fat measurements yield identical results.11 Note that simply adding gadolinium to the phantoms would have better matched the T1 of liver tissue and allowed a much shorter magnetic resonance scan time, but gadolinium has a large atomic number, which would have confounded DECT measurements.
Mice imaging was performed as above with minor changes in the following parameters: TR, 24.4 milliseconds; flip angle, 5 degrees; 256 × 154 matrix; FOV, 16 × 9.6 cm; 28 coronal slices (0.8 mm thick) for a total scan time of 6 minutes; and true spatial resolution, 0.6 × 0.6 × 0.8 mm3.
Separated but coregistered fat and water images are generated through IDEAL reconstruction.13,29,30 When corrections for confounding factors have been performed, the FF represents the proton density FF, which is equivalent to the ratio of the unconfounded signal from all MRI-visible protons of fat to the sum of the unconfounded signal from all MRI-visible protons of fat and water.
Proton density FF maps were generated by accounting for all known confounding factors, such as the complex spectrum of fat,31,32 T2* decay,8,31 noise bias,6 T1-related bias,6,31 and eddy currents that can corrupt the ability of MRI to quantify fat. These correction methods have been addressed and previously validated in phantoms,24 animals,11,27 and human studies.13,33 The liver fat spectrum described by Hamilton et al34 was used for both the phantom and the mice because peanut oil and adipose tissue have similar spectrums.32 For brevity and clarity, we will refer to proton density FF as MRI-FF in this work.
Data Analysis
For the MRI-FF and DECT phantom images, a region of interest (ROI) was placed in each of the fat phantoms and the mean value was recorded. The ROI was chosen on the attenuation image to align subjectively with the MRI ROI and was then copied onto the same location within the fat density and Zeff images. For the mice data, an ROI was placed on the coronal MRI-FF maps and the coronal-reformatted DECT attenuation image (0.8 mm thick) in both the caudate and left lateral liver lobes after visually colocalizing the images based on anatomy. As described for the phantom, the ROI was then copied to the fat density and Zeff images. Lastly, axial attenuation images from single-energy CT and DECT were compared by using coregistered ROIs placed in the left lateral liver lobe for each modality. The size of each ROI was chosen to include as much of the lobe as possible while avoiding the blood vessels.
Statistical Analysis
Phantom data were analyzed using linear regression performed separately for each magnetic resonance (MRI-FF) and DECT (attenuation, fat density, and Zeff) imaging attribute against the known fat percentage. The slope and intercept with 95% confidence intervals (CIs) are reported. For MRI-FF, where excellent agreement is expected in a phantom,35 2-sided t tests at the 0.05 significance level were used to determine whether the estimated slope and intercept were significantly different from 1.0 and 0.0, respectively.
In vivo mouse data were analyzed using simple linear regression analysis to obtain correlation coefficients relating the following measurements in the caudate lobe of the liver:
DECT attenuation versus TG
MRI-FF versus TG
DECT attenuation versus MRI-FF
Fat density versus TG
Fat density versus MRI-FF
Zeff versus TG
Zeff versus MRI-FF
where TG represents the TG FF measurements. Simple regression was performed because a multivariate analysis including various features of histology (ballooning, inflammation, and fibrosis) yielded no dependence. Simple regression was also used to compare single-energy attenuation and DECT attenuation values, and 2-sided t tests were performed for the slope and intercept as described above. Nonlinear regressions were performed using Box-Tidwell transformations of the predictor for DECT attenuation and MRI-FF compared with total percentage of cells affected by steatosis as graded by the pathologist in the left lateral lobe. Because zero values were present in the data, creating problems for Box-Tidwell transformations that require logarithm analysis, all measurements (attenuation, MRI-FF, and steatosis) were increased by a value of 1. Statistical computations and graphics were obtained in R 2.12.1 (R Development Core Team, 2009).
RESULTS
Figure 1 shows images from the water-fat phantoms, qualitatively comparing MRI and DECT. Excellent correlation between TG content and each of the image measurements (MRI-FF, DECT attenuation, fat density, and Zeff) was found, with r2 values of 0.96 (P ≤ 0.003, Table 1) or better in each case. In the case of MRI-FF versus known TG, perfect agreement was observed, as the slope and intercept were not statistically different from 1 and 0, respectively.
FIGURE 1.
MRI-FF map and DECT images of a phantom consisting of glass vials with known volume concentration of fat (0%, 10%, 20%, 30%, and 50%). Excellent correlation in each case can be observed both qualitatively and quantitatively (Table 1).
TABLE 1.
Phantom Results Comparing DECT and MRI Measurements With Known TG Volume Fraction
| Y vs X | r 2 | P * | Slope (95% CI) | Intercept (95% CI) |
|---|---|---|---|---|
| HU vs TG | 0.999 | <0.001 | −1.48 (−1.50 to −1.45) | 10.7 (9.9 to 11.5) |
| Fat density vs TG | 0.980 | 0.001 | 18.9 (15.7 to 22.0) | 112 (25 to 199) |
| Zeff vs TG | 0.96 | 0.003 | −0.023 (−0.029 to −0.018) | 7.5 (7.3 to 7.6) |
| MRI-FF vs TG | 0.995 | <0.001 | 1.085 (0.997 to 1.172)† | −0.34 (−2.65 to 2.21)‡ |
P < 0.05 indicates statistical significance.
P > 0.05 indicating no significant difference between obtained slope and 1.0.
P > 0.05 indicating no significant difference between obtained intercept and 0.0.
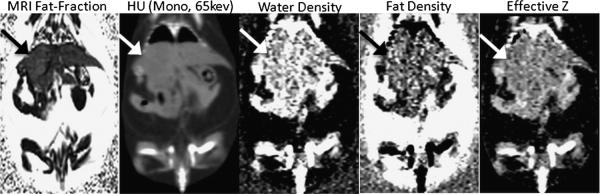
Example in vivo images are in Figure 2 from an 8-week-old ob/ob mouse. Qualitatively, coronal MRI-FF maps and coronally reformatted DECT maps (monochromatic attenuation, water density, fat density, and Zeff) demonstrated good to excellent image quality. Note, however, that the lungs are not distinguishable from the liver in the water density, fat density, or Zeff images using the dual-material (fat, water) decomposition method.
FIGURE 2.
Representative MRI-FF map and DECT images of an ob/ob mouse in the coronal plane. The arrow denotes the liver.
Figure 3 displays a composite set of MRI-FF and DECT maps, with corresponding histological slides of the liver in a representative mouse from each of the 3 groups: low-, medium-, and high-fat mice. One can readily observe that MRI-FF and DECT fat density signal values increased with increasing steatosis, whereas both DECT attenuation and Zeff signal demonstrated an inverse relationship, showing decreasing signal with increasing fat content. The expected trend is also visually evident in the hematoxylin and eosin images, which show an increasing number of cells containing vacuoles of TGs (0%, 13%, and 65% for low, medium, and high, respectively).
FIGURE 3.
Representative cropped coronal MRI-FF and DECT images with corresponding histological slides for a representative mouse from each of 3 groups: low, medium, and high fat.
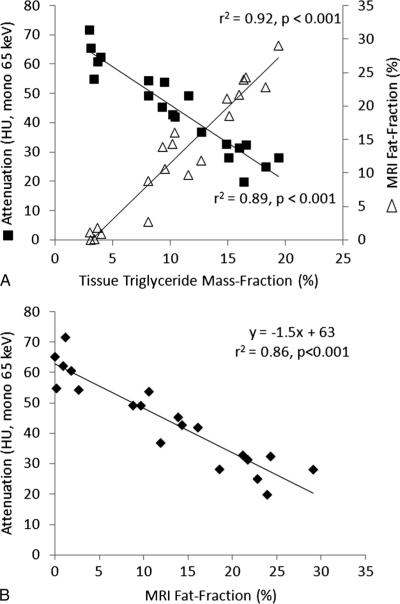
Excellent correlation with tissue TG concentration was observed from the mean ROI measurements for both MRI-FF (r2 = 0.92, P < 0.001) and DECT attenuation (r2 = 0.89, P < 0.001) (Fig. 4A). Therefore, linear regression for DECT attenuation versus MRI-FF also yielded a high correlation (r2 = 0.86, P < 0.001; Fig. 4B and Table 2) with a slope of −1.5 (95% CI, −1.7 to −1.2) HU/% and an intercept of 63 (95% CI, 59–67) HU. When comparing DECT attenuation and single-energy attenuation values, excellent correlation (r2 = 0.88, P < 0.001) and strong agreement were observed, with a slope and intercept not statistically different from 1 and 0, respectively (Fig. 5). Although correlation was excellent, in vivo results for MRI-FF versus TG yielded a slope and intercept that were statistically different from 1 and 0, respectively, although this was to be expected because they measure related but different parameters in the invivo setting. Invivo fat density and Zeff measurements demonstrated good, but inferior, correlation (r2 ≤ 0.67, P < 0.001) with both MRI and tissue TG concentration (Table 2).
FIGURE 4.
DECT attenuation and MRI-FF versus tissue TG (A) and DECT attenuation versus MRI-FF (B) for all mice. Excellent correlation was observed in each case (r2 ≥ 0.86, P < 0.001). A relationship of y = −1.5× + 63 was observed between attenuation and MRI-FF.
TABLE 2.
In Vivo Results Comparing DECT and MRI Measurements With TG Mass Fraction
| Y vs X | r 2 | P * | Slope (95% CI) | Intercept (95% CI) |
|---|---|---|---|---|
| HU vs TG | 0.89 | <0.001 | −2.6 (−3.0 to −2.2) | 72 (67 to 77) |
| MRI-FF vs TG | 0.92 | <0.001 | 1.7 (1.4 to 1.9) | −5.2 (−7.9 to −2.5) |
| HU vs MRI-FF | 0.86 | <0.001 | −1.5 (−1.7 to −1.2) | 63 (59 to 67) |
| Fat density vs TG | 0.57 | <0.001 | 30 (18 to 43) | −78 (−225 to 70) |
| Fat density vs MRI-FF | 0.66 | <0.001 | 19 (12 to 25) | 7.9 (−90.7 to 106.4) |
| Zeff VS TG | 0.57 | <0.001 | −0.03 (−0.05 to −0.02) | 7.7 (7.5 to 7.9) |
| Zeff vs MRI-FF | 0.67 | <0.001 | −0.02 (−0.03 to −0.01) | 7.6 (7.5 to 7.7) |
P < 0.05 indicates statistical significance.
FIGURE 5.

DECT attenuation versus single-energy CT attenuation for all mice. Linear regression yielded excellent correlation (r2 = 0.88, P < 0.001) and agreement with a slope of 0.92 (95% CI, 0.76–1.08) and an intercept of 1.7 (−6.3 to 9.6). There was no significant difference between the measured slope and 1.0 (P = 0.33) and no significant difference between the measured intercept and 0.0 (P = 0.68).
Histology results across all mice livers are summarized in Table 3. Total steatosis measurements ranged from 0% to 65% of cells affected, and ballooning from 0 to 2. Inflammation and fibrosis were not observed in any of the mouse livers. Figure 6 shows the nonlinear fit and correlation for both DECT attenuation versus histology (y + 1 = 70.8(x + 1)−0.23; r2 ≤ 0.88, P < 0.001) and MRI-FF versus histology (y + 1 = 1.48(x + 1)0.68; r2 ≤ 0.95, P < 0.001).
TABLE 3.
Histology Results From the Left Lobe of the Liver for Each Mouse in The Study
| Steatosis* |
|||||
|---|---|---|---|---|---|
| Group | Mouse | Total | Macro | Micro | Ballooning |
| Control | 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | 0 | |
| Medium fat | 7 | 12 | 0 | 12 | 1 |
| 8 | 13 | 3 | 10 | 1 | |
| 9 | 14 | 1 | 13 | 1 | |
| 10 | 15 | 1 | 14 | 1 | |
| 11 | 16 | 8 | 8 | 1 | |
| 12 | 18 | 1 | 17 | 0 | |
| 13 | 23 | 3 | 20 | 1 | |
| High fat | 14 | 36 | 1 | 35 | 1 |
| 15 | 45 | 5 | 40 | 1 | |
| 16 | 55 | 1 | 54 | 1 | |
| 17 | 60 | 25 | 35 | 0 | |
| 18 | 65 | 1 | 64 | 1 | |
| 19 | 65 | 50 | 15 | 1 | |
| 20 | 68 | 8 | 60 | 2 | |
Histology was performed according to the scoring system used by Kleiner et al.28 Inflammation and fibrosis were not observed in any of the mouse livers.
The total percentage of cells containing fat vacuoles were further separated into cells affected by microvesicular and macrovesicular steatosis.
FIGURE 6.
DECT attenuation versus histology (A) and MRI-FF versus histology (B) across all mice. Nonlinear regression yielded excellent correlation for attenuation (r2 = 0.88, P < 0.001) and MRI-FF (r2 = 0.95, P < 0.001).
DISCUSSION
In this study, we have shown that all DECT and MRI measurements correlated extremely well with TG content in a phantom, but only the monochromatic attenuation (HU) and MRI-FF measurements demonstrated excellent correlation in vivo. Monochromatic attenuation and MRI-FF also correlated very well with histological grading of steatosis. Given the strong correlation and agreement between DECT and single-energy attenuation, these results indicate that CT attenuation is superior at quantifying TG content than DECT fat density and Zeff measurements. This commercial implementation of DECT, therefore, does not enable quantification of liver fat in vivo beyond what conventional CT can provide. In addition, this study also demonstrates that previously validated quantitative MRI methods may serve as an excellent reference standard for liver fat quantification in future CT validation studies.
This work also demonstrates what has been long established: CT is sensitive to the presence of fat in tissue.16,17 It does, for the first time, however, provide the first, direct in vivo comparison of DECT, MRI-FF, TG concentration, and histology. Although we do not recommend the primary use of DECT or conventional CT for dedicated quantification of hepatic steatosis, there are many clinical circumstances in which patients undergo a clinical CT examination, offering the opportunity to extract additional important information on the presence and concentration of liver fat. Computed tomography, as well as, increasingly, DECT, plays a central role in the evaluation of abdominal pathology and increasingly for noncontrast techniques such as CT colonography. Given the high and increasing prevalence of fatty liver disease, measuring hepatic fat content from CT scans performed for other purposes could provide an excellent screening opportunity. Computed tomography may be advantageous as the primary means of fat quantification in small organs with complex geometry such as the pancreas and adrenal glands.
Although CT attenuation measurements may not be as sensitive as biopsy or MRI because of confounding factors discussed below,36 multiple studies have demonstrated that liver attenuation is an excellent predictor of liver fat content, with specificities approaching 100% for identifying moderate to severe hepatic steatosis using HU values between 40 and 48.37–39 When the radiologist encounters a low-attenuation liver, data from these previous studies and the current work can provide guidance on interpreting the results for the evaluation of fatty liver disease, which is gaining increasing recognition as a precirrhotic and precancerous condition.3
In contrast to phantom results, the in vivo MRI-FF versus TG results did not show perfect agreement, although this was fully expected. Triglyceride mass fraction is determined by dividing the chemically extracted lipids by the mass of the entire liver tissue specimen. Certain components present in the tissue specimen are invisible to proton MRI, such as bound or aggregated lipids (eg. lipid membranes and lipoproteins), because of their very short T2.40,41 Therefore, although proton density FFs measured with MRI represent a fundamental property of the tissue and reflect the concentration of TGs in the tissue, there is not a 1-to-1 correspondence to TG concentration measured by TG tissue extraction.
There are several limitations to this study, including the fact that the mouse model used, although highly representative of NAFLD, may not accurately represent liver disease in humans. For example, hepatic iron overload and certain drugs such as amiodarone and gold therapy are known to increase the attenuation of the liver,19,42−45 as does the presence of glycogen.18 In an ex vivo phantom, Fischer et al21 successfully used DECT to accurately quantify hepatic fat content in the presence of iron using an iron-specific dual-energy 3-material decomposition algorithm. Although ex vivo results may not be readily extended to the in vivo setting, as demonstrated in this study, the results are encouraging. Although the results of this work are also highly encouraging and indicate the ability of attenuation (HU) to accurately quantify fat in the liver, additional studies in patients, with tissue and/or quantitative MRI correlation, are clearly warranted.
In summary, in this work, we have explored the ability of DECT to quantify hepatic steatosis using a mouse model of NAFLD. Attenuation was superior to decomposed DECT fat density and Zeff images in vivo, demonstrating excellent correlation with tissue TG concentration and histological grading of steatosis. Furthermore, we have demonstrated excellent correlation of attenuation (HU) with a validated quantitative chemical shift–based MRI method, demonstrating the ability of MRI to serve as an excellent reference standard for future CT validation studies. These results indicate that this commercial version of DECT does not improve the accuracy of fat quantification in the liver beyond what CT attenuation can already provide.
ACKNOWLEDGMENTS
We gratefully acknowledge statistical assistance from Alejandro Munoz Del Rio, technical assistance from our CT technologists Sara Asuncion and Kari Pulfer, and helpful discussions with Tim Szczykutowicz and Dr Perry Pickhardt. We are also thankful for research support from GE Healthcare and the following funding organizations: UW Radiology R&D, National Institutes of Health, and the Coulter Foundation.
Conflicts of interest and sources of funding: This research was funded by UW Radiology R&D, the National Institutes of Health (R01 DK083380, R01 DK088925, RC1 EB010384, T32 CA009206), and the Coulter Foundation.
REFERENCES
- 1.Boyce CJ, Pickhardt PJ, Kim DH, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol. 2010;194:623–628. doi: 10.2214/AJR.09.2590. [DOI] [PubMed] [Google Scholar]
- 2.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 3.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 4.El-Badry AM, Breitenstein S, Jochum W, et al. Assessment of hepatic steatosis by expert pathologists: the end of a gold standard. Ann Surg. 2009;250:691–697. doi: 10.1097/SLA.0b013e3181bcd6dd. [DOI] [PubMed] [Google Scholar]
- 5.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 6.Liu CY, McKenzie CA, Yu H, et al. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 7.Reeder SB, Wen Z, Yu H, et al. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn Reson Med. 2004;51:35–45. doi: 10.1002/mrm.10675. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, McKenzie CA, Shimakawa A, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26:1153–1161. doi: 10.1002/jmri.21090. [DOI] [PubMed] [Google Scholar]
- 9.Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 10.Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magn Reson Med. 1991;18:371–383. doi: 10.1002/mrm.1910180211. [DOI] [PubMed] [Google Scholar]
- 11.Hines CD, Yu H, Shimakawa A, et al. Quantification of hepatic steatosis with 3-T MR imaging: validation in ob/ob mice. Radiology. 2010;254:119–128. doi: 10.1148/radiol.09090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang BK, Yu ES, Lee SS, et al. Hepatic fat quantification: a prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Invest Radiol. 2012;47:368–375. doi: 10.1097/RLI.0b013e31824baff3. [DOI] [PubMed] [Google Scholar]
- 13.Meisamy S, Hines CD, Hamilton G, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–775. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoo T, Bydder M, Hamilton G, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251:67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoo T, Shiehmorteza M, Hamilton G, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258:749–759. doi: 10.1148/radiol.10100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bydder GM, Kreel L, Chapman RWG, et al. Accuracy of computed-tomography in diagnosis of fatty liver. Br Med J. 1980;281:1042. doi: 10.1136/bmj.281.6247.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piekarski J, Goldberg HI, Royal SA, et al. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137:727–729. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 18.Doppman JL, Cornblath M, Dwyer AJ, et al. Computed tomography of the liver and kidneys in glycogen storage disease. J Comput Assist Tomogr. 1982;6:67–71. doi: 10.1097/00004728-198202000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Mendler MH, Bouillet P, Le Sidaner A, et al. Dual-energy CT in the diagnosis and quantification of fatty liver: limited clinical value in comparison to ultra-sound scan and single-energy CT, with special reference to iron overload. J Hepatol. 1998;28:785–794. doi: 10.1016/s0168-8278(98)80228-6. [DOI] [PubMed] [Google Scholar]
- 20.Coursey CA, Nelson RC, Boll DT, et al. Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics. 2010;30:1037–1055. doi: 10.1148/rg.304095175. [DOI] [PubMed] [Google Scholar]
- 21.Fischer MA, Gnannt R, Raptis D, et al. Quantification of liver fat in the presence of iron and iodine: an ex-vivo dual-energy CT study. Invest Radiol. 2011;46:351–358. doi: 10.1097/RLI.0b013e31820e1486. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Gao Z, Zou Q, et al. Quantitative diagnosis of fatty liver with dual-energy CT. An experimental study in rabbits. Acta Radiol. 2003;44:92–97. [PubMed] [Google Scholar]
- 23.Raptopoulos V, Karellas A, Bernstein J, et al. Value of dual-energy CT in differentiating focal fatty infiltration of the liver from low-density masses. AJR Am J Roentgenol. 1991;157:721–725. doi: 10.2214/ajr.157.4.1892025. [DOI] [PubMed] [Google Scholar]
- 24.Hines CD, Yu H, Shimakawa A, et al. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J Magn Reson Imaging. 2009;30:1215–1222. doi: 10.1002/jmri.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanji AA. Animal models of nonalcoholic fatty liver disease and steatohepatitis. Clin Liver Dis. 2004;8:559–574. ix. doi: 10.1016/j.cld.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Menahan LA. Age-related changes in lipid and carbohydrate metabolism of the genetically obese mouse. Metabolism. 1983;32:172–178. doi: 10.1016/0026-0495(83)90225-1. [DOI] [PubMed] [Google Scholar]
- 27.Hines CD, Agni R, Roen C, et al. Validation of MRI biomarkers of hepatic steatosis in the presence of iron overload in the ob/ob mouse. J Magn Reson Imaging. 2012;35:844–851. doi: 10.1002/jmri.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histo-logical scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 29.Reeder SB, McKenzie CA, Pineda AR, et al. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 2007;25:644–652. doi: 10.1002/jmri.20831. [DOI] [PubMed] [Google Scholar]
- 30.Reeder SB, Pineda AR, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54:636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 31.Bydder M, Yokoo T, Hamilton G, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26:347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H, Shimakawa A, McKenzie CA, et al. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60:1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeder SB, Cruite I, Hamilton G, et al. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton G, Yokoo T, Bydder M, et al. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed. 2011;24:784–790. doi: 10.1002/nbm.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeder SB, Hines CD, Yu H, et al. On the definition of fat-fraction for in-vivo fat quantification with magnetic resonance imaging; Honolulu, HI. April 18–24, 2009; Paper presented at The International Society for Magnetic Resonance in Medicine 17th Annual Meeting. [Google Scholar]
- 36.Yoshimitsu K, Kuroda Y, Nakamuta M, et al. Noninvasive estimation of hepatic steatosis using plain CT vs. chemical-shift MR imaging: significance for living donors. J Magn Reson Imaging. 2008;28:678–684. doi: 10.1002/jmri.21457. [DOI] [PubMed] [Google Scholar]
- 37.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 38.Pickhardt PJ, Park SH, Lee SS, et al. Specificity of noncontrast CT liver attenuation for moderate-severe hepatic steatosis (fatty liver). Paper presented at the RSNA Scientific Assembly; Chicago, IL. November 28–December 2, 2011. [Google Scholar]
- 39.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 40.Reeder SB, Hines CD, McKenzie CA, et al. Tissue- and magnetic resonance-based metrics for quantifying hepatic content: implications for validation studies using tissue as the reference standard. Paper presented at the International Society for Magnetic Resonance in Medicine 19th Annual Meeting; Montreal, Canada. May 7–13, 2011. [Google Scholar]
- 41.Yang Y, Bai G, Zhang X, et al. 1H NMR spectroscopic evidence of interaction between ibuprofen and lipoproteins in human blood plasma. Anal Biochem. 2004;324:292–297. doi: 10.1016/j.ab.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Boll DT, Merkle EM. Diffuse liver disease: strategies for hepatic CT and MR imaging. Radiographics. 2009;29:1591–1614. doi: 10.1148/rg.296095513. [DOI] [PubMed] [Google Scholar]
- 43.Hamer OW, Aguirre DA, Casola G, et al. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26:1637–1653. doi: 10.1148/rg.266065004. [DOI] [PubMed] [Google Scholar]
- 44.Kuhlman JE, Teigen C, Ren H, et al. Amiodarone pulmonary toxicity: CT findings in symptomatic patients. Radiology. 1990;177:121–125. doi: 10.1148/radiology.177.1.2399310. [DOI] [PubMed] [Google Scholar]
- 45.De Maria M, De Simone G, Laconi A, et al. Gold storage in the liver: appearance on CT scans. Radiology. 1986;159:355–356. doi: 10.1148/radiology.159.2.3961168. [DOI] [PubMed] [Google Scholar]