Abstract
Expression of the N-methyl-D-aspartate receptor (NMDA) receptor in trigeminal nuclei has been shown to play a role in the mechanisms of trigeminal pain. Here, we examined the hypothesis that the upregulation of the NR1 subunit of the NMDA receptor (NR1) in the trigeminal subnucleus caudalis (Sp5c) following inflammation of the temporomandibular joint (TMJ) region would be regulated by interleukin-6 (IL-6) and the nuclear factor kappa B (NF-κB). Inflammation of a unilateral TMJ region was produced in rats by injecting 50 μl of complete Freund’s adjuvant (CFA) into a TMJ and adjacent tissues, which resulted in persistent pain behavior as assessed using algometer before (baseline) and on day 1, 3 and 7 after the CFA injection. The CFA injection also induced a significant upregulation of NR1 and NF-κB on day 3 and 7, and of IL-6 on day 1, 3, and 7, within the ipsilateral Sp5c, as compared with the sham TMJ injection group. Once daily intracisternal injection of an IL-6 antiserum or NF-κB inhibitor (PDTC) for six days, beginning on day 1 immediately after the CFA injection, prevented both the upregulation of NR1 in the ipsilateral Sp5C and pain behavior. Moreover, once daily intracisternal IL-6 administration for six days in naïve rats induced the NR1 upregulation and pain behavior similar to that after TMJ inflammation. These results indicate that the upregulation of IL-6 and NF-κB after inflammation of the unilateral TMJ region is a critical regulatory mechanism for the expression of NR1 in the ipsilateral Sp5c, which contributed to the development of TMJ pain behavior in rats.
Keywords: NF-kappaB Inhibitor, NF-kappa B, Neuropathic pain, IL-6, NMDA receptor, NR1, Trigeminal, Temporomandibular joint inflammation, TMJ
INTRODUCTION
Temporomandibular disorder (TMD) refers to a heterogeneous group of clinical conditions including pain and limited movement of the temporomandibular joint (TMJ). Besides the increased pain sensitivity and referred pain beyond the affected TMJ, both autonomic and endocrine functions could be altered as well due to persistent TMJ inflammation. While the exact mechanisms of TMJ pain remain unclear, a growing body of evidence indicates that persistent TMD pain conditions may be mediated by both peripheral and central mechanisms [11, 42].
Inflammation of the TMJ region is a likely triggering factor leading to the pathogenesis of persistent TMJ pain [9, 21, 24, 41, 48], which may result in the increased neuronal excitability in trigeminal nuclei including the subnucleus caudalis (Sp5C) [12]. Although there are anatomic and functional similarities between the spinal and trigeminal somatosensory system, segmental distributions of the somatic sensory input are less well organized in the trigeminal sensory system, making it possible that the inflammation in the TMJ region may lead to changes in various trigeminal subnuclei including Sp5C [3, 10, 20, 37].
It has been shown that the N-methyl-D-aspartate receptor (NMDAR) plays a significant role in the cellular mechanisms of trigeminal nociception [4, 43, 52]. The NR1 subunit of the NMDA receptor (NR1) has also been shown to be involved in social behaviors in mice [13]. Recently, cytokines such as interleukin-6 (IL-6) have been shown to regulate nociception through both peripheral and central mechanisms. For example, hyperalgesia was induced by the intracerebroventricular injection of interleukin-1 beta (IL-1β) or prostaglandin E2 in rats, which was blocked by pretreatment with interleukin-1 receptor antagonist [27, 28]. IL-6 and tumor necrosis factor-alpha also induced hyperalgesia in a prostagnoid-dependent manner [29]. In addition, nuclear factor-kappa B (NF-κB) has been shown to be a key regulator of gene transcriptions in the transcriptional response to inflammation [16, 17].
To date, the regulatory mechanism of the NR1 expression within trigeminal nuclei remains largely unclear. Recent studies also demonstrate that astroglial activation within the trigeminal Vi/Vc transition zone occurred following masseter or tooth pulp inflammation [5,15], which modulated the NMDAR phosphorylation via IL-1β [15]. Thus, it is possible that cytokines and NF-κB could regulate the trigeminal expression of NR1. In the present study, we examined the hypothesis that the upregulation of NR1 in Sp5c following inflammation of the unilateral TMJ region would be regulated by interleukin-6 (IL-6) and NF-κB.
METHODS
Experimental animals
Adult male Sprague-Dawley rats (S.D., Charles River Lab, Wilmington, MA, 200–300g) were used. The experimental protocol was approved by our Institutional Animal Care and Use Committee and was carried out in accordance with the National Institutes of Health Guideline for the Care and Use of Laboratory Animals. Animals were housed under controlled temperature (21°C±2°C), relative humidity (50%±10%) and artificial light (12 h light/dark cycle, lights on at 7 A.M.). All animals had ad libitum access to distilled water and a standard rat diet. The rat’s brain atlas by Paxinos and Watson [33] was used to identify the trigeminal area of interest for sample collections and immunohistochemistry. The experiments were carried out with the experimenter blinded to the treatment conditions.
A rat model of inflammation of a unilateral TMJ region
Chronic inflammation of the TMJ region was induced by the injection into the TMJ space of pre-prepared CFA (Sigma, St Louis) in a suspension (oil/saline, 1:1). A volume of 50 μl (25 μg heat-killed mycobacterium) was injected for each rat under pentobarbital sodium (50 mg/kg, i.p.) anesthesia. The TMJ region was identified by palpation. A 22-gauge needle was percutaneously advanced into the TMJ space immediately inferior to the posterior border of the zygomatic arch until it reached the mandibular condyle. For sham injection, the vehicle for the CFA preparation, incomplete Freund’s adjuvant (IFA, Sigma, St Louis), was injected into unilateral TMJ using the same technique and injection volume. The needle was withdrawn after the injection. Both CFA and IFA injection groups were exposed to the same anesthesia and handling in the experiment. Previous studies have shown that this volume of CFA injected into a TMJ produced persistent hyperalgesia [31] and the elevated Fos expression in the lower trigeminal brainstem complex for at least 7–10 days [53]. In our study, the presence of TMJ inflammation was examined during post-injection days including joint swelling and sensitivity to mechanical stimulation.
Behavioral test
Mechanical hyperalgesia was assessed between 9:00 and 11:00 am. The rat’s head was positioned against the experimenter’s hand during the test and the experimenter switched his hand in order to test the ipsilateral versus contralateral (to CFA or sham) TMJ region. Three habituation sessions (15min/session) in three consecutive days were conducted before the baseline test. A digital algometer (Wagner Instruments, Greenwich, CT) was used, which generated a linearly increasing force delivered through a flat plastic tip (similar to the Randall-Selitto test with a broader tip) against the ipsilateral or contralateral TMJ. For all groups, the site where the algometer tip was placed was in the middle of a TMJ right at the edge of the zygomatic arch. A threshold force was defined as the force (in gm) that resulted in rat’s moving from the source of stimulation [37] with the cut-off force as 2,500 g.
Intracisternal drug delivery
For the intracisternal drug administration, PE-10 tubing was inserted into the intracisternal space for each rat. Under pentobarbital anesthesia, a rat was fixed on a stereotaxic apparatus. A small incision was made to expose the atlantooccipital membrane. The PE-10 tube was inserted intracisternally, ending just dorsal to the obex [6, 14, 18, 47]. The outside end of the tube was secured to the skull by using 3-0 silk. Skin wound was closed with wound clips. Rats (fewer than 5%) exhibiting neurological deficits and distress (e.g., poor eating, grooming, paralysis) were excluded from the experiment. Intracisternal injection was made in awake rats using a microsyringe (50μl) with 10 μl drug followed by a 10 μl saline flush through the tube dead space.
Experimental design
To examine the role of IL-6 and NF-κB in the expression of NR1 within Sp5c, seven groups of rat (n=5–7) were used, including 1) CFA/TMJ injection alone, 2) sham (IFA) TMJ injection alone, 3) CFA/TMJ injection plus 0.5 μg IL-6 goat anti-rat IL-6 serum (intracisternal, R&D System Inc., Minneapolis, MN), 4) CFA/TMJ injection plus 10 μl normal goat serum (intracisternal), 5–6) CFA/TMJ injection plus 7.5 or 15 μg PDTC in 10 μl saline (an NF-κB inhibitor; intracisternal; Sigma, St Louis)[16], and 7) naïve rats plus 7.5 μg PDTC in 10 μl saline. The PDTC doses were chosen based on our pilot experiment and the literature [16]. All intracisternal injections were administered once daily for six consecutive days, beginning on day 1 immediately after a TMJ injection. Behavioral tests were performed on day 0 (baseline), 1, 3, and 7 before a drug or vehicle injection in order to examine the accumulative effect of a drug on the development of pain behaviors.
To examine whether exogenous IL-6 would induce the NR1 upregulation in Sp5c, naïve rats (without TMJ injection) were given IL-6 (rat recombinant interleukin 6, R&D System Inc., Minneapolis, MN; 33 ng) intracisternally once daily for six days. Behavioral tests were performed on day 0 (baseline), 1, 3, and 7 of IL-6 injections (approximately 24 hrs after last IL-6 injection). In both experiments, trigeminal tissues were harvested within 2 hr after the final behavioral test.
Immunohistochemistry
Rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused transcardially with cold phosphate buffered saline (PBS, 0.01M, pH 7.35), which was followed by 4% paraformaldehyde in phosphate buffer (PB, 0.1M, pH 7.35). The brain regions containing Sp5C were harvested and post-fixed overnight in the same fixative and then cryoprotected in 30% sucrose in 0.1M PB until tissue blocks sank to the bottom. Sections were cut on a cryostat (Leica, 30 μm). Coronal sections were floated in PBS and processed for immunostaining.
Immunocytochemical staining was used to detect IL-6 (1:1000, goat polyclonal; R & D Systems, Minneapolis, MN), NF-κB (1:1000, rabbit polyclonal, Santa Cruz Biotechnology, Inc.), and NR1 (1:500, mouse monoclonal; United States Biological) within Sp5c. The antibodies were raised against rats. Tissue sections were blocked with 1% BAS in 0.3% Triton X-100 for 1 hr at room temperature and incubated overnight at 4°C with a primary antibody. For controls, a primary antibody was omitted. Sections were then incubated for one hour at room temperature with a corresponding FITC- or Cy3-conjugated secondary antibody (1:400; Chemicon, Temecula, CA). For double staining, a second primary antibody was added after incubation with the first primary antibody following the same procedure described above. Six non-adjacent sections were randomly selected, analyzed using a fluorescence microscope (Olympus, Tokyo, Japan), recorded with a digital camera, and processed using Adobe Photoshop.
Western Blot
Rats were anesthetized using sodium pentobarbital before being decapitated. Trigeminal tissue samples (ipsilateral versus contralateral Sp5c, dorsal lateral portions of the oblongata and rostral region of the spinal cord) were removed and immediately placed on dry ice and stored at −80°C until use. The samples were taken from the more caudally Sp5C area, as compared with that reported by Guo et al [15]. Samples were homogenized in SDS sample buffer containing a mixture of protease inhibitors (Sigma). Protein samples were separated on SDS-PAGE gels (4–10% gradient gel) and transferred to polyvinylidene difluoride filters (Millipore, Bedford, MA). The filters were blocked with 5% milk for one hour at room temperature and incubated overnight at 4°C with the same NR1, IL-6, or NF-κB antibody used for immunostaining with similar concentrations (see above), followed by HRP-conjugated secondary antibody (1:7000; Amersham Biosciences, Arlington Heights, IL) incubated for one hour at room temperature. The blots were visualized in ECL solution (NEN, Boston, MA) for one minute and exposed onto hyperfilms (Amersham Biosciences) for 1–10 min. The blots were then incubated in a stripping buffer (67.5 mM Tris, pH 6.8, 2% SDS, and 0.7% β-mercaptoethanol) for 30 min at 50 °C and reprobed with mouse anti-β-actin antibody (1:20,000; Abcam Inc, Cambridge, MA) as a loading control. All Western analysis was made in triplicates.
Statistical Analysis
For the behavioral data analysis, repeated measure two-way ANOVA was used followed by the post-hoc Tukey test (SPSS) to examine the interaction between time points and treatment effects. For Western blot, the band density was measured with Photoshop and normalized against corresponding loading controls. Differences were compared using one-way ANOVA (SPSS). The statistical significance was set at the level of α=0.05.
RESULTS
Effect of IL-6 antiserum or PDTC on TMJ pain behavior
The CFA, but not IFA, injection into a unilateral TMJ induced pain behavior on the ipsilateral TMJ site on day 1, 3 and 7 after the injection, as measured by an algometer (Fig. 1, P<0.05; n=5). In contrast, there was no change in pain behavior over time on the contralateral TMJ site of rats receiving the CFA injection (Fig. 1, P>0.05).
Fig. 1. Time course of TMJ pain behavior.
Escape force was measured by using an algometer at both ipsilateral and contralateral TMJ site to the CFA injection. Escape force was also examined on the ipsilateral TMJ site of a vehicle injection. * P<0.05, as compared with baseline (Bas).
Repeated intracisternal administration (once daily × 6 days) of an IL-6 antiserum (0.5 μg), but not normal goat control serum, attenuated pain behavior over time (Fig. 2a, P<0.05; n=5–7). Similarly, pain behavior on the ipsilateral TMJ site induced by the CFA injection was attenuated by the repeated intracisternal administration (once daily × 6 days) of 7.5 μg PDTC (an NF-κB inhibitor) (Fig. 2b, P<0.05, n=5–6). A higher PDTC dose (15 μg) did not further improve the behavioral response in the CFA injection group (data not shown). Repeated intracisternal administration of 7.5 μg PDTC alone did not change the escape threshold in naive rats (Fig. 2b, P>0.05, n=6). In addition, the nociceptive response on the contralateral side of CCI rats treated with IL-6 antiserum did not differ from that of sham rats without IL-6 antiserum, and IL-6 antiserum alone did not affect the baseline nociceptive response in both ipsilateral and contralateral sides of sham rats in our pilot experiment (data not shown). These results indicate that both IL-6 and NF-κB were contributory to the development of TMJ pain behavior after the CFA injection in rats.
Fig. 2. Effect of IL-6 antiserum, PDTC, or exogenous IL-6 on TMJ pain behavior.
A, B. TMJ pain behavior was attenuated by the intracisternal injection of either an IL-antiserum (A) or the NF-κB inhibitor PDTC, given once daily for six days. C. Intracisternal administration of exogenous IL-6 induced TMJ pain behavior in naïve rats. * P< 0.05, as compared with baseline (BAS).
Effect of exogenous IL-6 on TMJ pain behavior
To examine whether IL-6 would induce similar pain behavior in naïve rats as compared to that induced by the CFA injection, exogenous IL-6 (33 ng) was administered intracisternally once daily for six consecutive days. This treatment regimen induced TMJ pain behavior when examined on day 3 and 7, but not day 1, as compared with control naïve rats injected with vehicle (Fig. 2c, n=7). Both the time course and degree of pain behavior were similar to those in rats with the CFA injection into a unilateral TMJ.
Co-expression of NR1 and NF-κB or IL-6 within Sp5c
NR1, NF-κB, and IL-6 were expressed within Sp5C. NR1 was co-expressed with NF-κB in the same region. While there were fewer NR1-positive than IL-6-positive cells within Sp5c, the majority of NR1-positive cells were also IL-6-positive (Fig. 3). Collectively, most cells within Sp5c co-expressed NR1 with NF-κB or IL-6. The exact cellular association of these expressions was not examined in the current experiment.
Fig. 3. Co-expression of NR1 and NF-κB or IL-6 within Sp5c.
NFκB, IL-6 and NMDAR expression within the ipsilateral Sp5c of rats with TMJ inflammation. Bar, 100 μm.
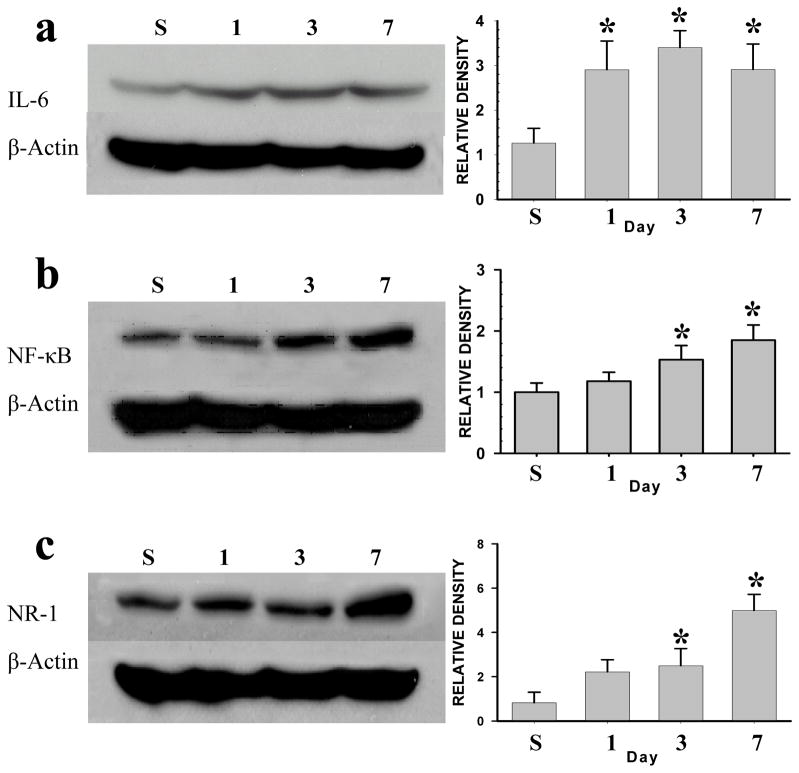
Upregulation of IL-6, NF-κB, and NR1 after TMJ inflammation
As compared with sham TMJ injection, the CFA injection induced a time-dependent upregulation of IL-6 (Fig. 4a), NF-κB (Fig. 4b), and NR1 (Fig. 4c) within the ipsilateral Sp5c (each P< 0.05; n=5–6). There were no upregulation of these cellular elements in the contralateral Sp5C after the TMJ injection (data not shown). Of interest is that the onset of the IL-6 upregulation preceded that of NF-κB and NR1 in that the upregulation of IL-6 began on day 1, whereas on day 3 for NF-κB and NR1, after the CFA injection (Fig. 4).
Fig. 4. Upregulation of IL-6, NF-κB, and NR1 after TMJ inflammation.
IL-6 (a), NF-κB (b), and the NR1 subunit of NMDAR (c) were upregulated in the ipsilateral Sp5c after TMJ inflammation. * P<0.05, as compared with the vehicle (S) TMJ injection on day 7.
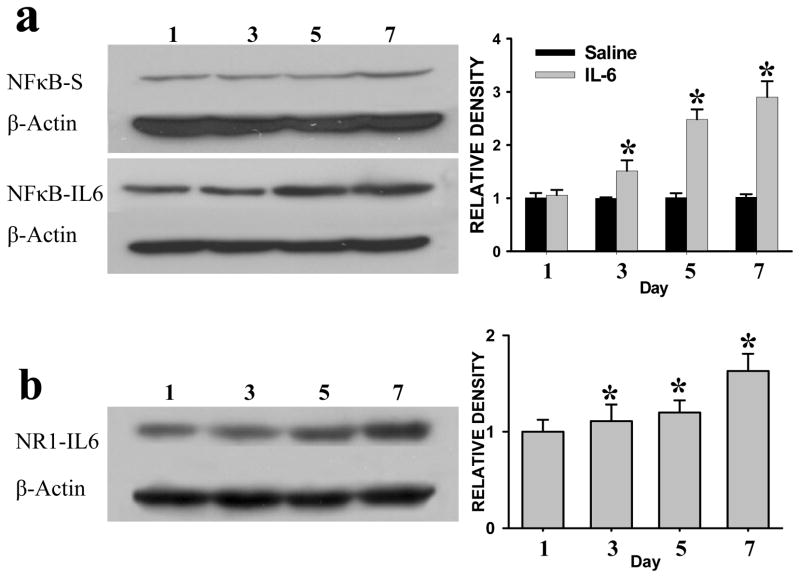
Upregulation of NF-κB and NR1 by exogenous IL-6 in naïve rats
Once daily intracisternal administration of exogenous IL-6 (33 ng), but not vehicle, induced the upregulation of NF-κB (Fig. 5a) and NR1 (Fig. 5b) within Sp5c of naïve rats (without CFA or sham injection). The statistically significant increase in the expression of NF-κB and NR1 began on day 3 of the IL-6 treatment regimen (Fig. 5a,b; each P< 0.05; n=5). These results indicate that exogenous IL-6 mimicked the effect of the CFA injection on the expression of NF-κB and NR1 within Sp5c as well as the development of TMJ pain behavior (see Fig. 2a).
Fig. 5. Upregulation of NF-κB and NR1 by exogenous IL-6 in naïve rats.
The expression of NF-κB and NR1 was upregulated in Sp5c after repeated intracisternal administration of exogenous IL-6 (once daily × 6 days). * P<0.05 as compared with that on day 1.
Prevention of the NR1 upregulation by IL-6 antiserum or PDTC
Once daily intracisternal administration of IL-6 antiserum (0.5 μg) or PDTC (15 μg) for six days prevented the upregulation of NR1 within the ipsilateral Sp5c, when examined on day 7 after the CFA injection (Fig. 6a,b; each P<0.05; n=5–7). Moreover, the same anti-IL-6 treatment regimen prevented the upregulation of NF-κB within the ipsilateral Sp5c on day 7 as compared with the control group (Fig. 6a; P<0.05; n=5). These results indicate that IL-6 played a critical role in the upregulation of NF-κB and NR1 within the ipsilateral Sp5c in response to unilateral TMJ inflammation.
Fig. 6. Attenuation of the NR1 and NF-κB upregulation by IL-6 antiserum or PDTC.
A. The upregulation of NF-κB and NR1 in the ipsilateral Sp5c was reduced by IL-6 antiserum (intracisternal, once daily × 6 days), when examined on day 7. B. The NR1 upregulation in the ipsilateral Sp5c was reduced by PDTC (intracisternal, once daily × 6 days), when examined on day 7. * P<0.05, as compared with sham TMJ injection on day 7; + P<0.05 as compared with the CFA/TMJ group with intracisternal vehicle. S: sham TMJ injection plus intracisternal vehicle; T: CFA/TMJ injection plus intracisternal vehicle; T/A: CFA/TMJ injection plus intracisternal IL-6 antiserum; T/P: CFA/TMJ injection plus intracisternal PDTC.
DISCUSSION
We have demonstrated that inflammation to a unilateral TMJ region induced the upregulation of NR1 and NF-κB on day 3 and 7, and of IL-6 on day 1, 3, and 7, within the ipsilateral Sp5c. Intracisternal injection of an IL-6 antiserum or NF-κB inhibitor (PDTC) for six days prevented both the upregulation of NR1 in the ipsilateral Sp5C and pain behavior. Moreover, once daily intracisternal IL-6 administration for six days in naïve rats induced the upregulation of NF-κB and NR1 and pain behavior similar to that after TMJ inflammation. These results indicate that the upregulation of IL-6 and NF-κB is an upstream event critical to the expression of NR1 in the ipsilateral Sp5C after unilateral TMJ inflammation, which contributed to the development of TMJ pain behavior in rats.
There were several methodological limitations. First, the TMJ inflammation model is likely to involve both the TMJ and surrounding tissues. Second, PDTC is one of several NF-κB inhibitors and future studies may further examine this issue using additional NF-κB inhibitors. Of interest to note is that there was a greater reversal effect of IL-6 antiserum on day 7 than on days 1 and 3 (Fig. 2), which might be due to the accumulative effect of IL-6 antiserum on the behavioral changes during the course of intracisternal treatment. Third, the extent of Sp5C examined in this study may be limited particularly in the Western blot study due to the technical limitation of tissue harvest in this area. Fourth, the cellular relationship between the IL-6 receptor and NR1 (e.g., neuronal versus subtypes of glial cells) was not examined in the present study, which should be determined in future studies.
NMDAR has been known to regulate pain behaviors induced by peripheral nerve injury [22, 49, 51]. Mechanistically, activation of NMDAR initiates intracellular cascades through calcium influx and activation of protein kinases, which in turn modulates cell membrane excitability and enhances nociceptive transmission [22, 49]. More recently, the expression of IL-6 and NMDAR has been shown to be upregulated within the spinal cord dorsal horn after peripheral nerve injury, which contributed to the mechanisms of pain behavior after nerve injury [46]. The present study indicates that a similar mechanism is likely to be contributory to TMJ pain within the trigeminal system. With regard to the cellular location of the NMDAR expression, the immunohistochemical data indicates that much of NR1 immunoreactivity was associated with neuronal cells and co-localized with IL-6 or NF-κB. A previous study has shown that IL-6 receptor is expressed in neurons of the human fetus brain and spinal cord [7].
Proinflammatory cytokines have been shown to play a significant role in the inflammatory responses through intracellular mediators such as NF-κB [2,23]. Specifically, interleukins such as IL-6 have been implicated in both peripheral and central responses to peripheral nerve injury [1, 8, 26, 30, 35, 36, 45]. In addition, interleukins enhance conditioned fear memory mediated in part through the effects of glucocorticoids [39] and contribute to the development of pain behaviors in several animal models [25, 32, 36, 40, 44]. Intracellularly, NF-κB is an important regulator of gene transcription involved in the immune/inflammatory response and cell survival [16, 17]. Our results indicate that a critical central locus of the IL-6 and NF-κB effect on TMJ pain is within the ipsilateral Sp5c. This finding is consistent with those studies showing that Sp5c is involved in glial activation in the trigeminal nociceptive processing [50] and pain behavior [19, 34]. Recent studies also demonstrate that astroglial activation within the trigeminal Vi/Vc transition zone occurred following masseter or tooth pulp inflammation [5,15], which modulated the NMDAR phosphorylation via IL-1β [15]. Thus, our results suggest a possible sequence of events related to the expression of NR1 in Sp5c after TMJ inflammation. That is, peripheral inflammation may increase the expression of cytokines such as IL-6 within the central trigeminal system leading to the expression of NMDAR through intracellular mediators such as NF-κB. The differences in the onset of the IL-6, NF-κB, and NR1 expression after TMJ inflammation support this possibility. It should be noted that the present data do not rule out the possibility of an NF-κB-independent pathway in relation to the IL-6 effect on the NMDAR expression. This issue remains to be examined in future studies.
In summary, the present data suggest that cellular mediators such as IL-6 and NF-κB are contributory to the regulation of the NMDAR expression in Sp5c, which is critically involved in the mechanisms of pain behavior after TMJ inflammation. The possibility that other proinflammatory cytokines and intracellular signaling pathways [25, 32, 40] would be involved in the expression of NR1 and the development of TMJ pain can not be ruled out. Moreover, future studies should examine the source and regulatory mechanism of the IL-6 expression in the trigeminal system after TMJ inflammation.
Acknowledgments
This study is supported by NIH RO1 grants DE18214, DE18538, and NS45681.
Footnotes
The authors claim no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arruda JL, Colburn RW, Rickman AJ, Rutkoski MD, Deleo JA. Increase of interleukin-6 mRNA in the spinal cord following peripheral injury in the rat: potential role of IL-6 in neuropathic pain. Brain Res Mol Brain Res. 1998;62:228–35. doi: 10.1016/s0169-328x(98)00257-5. [DOI] [PubMed] [Google Scholar]
- 2.Barkhudaryan N, Dunn AJ. Molecular mechanisms of actions of interleukin-6 on the brain, with special reference to serotonin and the hypothalamo-pituitary-adrenocortical axis. Neurochem Res. 1999;24:1169–80. doi: 10.1023/a:1020720722209. [DOI] [PubMed] [Google Scholar]
- 3.Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000;88:221–4. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- 4.Cairns BE, Sessle BJ, Hu JW. Temporomandibular-evoked jaw muscle reflex: role of brain stem NMDA and non-NMDA receptors. Neuroreport. 2001;12:1875–8. doi: 10.1097/00001756-200107030-00022. [DOI] [PubMed] [Google Scholar]
- 5.Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, Sessle BJ. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J Neurosci. 2007;27:9068–9076. doi: 10.1523/JNEUROSCI.2260-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HS, Lu JS, Lee HJ, Kim BC, Park JS, Ahn DK. Effects of intracisternal injection of interleukins-6 on nociceptive jaw opening reflex and orofacial formalin test in freely moving rats. Brain Res Bull. 2002;59:365–70. doi: 10.1016/s0361-9230(02)00931-0. [DOI] [PubMed] [Google Scholar]
- 7.Dame JB, Juul SE. The distribution of receptors for the pro-inflammatory cytokines interleukin (IL)-6 and IL-8 in the developing human fetus. Early Hum Dev. 2000;58:25–39. doi: 10.1016/s0378-3782(00)00064-5. [DOI] [PubMed] [Google Scholar]
- 8.Deleo JA, Colburn RW, Nichols M, Malhotra A. Interleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy model. J Interferon Cytokine Res. 1996;16:695–700. doi: 10.1089/jir.1996.16.695. [DOI] [PubMed] [Google Scholar]
- 9.Denucci DJ, Dionne RA, Dubner R. Identifying a neurobiologic basis for drug therapy in TMDs. J Am Dent Assoc. 1996;127:581–93. doi: 10.14219/jada.archive.1996.0270. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson KW. Rheumatoid diseases and the temporomandibular joint: a review. Cranio. 1995;13:264–9. doi: 10.1080/08869634.1995.11678078. [DOI] [PubMed] [Google Scholar]
- 11.Dubner R. The neurobiology of persistent pain and its clinical implications. Suppl Clin Neurophysiol. 2004;57:3–7. doi: 10.1016/s1567-424x(09)70337-x. [DOI] [PubMed] [Google Scholar]
- 12.Dubner R, Ren K. Brainstem mechanisms of persistent pain following injury. J Orofac Pain. 2004;18:299–305. [PubMed] [Google Scholar]
- 13.Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Flores CA, Wang XM, Zhang KM, Mokha SS. Orphanin FQ produces gender-specific modulation of trigeminal nociception: behavioral and electrophysiological observations. Neuroscience. 2001;105:489–98. doi: 10.1016/s0306-4522(01)00179-8. [DOI] [PubMed] [Google Scholar]
- 15.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–18. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–222. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 17.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 18.Kim HD, Lee HJ, Choi HS, Ju JS, Jung CY, Bae YC, Ahn DK. Interleukin-1β injected intracisternally inhibited NMDA-evoked behavioral response in the orofacial area of freely moving rats. Neurosci Lett. 2004;360:37–40. doi: 10.1016/j.neulet.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 19.Lan L, Yuan H, Duan L, Cao R, Gao B, Shen J, Xiong Y, Chen LW, Rao ZR. Blocking the glial function suppresses subcutaneous formalin-induced nociceptive behavior in the rat. Neurosci Res. 2007;57:112–29. doi: 10.1016/j.neures.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Leah JD, Porter J, de-Pommery J, Menétrey D, Weil-Fug uzza J. Effect of acute stimulation on Fos expression in spinal neurons in the presence of persisting C-fiber activity. Brain Res. 1996;719:104–11. doi: 10.1016/0006-8993(96)00111-4. [DOI] [PubMed] [Google Scholar]
- 21.Lobbezoo F, Drangsholt M, Peck C, Sato H, Kopp S, Svensson P. Topical review: new insights into the pathology and diagnosis of disorders of the temporomandibular joint. J Orofac Pain. 2004;18:181–91. [PubMed] [Google Scholar]
- 22.Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353–64. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- 23.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–32. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 24.Milam SB, Schmitz JP. Molecular biology of temporomandibular joint disorders: proposed mechanisms of disease. J Oral Maxillofac Surg. 1995;53:1448–54. doi: 10.1016/0278-2391(95)90675-4. [DOI] [PubMed] [Google Scholar]
- 25.Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier S, Watkins LR. Spinal glia and proinflammatory cytokine mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–40. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy PG, Ramer MS, Borthwick L, Gauldie J, Richardson PM, Bisby MA. Endogenous interleukin-6 contributes to hypersensitivity to cutaneous stimuli and changes in neuropeptides associated with chronic nerve constriction in mice. Eur J Neurosci. 1999;11:2243–53. doi: 10.1046/j.1460-9568.1999.00641.x. [DOI] [PubMed] [Google Scholar]
- 27.Oka T, Aou S, Hori T. Intracerebroventricular injection of interleukin-1 beta induces hyperalgesia in rats. Brain Res. 1993;624:61–8. doi: 10.1016/0006-8993(93)90060-z. [DOI] [PubMed] [Google Scholar]
- 28.Oka T, Aou S, Hori T. Intracerebroventricular injection of prostaglandin E2 induces thermal hyperalgesia in rats: the possible involvement of EP3 receptors. Brain Res. 1994;663:287–92. doi: 10.1016/0006-8993(94)91275-0. [DOI] [PubMed] [Google Scholar]
- 29.Oka T, Oka K, Hosoi M, Hori T. Intracerebroventricular injection of interleukin-6 induces thermal hyperalgesia in rats. Brain Res. 1995;692:123–8. doi: 10.1016/0006-8993(95)00691-i. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Pro- and anti-inflammatory cytokines gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol. 2001;169:386–91. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto K, Imbe H, Tashiro A, Kumabe S, Senba E. Blockade of peripheral 5HT3 receptor attenuates the formalin-induced nocifensive behavior in persistent temporomandibular joint inflammation of rat. Neurosci Lett. 2004;367:259–63. doi: 10.1016/j.neulet.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289–93. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. New York: Academic Press; 1997. [Google Scholar]
- 34.Piao ZG, Cho IH, Park CK, Hong JP, Choi SY, Lee SJ, Lee S, Park K, Kim JS, Oh SB. Activation of glia and microglial P38 MAPK in medullary dorsal horn contributes to tactile hypersensitivity following trigeminal sensory nerve injury. Pain. 2006;121:219–31. doi: 10.1016/j.pain.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Raghavendra V, Rutkowski MD, Deleo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–89. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–21. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 38.Shinoda M, Ozaki N, Asai H, Nagamine K, Sugiura Y. Changes in P2X3 receptor expression in the trigeminal ganglion following monoarthritis of the temporomandibular joint in rats. Pain. 2005;116:42–51. doi: 10.1016/j.pain.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 39.Song C, Phillips AG, Leonard B. Interleukin 1 beta enhances conditioned fear memory in rats: possible involvement of glucocorticoids. Eur J Neurosci. 2003;18:1739–43. doi: 10.1046/j.1460-9568.2003.02886.x. [DOI] [PubMed] [Google Scholar]
- 40.Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination of soluble tumor necrosis factor receptor exhibits an anti-allodynia action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–39. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- 41.Ta LE, Dionne RA. Treatment of painful temporomandibular joints with a cyclooxygenase-2 inhibitor: a randomized placebo-controlled comparison of celecoxib to naproxen. Pain. 2004;111:13–21. doi: 10.1016/j.pain.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 42.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 43.Tsai CM, Chiang CY, Yu XM, Sessle BJ. Involvement of trigeminal subnucleus caudalis (medullary dorsal horn) in craniofacial nociceptive reflex activity. Pain. 1999;81:115–28. doi: 10.1016/s0304-3959(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 44.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Dis. 2003;2:973–85. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo G, Yang L, Mao J. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. J Neurosci. 2004;24:8595–605. doi: 10.1523/JNEUROSCI.3058-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Lim G, Zeng Q, Sung B, Yang L, Mao J. Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury. J Neurosci. 2005;25:488–95. doi: 10.1523/JNEUROSCI.4127-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang XM, Zhang ZJ, Baines R, Nokha SS. Effect of antisense knockdown of alpha(2a)- and alpha(2c)-adrenoceptors on the antinociceptive action of clonidine on trigeminal nociception in the rat. Pain. 2002;98:27–35. doi: 10.1016/s0304-3959(01)00464-x. [DOI] [PubMed] [Google Scholar]
- 48.Woda A, Pionchon P. A unified concept of idiopathic orofacial pain: clinical features. J Orofac Pain. 1999;13:172–84. discussion 185–95. [PubMed] [Google Scholar]
- 49.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 50.Xie YF, Zhang S, Chaing CY, Hu JW, Dostrovsky JO, Sessle BJ. Involvement of glia in central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn) Brain Behav Immuno. 2007;21:634–41. doi: 10.1016/j.bbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T, Yaksh TL. Studies on the spinal interaction of morphine and the NMDA antagonist MK-801 on the hyperesthesia observed in a rat model of sciatic mononeuropathy. Neurosci Lett. 1992;135:67–70. doi: 10.1016/0304-3940(92)90137-v. [DOI] [PubMed] [Google Scholar]
- 52.Yu XM, Sessle BJ, Haas DA, Izzo A, Vernon H, Hu JW. Involvement of NMDA receptor mechanisms in jaw electromyographic activity and plasma extravasation induced by inflammatory irritant application to temporomandibular joint region of rats. Pain. 1996;68:169–78. doi: 10.1016/S0304-3959(96)03181-8. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Q, Imbe H, Dubner R, Ren K. Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: implications for persistent orofacial pain. J Comp Neurol. 1999;412:276–91. doi: 10.1002/(sici)1096-9861(19990920)412:2<276::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]