Abstract
Structure-activity relationship study shows that the catechol group in 7,8-dihdyroxyflavone, a selective small TrkB receptor agonist, is critical for the agonistic activity. To improve the poor pharmacokinetic profiles intrinsic to catechol-containing molecules and elevate the agonistic effect of the lead compound, we initiated the lead optimization campaign by synthesizing various bioisosteric derivatives. Here we show that the optimized 2-methyl-8-(4′-(pyrrolidin-1-yl)phenyl)chromeno[7,8-d]imidazol-6(1H)-one derivative possesses the enhanced TrkB stimulatory activity. Chronic oral administration of this compound significantly reduces the immobility in forced swim test and tail suspension test, two classical antidepressant behavioral animal models, which is accompanied by robust TrkB activation in hippocampus of mouse brain. Further, in vitro ADMET studies demonstrate that this compound possesses the improved features compared to the previous lead compound. Hence, this optimized compound may act as a promising lead candidate for in-depth drug development for treating various neurological disorders including depression.
Keywords: TrkB agonist, BDNF, synthetic derivatives, antidepressant, neurogenesis
Introduction
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family, which includes nerve growth factor (NGF), NT-3 and NT-4/5. BDNF binding to its cognate receptor, TrkB, triggers its dimerization through conformational changes and autophosphorylation of tyrosine residues, resulting in activation of the three major signaling pathways, MAPK, PI3K and PLC-γ1. Accumulating evidence indicates that these molecules are also critical for antidepressant drug efficacy. Antidepressants induce BDNF mRNA expression, as well as autophosphorylation and activation of TrkB,1,2 and the behavioral effects of antidepressants in the forced swim test are attenuated in mice with only one active BDNF allele (BDNF+/− mice), completely lacking BDNF specifically in forebrain or expressing a dominant negative form of TrkB (trkB.T1).2, 3 Ablation of TrkB, specifically in hippocampal neuronal progenitor cells renders the mice behaviorally non-responsive to chronic antidepressant treatment.4 Hence, these results suggest a model whereby chronic antidepressant treatment induces BDNF expression and long-term activation of TrkB, leading to an antidepressant effect 5–7.
The preclinical evidence strongly supports the idea that BDNF might be useful as a therapeutic agent for a variety of neurological disorders 8, 9. To search for small molecules that mimic the biological functions of BDNF, we invented a cell-based assay and identified two small molecular TrkB agonists with different structural backbones 10, 11. Our studies demonstrate that these small molecules exert potent neuroprotective effects in stroke, PD and possess robust antidepressant effect 10, 12. Both 7,8-dihydroxyflavone (7,8-DHF) 10, 12 and deoxygedunin induce neurogenesis and exhibit antidepressant-like profile in a TrkB-dependent manner 11, 12. Most recently, it has been shown that 7,8-DHF mimics BDNF and improves the cognitive defect in AD 13. Further, 7,8-DHF dampens the development of the “depressive” phenotype 14. Like BDNF, 7,8-DHF rescues synaptic plasticity in aged animals 15. Notably, 7,8-DHF exhibits therapeutic efficacy in a mouse model of Rett syndrome, caused by mutations in the MeCP2 gene; MeCP2 mutant mice have reduced levels of BDNF 16. Hence, these studies demonstrate that our small TrkB agonists mimic BDNF and exert neuroprotective roles in various neurological diseases and display an antidepressant-like profile.
Our preliminary structure-activity relationship (SAR) study shows that the 7,8-dihydroxy groups are essential for the agonistic effect.12 To improve the lead compound’s agonistic activity, we have conducted an extensive SAR study and synthesized numerous derivatives. We have successfully identified 4′-dimethylamino-7,8-dihydroxyflavone (4′-DMA-7,8-DHF) 12 that displays higher TrkB agonistic activity than the original hit 7,8-DHF. This novel compound also exhibits a more robust and longer TrkB activation effect in animals. Consequently, this new compound reveals more potent anti-apoptotic activity. Interestingly, chronic oral administration of 4′-DMA-7,8-DHF and the original hit 7,8-DHF strongly promotes neurogenesis in dentate gyrus and demonstrates marked antidepressant-like profile.12 However, catechol containing compounds usually have short in vivo half-life and are prone to be cleared in the circulatory system following oxidation, glucuronidation, sulfation or methylation.17, 18 The potential metabolic pathways implicated in catechol-containing 7,8-DHF clearance in the circulatory system may remain similar. To overcome the intrinsic pharmacokinetics (PK) shortcomings that catechol groups are associated with, we replaced 7,8-dihydroxy groups with an imidazole ring. In this report, we expand on our previous findings to include the synthesis of numerous imidazole-substituted flavonoids with improved in vitro ADMET profiles, efficacy in activating the TrkB receptor in mouse brain and elevated anti-depressant effects.
Results
Synthesis of imidazole-flavonoid and indole-flavonoid derivatives
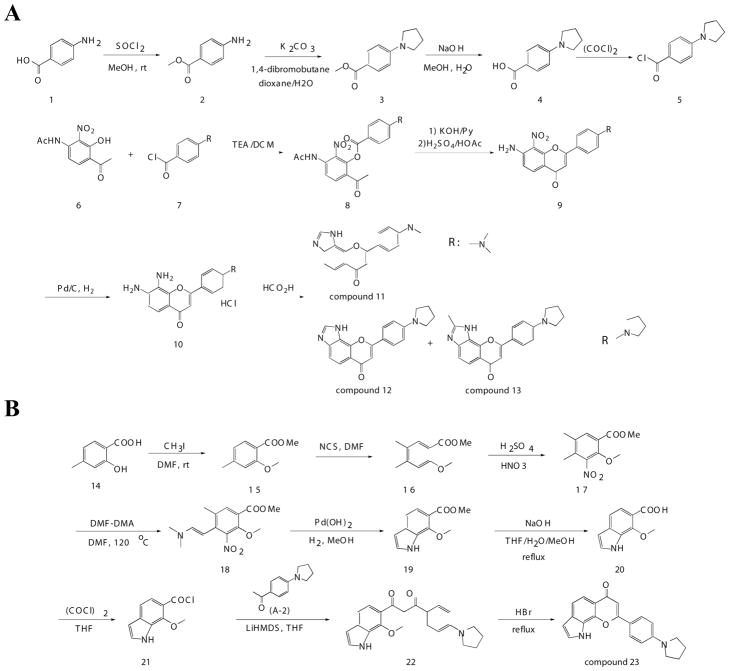
Previous study shows that the catechol group plays a critical role in regulating 7,8-DHF and its derivatives’ TrkB agonistic effect. Since catechol-containing compounds usually possess relatively poor pharmacokinetic (PK) profiles, we synthesized numerous analogues of this compound to enhance the desired biological and physical properties of the lead compound, 4′-DMA-7,8-DHF. The in vivo PK data for this compound are included in Table 1. To synthesize the intermediate 4-dimethylamino- or 4-pyrrolidin-1-yl- benzoyl chloride, we conducted the reactions described in the top of Figure 1A. 4-(dimethylamino)benzoyl chloride (7, R: dimethylamino-) was coupled to N-(4-acetyl-3-hydroxy-2-nitrophenyl)acetamide (6) in the presence of triethylamine to yield 3-acetamido-6-acetyl-2-nitrophenyl 4-(dimethylamino)benzoate (8), which was subsequently cyclized to afford 7-amino-2-(4-(dimethylamino)phenyl)-8-nitro-4H-chromen-4-one (9). This compound was reduced to generate 7,8-diamino-2-(4-(dimethylamino)phenyl)-4H-chromen-4-one hydrochloride (10). A solution of this compound was refluxed in HCO2H for 1 h to produce a yellow solid, which was recrystallized to yield pure 8-(4′-(dimethylamino)phenyl)chromeno [7,8-d]imidazol-6(1H)-one (compound 11). The 4′-pyrrolidino imidazole derivatives were synthesized with similar routes, producing a mixture of imidazole-flavonoid (compound 12) and methyl-imidazole-flavonoid (compound 13) with 60 : 40 ratio (Figure 1A), which was separated with pre-HPLC to give compound 12 as an orange solid and a dark-yellow solid compound 13. The yield for 2-methyl-imidazole side product in 4′-dimethylamino derivative was very low compared to compound 11. For compound 12, 16 hydrogen atoms were present in the 1HNMR, while for compound 13, 18 protons appeared. We reasoned the methyl group was on the imidazole ring, because the proton between the two nitrogen atoms of imidazole ring missed (8.81 ppm in compound 12) and the chemical shift of methyl group was consistent with the structure. To prepare the intermediate 7-methoxy-6-indole-carboxylic chloride (21), we employed compound 14 as a starting material, via the reactions described in the top of Figure 1B. The obtained intermediate compound 21 was then coupled to 4′-pyrrolidino-phenyl methyl ketone in the presence of LiHMDS, and the reaction mixture was quenched with NH4Cl water solution to give compound 22 (1-(4′-pyrrolidino-phenyl)-3-(7′-methoxy-6-indolyl)propane-1,3-dione), which was subsequently cyclized in the presence of HBr to afford compound 23 (Figure 1B). The yield for each step in the synthesis is indicated in the experimental section.
Table 1.
In vivo Pharmacokinetic studies
| Compound I.D. | Route of Administration | Dose (mg/kg) | Subject | T1/2 (min) | CL (mL/min/kg) | Vz (mL/kg) | Vss (mL/kg) | AUClast (min* ng/mL) | AUCINF (min* ng/mL) |
|---|---|---|---|---|---|---|---|---|---|
| 4′-dimethylamino-7,8- dihydroxflavone HBr | IV | 1 | Mice | 9 | 168 | 2117 | 803 | 5930 | 5943 |
| 13 | IV | 3 | Mice | 103 | 156 | 23085 | 10011 | 18746 | 19229 |
| Route of Administration | Dose (mg/kg) | Subject | Bioavailability (%) | Tmax (min) | Cmax (ng/mL) | T1/2 (min) | AUClast (min* ng/mL) | ACINF (min* ng/mL) | |
| 4′-dimethylamino-7,8- dihydroxflavone HBr | PO | 5 | Mice | NC | NC | NC | NC | NC | NC |
| 13 | PO | 10 | Mice | 2 | 120 | 7 | 58 | 1144 | 1293 |
Notes: Pharmacokinetic parameters were derived from non-compartmental model using WinNonlin 5.2.
T1/2 = Elimination half-life
CL = Total body clearance
Vz = Volume of distribution
Vss = An estimate of the volume of distribution at steady state
AUClast = Area under the concentration-time curve from the time of dosing to the time of last observation that is greater than the limit of quantitation*
AUCINF = Area under the concentration-time curve from the time of dosing, extrapolated to infinity*
Linear/Log trapezoidal method was used for AUC calculation.
Tmax = Time of maximum observed concentration
Cmax = Concentration corresponding to Tmax
NC = Not calculable, because plasma concentration are too low
Terminal points = the number of observations used to calculate the terminal slope.
Figure 1. Organic synthesis of various flavonoids.
A, Schematic diagram of synthetic routes for 4′-dimethylamino-7,8-imidazole flavone (compounds 11, 12 and 13). R represents dimethylamino group or 4-pyrrolidin-1-yl-group. B, Schematic diagram of synthetic route for compound 23.
8-(4′-(dimethylamino)phenyl)chromeno [7,8-d]imidazol-6(1H)-one displays increased TrkB stimulatory effect
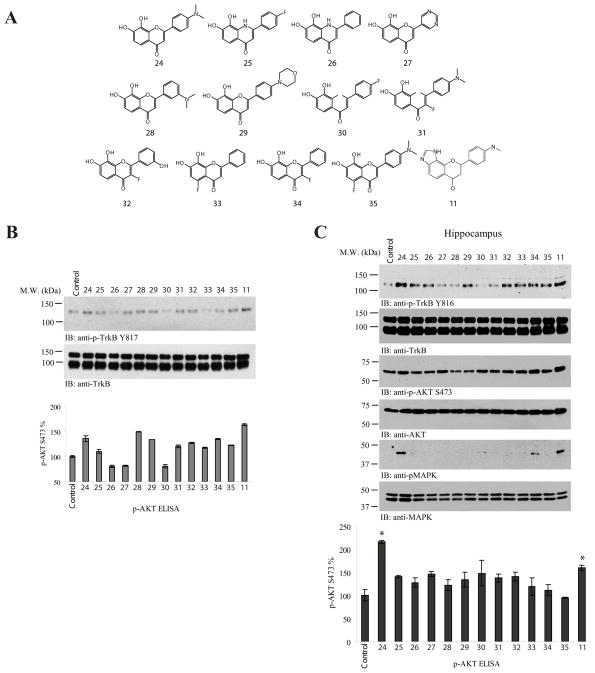
Our previous study shows that the electron donor dimethylamino group on 4′ position of B ring significantly elevates the agonistic effect. 12 Hence, we wanted to test 3′-dimethylamino or 4′-morpholino group’s effect on 7,8-DHF’s TrkB agonistic activity. Further, we wondered the effect of the electron-withdrawing group, fluoro, on 7,8-DHF or 4′-DMA-7,8-DHF’s agonistic activity (Figure 2A). To compare the TrkB activation by these compounds, we prepared primary cortical cultures and treated them with 500 nM of various compounds for 15 min and collected the cell lysates. Compound 11 exhibited stronger effect in triggering TrkB activation than the lead compound 4′-DMA-7,8-DHF (24 in Figure 2A). 3′-dimethylamino-7,8-DHF (28) or 4′-morpholino-7,8-DHF (29) exhibited comparable activity as the lead compound 24. Fluoride substitution at position 3 or 5 (32, 33, 34 and 35) did not significantly affect 7,8-DHF’s TrkB agonistic activity. Fluoride substitution at the 4′ position on B ring (30) inhibited its activity, which might be due to its electron-withdrawing effect. As we showed before,12 replacing an O atom with an N atom in the C ring (compound 25 and 26) diminished agonistic activity (Figure 2B, upper panel). The p-Akt ELISA results were similar to the TrkB activation pattern (Figure 2B, lower panel). To further explore these compounds’ effects on TrkB activation in mouse brain, we orally administrated 1 mg/kg of each compound and monitored TrkB’s activity at 4 h. As expected, the lead compound 24 clearly activated the TrkB receptor; 4′-dimethylamino-7,8-imidazole-flavone (11) also robustly activated TrkB. The rest of compounds displayed a similar effect as was observed by the in vitro assay (Figure 2C, top panel). Accordingly, the downstream p-Akt and p-MAPK signalings were activated by both compound 24 and 11. p-Akt ELISA analysis also correlated with the observations of p-TrkB immunoblotting (Figure 2C, bottom panel).
Figure 2. 8-(4-(dimethylamino)phenyl)chromeno [7,8-d]imidazol-6(1H)-one exhibits elevated TrkB stimulatory activity.
A, The chemical structures of various synthetic flavonoids. B, 8-(4- (dimethylamino)phenyl)chromeno [7,8-d]imidazol-6(1H)-one displays stronger TrkB stimulatory activity than the lead compound 24. Primary cortical cultures from E17 rat embryos were treated with 500 nM of various synthetic flavone derivatives for 15 min. The cell lysates (20 μg) were analyzed by p-TrkB immunoblotting (top panel) and p-Akt ELISA (bottom panel). The data were from two sets of replicated experiments (mean ± SEM). C, 8-(4-(dimethylamino) phenyl)chromeno [7,8-d]imidazol-6(1H)-one strongly activates TrkB receptor in mouse brain. One mg/kg of various indicated compounds were orally administrated into C57 BL/6J mice and TrkB phosphorylation and its downstream signaling cascades including Akt and MAPK in the hippocampus of mouse brain were analyzed by immunoblotting at 4 h. Compounds 11 and 24 displayed the strongest TrkB stimulatory effect (1st panel). The downstream p-Akt and p-MAPK activity coupled to the TrkB activation patterns (3rd and 5th panels). P-Akt 473 ELISA in drug treated mouse brain was analyzed (7th panel) (*: P<0.05 vs control; one-way ANOVA). The data were from two sets of replicated experiments (mean ± SEM).
8-(4′-(dimethylamino)phenyl)chromeno[7,8-d]imidazol-6(1H)-one is active in mouse models of depression with increased locomoter activity
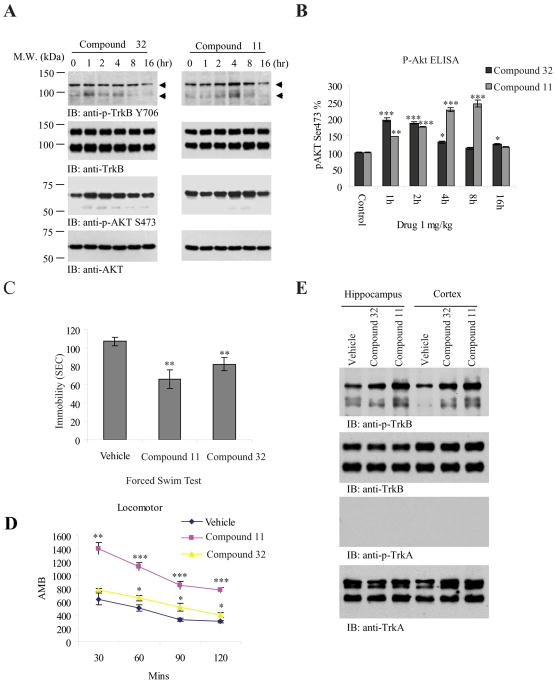
To gain insight into TrkB activation kinetics, 8-(4′-(dimethylamino)phenyl)chromeno[7,8-d]imidazol-6(1H)-one (11), was administered to C57BL6 mice at 1 mg/kg via oral gavage; For comparison, we employed compound 32 in the same procedure. The mouse brains were collected and TrkB activation and its downstream Akt signaling were analyzed by immunoblotting. Compound 11 activated TrkB receptor in a time-dependent manner peaking at 4 h and faded away at 16 h. The p-Akt signal was in alignment with the upstream p-TrkB activity. Compound 32 displayed less effect on TrkB activation than compound 11 (Figure 3A, top and 3rd panels). P-Akt ELISA correlated with p-Akt immunoblotting results for both compounds. Compound 11 gradually activated Akt and climaxed at 8 h, where the p-Akt signal was elevated by about 250% compared to the control. Compound 32 also elicited p-Akt activation peaking at 1 h, declining at 4 h and returning to the baseline at 8 h. The peak magnitude of Akt activation by compound 32 was significantly less than compound 11 (Figure 3B). Forced swim test (FST) is broadly used for screening potential antidepressant drugs and is widely used to measure antidepressant activity. The FST is a good screening tool with good reliability and predictive validity19, 20. To explore whether these compounds possess any antidepressant effect, we chronically treated C57/BL6 J mice with 5 mg/kg of the compound, once a day for 3 weeks. At the end of the treatment, we conducted the locomoter activity assay, followed by a forced swim test. We found that both compounds significantly reduced the immobility and the effect of compound 11 was more robust than compound 32 (Figure 3C). Nonetheless, compound 11 substantially augmented locomoter activity compared to compound 32 and vehicle control (Figure 3D). Immunoblotting with brain tissues from both cortex and hippocampus demonstrated that both compounds 11 and 32 clearly escalated TrkB phosphorylation after 3 weeks of drug treatment compared to vehicle control, but compound 11 displayed a stronger effect than compound 32. The TrkA receptor was not activated by any of these compounds (Figure 3E), demonstrating that they are TrkB receptor-specific agonists.
Figure 3. 8-(4-(dimethylamino)phenyl)chromeno [7,8-d]imidazol-6(1H)-one strongly activates TrkB and reduces the immobility in forced swim test.
A, Time course assay of 8-(4-(dimethylamino)phenyl)chromeno [7,8-d]imidazol-6(1H)-one. One mg/kg of compound (32) and compound (11) were orally administrated into C57 BL/6J mice and TrkB phosphorylation and its downstream signaling cascades including Akt in mouse brain were analyzed by immunoblotting at various time points. TrkB activation by compound 11 peaked at 4 h, whereas the maximal TrkB activation by compound 32 in mouse brain occurred at 1–2 h. Arrows indicate the p-TrkB in mature glycosylated or unglycosylated forms (1st panels). The downstream Akt activation pattern tightly correlated with the upstream TrkB activation (3rd panels). B, P-Akt S473 in drug-treated mouse brain was by ELISA using 20 μg brain lysates. (*: P<0.05, **: P<0.01, ***, P<0.001 vs control; one-way ANOVA). The data were from two sets of replicated experiments (mean ± SEM). C, Forced swim test with compound 11 and 32. The test (6 min, immobility recorded in the last 4 min) were performed in male C57BL/6J mice that have been orally administrated with 5 mg/kg compound 11, compound 32 or vehicle solvent saline for 21 days. Data are presented as mean ± SEM (n=6, **P<0.01 vs vehicle, Student t-test). D. Locomotor activity assay. Drug-treated mice as stated in C were subjected locomotor activity at day 22. Compound 11 but not 32 significantly increased the locomotor activity compared to vehicle control. Data are presented as mean ± SEM (n=6, *: P<0.05, ***P<0.001, two-way ANOVA). E. TrkB but not TrkA is activated by compound 11 and 32 in mouse brain. The brain lysates from chronic drug-treated mice were analyzed by immunoblotting with anti-p-TrkA 794 and p-TrkB 816.
2-Methyl-8-(4′-(pyrrolidin-1-yl)phenyl)chromeno[7,8-d]imidazol-6(1H)-one demonstrates greater potency than 8-(4′-(dimethylamino)phenyl)chromeno[7,8-d]imidazol-6(1H)-one
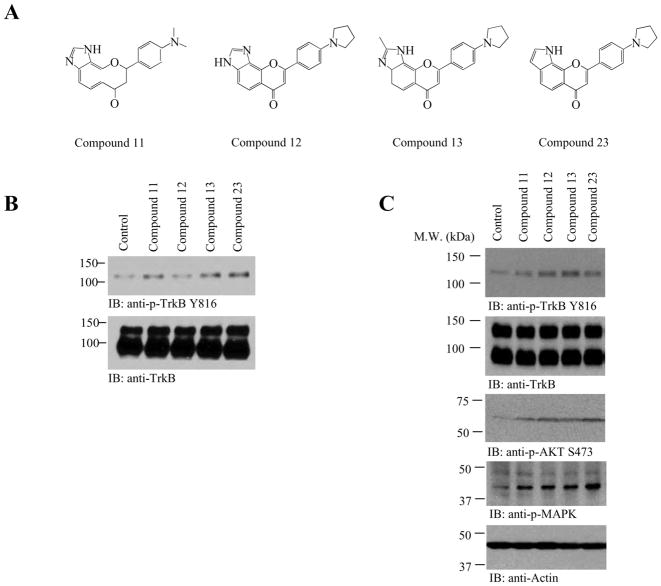
Though compound 11 possessed robust TrkB stimulatory effect and reduces immobility in forced swim test, it escalated locomotor activity after 3 weeks of administration. To alleviate this concern, we synthesized several imidazole or indole-substituted flavonoid compounds (Figure 4A). Immunoblotting and p-Akt ELISA analysis demonstrated that both compounds 13 and 23 displayed higher activity than compound 11 in triggering TrkB and Akt activation in primary neurons (Figure 4B). We made similar observations about TrkB receptor and Akt activation in mouse brain 2 h after oral administration of 1 mg/kg compounds (Figure 4C). Hence, we chose to focus on compound 13 and 23 to examine their antidepressant effect in both forced swim test and tail suspension test assays.
Figure 4. 2-Methyl-8-(4-(pyrrolidin-1-yl)phenyl)chromeno[7,8-d]imidazol-6(1H)-one (compound 13) triggers TrkB activation in primary neurons and mouse brain.
A, Chemical structures of various synthetic 4′-pyrrolidino-flavone derivatives. B, 2-methyl-8-(4-(pyrrolidin-1-yl)phenyl)chromeno[7,8-d]imidazol-6(1H)-one (compound 13) triggers TrkB activation in primary neurons. Rat primary neurons were treated with 500 nM various compounds for 15 min. Cell lysates (20 mg) were analyzed with various antibodies as indicated. C, 2-methyl-8-(4-(pyrrolidin-1-yl)phenyl)chromeno[7,8-d]imidazol-6(1H)-one (compound 13) triggers TrkB activation in mouse brain. One mg/kg of various compounds were orally administrated into C57 BL/6J mice and TrkB phosphorylation (1st panel) and its downstream signaling cascades including Akt and MAPK in the hippocampus of mouse brain were analyzed by immunoblotting at 2 h. The downstream p-Akt and p-MAPK activity coupled to the TrkB activation patterns (3rd and 4th panels).
2-Methyl-8-(4′-(pyrrolidin-1-yl)phenyl)chromeno[7,8-d]imidazol-6(1H)-one demonstrates antidepressant-like profile without altering locomoter activity
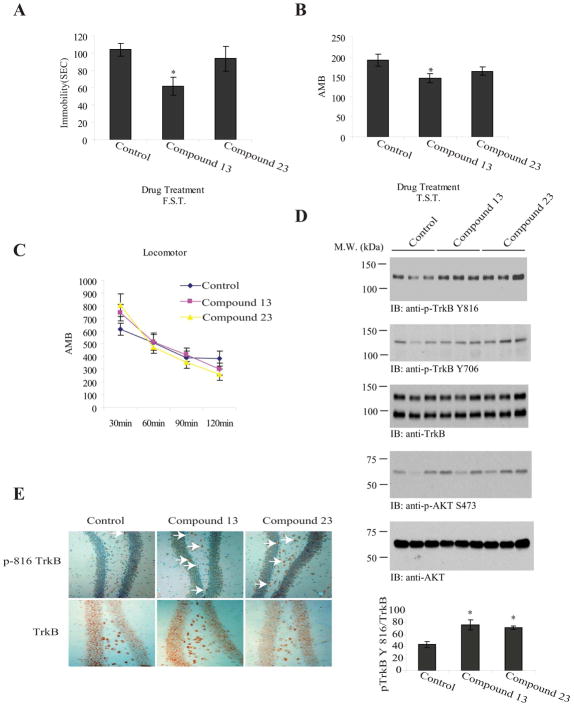
Next, we treated the mice with compounds 13 and 23 at dosage of 2.5 mg/kg via oral gavage once a day for 3 weeks and to explore their effect on locomotor activity. FST showed that compound 13 significantly reduced the immobility by 45% compared to vehicle control; by contrast, compound 23 had no effect (Figure 5A). The tail suspension test (TST) has become one of the most widely used models for assessing antidepressant-like activity in mice. The test is based on the fact that animals subjected to the short-term, inescapable stress of being suspended by their tail, will develop an immobile posture. Various antidepressant medications reverse the immobility and promote an escape-related behavior.21 We made similar observations of compound 13 significantly reducing the immobility versus vehicle control, whereas compound 23 lacked efficacy (Figure 5B). Further, locomotor activity analysis revealed that neither compound 13 nor 23 altered the motion activity compared to vehicle control (Figure 5C). Immunoblotting using both anti-p-TrkB Y816 and anti-p-TrkB Y706 antibodies with brain tissues demonstrated that both compounds 13 and 23 markedly activated TrkB. P-Akt immunoblotting also correlated with the upstream TrkB activation (Figure 5D, top, 2nd and 4th panels). Quantitative p-Akt ELISA assay supported that both compounds notably activated Akt, fitting with the observations by Western blotting (Figure 5D, bottom panel).
Figure 5. 2-methyl-8-(4-(pyrrolidin-1-yl)phenyl)chromeno[7,8-d]imidazol-6(1H)-one (compound 13) triggers TrkB activation in mouse brain and exhibits antidepressant effect.
A, Forced swim test (6 min, immobility recorded in the last 4 min) was performed in male C57BL/6J mice that have been orally administrated with 2.5 mg/kg compound 13, 23 or vehicle solvent saline for 21. Compound 13 but not compound 23 significantly decreased the immobility. Data are presented as mean ± SEM (n=8, *: P<0.05, Student’s t-test). B, Tail suspension test. The drug-treated mice were subjected tail suspension assay. Compound 13 but not 23 reduced the immobility. Data are presented as mean ± SEM (n=8, *: P<0.05, Student’s t-test). C. Locomotor activity assay. None of the tested compounds significantly altered the locomotor activity. D. Both compounds 13 and 23 activate TrkB and its downstream signaling cascades. 2.5 mg/kg of various compounds were orally administrated into C57 BL/6J mice and TrkB phosphorylation and its downstream effector Akt activation in the hippocampus were analyzed by immunoblotting after behavioral tests. Both compound 13 and 23 evidently elevated TrkB phosphorylation (1st panel). The downstream p-Akt activity was also upregulated by compounds 13 and 23 (4th panel). The ratio of P-TrkB/total TrkB in drug-treated mouse brain was analyzed (6th panel). The data were from two sets of replicated experiments and are expressed as mean ± SEM (*, P<0.05 vs control, Student’s t-test). E, Both compound 13 and 23 elevated TrkB phosphorylation in hippocampus. The chronic drug-treated mice were perfused and the brain sections were stained with anti-p-TrkB 816 and anti-TrkB antibodies. The p-TrkB activated neurons were labeled with white arrows.
Immunohistochemistry staining with anti-p-TrkB demonstrated that TrkB was activated by these two chemicals in dentate gyrus after 3 weeks of treatment (Figure 5E, white arrows). Therefore, chronic treatment with compound 13 promotes TrkB activation in the hippocampus of mice and exhibits potent antidepressant-like profile.
In vitro ADMET profiles of the imidazole derivatives
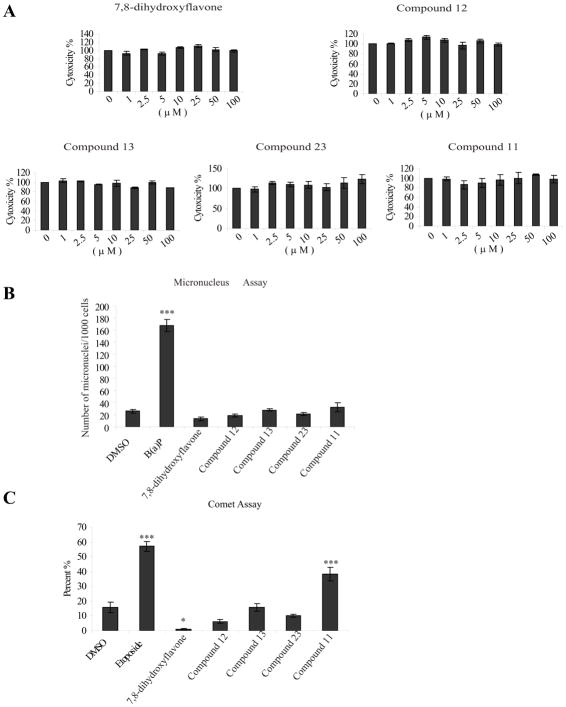
7,8-DHF and other flavonoids have recently been shown to possess impressive anti-genotoxic effects against DNA lesions and micronuclei induced in human hepatocellular carcinoma cells, HepG2, by a potent mutagen and carcinogen benzo[a]pyrene (B(a)P) 22. In order to gain insight into the liabilities of the drug candidates, we examined their in vitro toxicity. An LDH cytotoxicity assay, using HepG2 cells treated with various compounds for 24 h, demonstrated that none of the tested compounds induced noticeable cytotoxicity even up to 100 μM, suggesting that these compounds do not trigger cell death even at very high concentrations (Figure 6A). Micronuclei formation is an important endpoint in genotoxicity study. To assess whether these synthetic compounds possess any potential carcinogenicity, we conducted the micronucleus assay by treating HepG2 cells with 50 μM of various compounds for 24 h, followed by DAPI staining. Quantitative analysis revealed that none of the tested compounds exhibited significant effect, whereas the positive control B(a)P robustly induced micronuclei (Figure 6B). Next, we performed a Comet assay with 100 μM drug-treated HepG2 cells. As a positive control, we chose etoposide, a topoisomerase inhibitor, which causes DNA strand breaks. Interestingly, compound 11 but not other synthetic derivatives induced DNA lesions (Figure 6C). Hence, these toxicity experiments support that compounds 12, 13 and 23 possess negligible cytotoxicity or genotoxicity.
Figure 6. In vitro cytotoxicity and genotoxicity assay.
A, Cytotoxicity assay in hepatocyte HepG2 cells. HepG2 cells were treated with various concentrations of flavonoids for 24 h. The drug-treated cells were subjected LDH assay. Data are presented as mean ± SEM. (n=3). B, Micronuclei assay in hepatocyte HepG2 cells. HepG2 cells were treated with 50 μM of various compounds for 24 h. The nuclei were stained with DAPI and analyzed under a fluorescent microscope. Data are presented as mean ± SEM. (n=3, ***P<0.001 vs vehicle, One-way ANOVA). C, Comet assays. HepG2 cells were treated with 100 μM of various compounds for 24 h. The percentage of lesion DNA in tail was used as a parameter for measurement of DNA damage. Data are presented as mean ± SEM. (n=3, ***P<0.001 vs vehicle, One-way ANOVA).
To explore these compounds’ in vitro ADMET profiles, we conducted numerous in vitro assays. Human liver microsomal stability assay showed that after 1 h incubation, compound 23 had 1.2% remaining, whereas compound 13 had 25.4% unchanged, supporting that compound 13 is more metabolically stable than compound 23. Reactive metabolic screening assays support that compound 13 is quite stable in vitro, whereas compound 23 formed numerous adducts (Table 2 & 3). CYP screen inhibition demonstrated that at 3 μM, CYP1A2 was inhibited about 27.3% by compound 13, whereas compound 23 inhibited 37.4%. At 30 μM, the inhibition patterns contained similar trends. The detailed inhibition data are summarized in Table 4. To test the possible cardiovascular toxicity, we examined hERG inhibition triggered by compound 13. The hERG inhibition assay revealed that compound 13 did not possess any concentration-dependent inhibition activity of hERG. A human plasma protein binding experiment showed that both compounds 13 and 23 displayed approximately 99.9% of protein binding (Table 5). Hence, compound 13 possesses a much more preferable in vitro ADMET profile than compound 23, fitting with its remarkable in vivo antidepressant efficacy.
Table 2.
Summary of microsomal stability screening
| Compound | Test conc (μM) | Test species | Mean remaining parent with NADH (%) | Mean remaining parent NADPH- free (%) |
|---|---|---|---|---|
| 13 | 1 | Human | 25.4 | 92.2 |
| 23 | 1 | Human | 1.2 | 79.6 |
Table 3.
Summary of reactive metabolite identification
| Compound | Scan | Potential Reactive Metabolites Identified | m/zb | Comment |
|---|---|---|---|---|
|
| ||||
| 13 | Precursor | No | No adduct detected | |
| Neutral loss | No | |||
|
| ||||
| 23 | Precursor | Yes | 627, 643 | |
| Neutral loss | Yes | 605, 629, 659, 675 | ||
Table 4.
Summary of CYP screening
| Compound | Test conc (μM) | CYP3A4- Modaxolam | CYP3A4- Testoterone | CYP2C9 | CYP2C19 | CYP2D6 | CYP1A2 |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 13 | 30 μM | 40.5% | 43.3% | 73.6% | 57.5% | 32.0% | 66.1% |
| 3 μM | 10.8% | 4.5% | 12.4% | 11.1% | 8.7% | 27.3% | |
|
| |||||||
| 23 | 30 μM | 48.1% | 18.7% | 52.9% | 90.0% | 81.6% | 75.9% |
| 3 μM | 3.9% | 3.4% | 11.9% | 16.2% | 7.2% | 37.4% | |
Table 5.
Summary of plasma protein binding
| Compound | Test conc (μM) | Test species | Fuplasma (%) | Mean plasma fraction bound (%) | Recovery (%) | Binding classification |
|---|---|---|---|---|---|---|
| 13 | 5 μM | Human | 0.03 | 99.9 | 86.3 | High Binding |
| 23 | 5 μM | Human | 0.07 | 99.9 | 97 | High Binding |
To investigate whether compound 13 possesses an improved in vivo PK profiles compared to the lead compound 24, we conducted in vivo pharmacokinetic studies in mice. The PK parameters are summarized in Table 1. For the lead compound 24, we employed the dosage of 1 mg/kg via i.v. injection and 5 mg/kg for P.O. route. At 1 mg/kg, the in vivo half-life, t ½, of the lead compound 24 in circulation was around 9 min and AUClast was about 5930 (min*ng/ml), but the bioavailability of the lead compound 24 was not measurable at 5 mg/kg (Table 1 & 6). Thus, we increased the doses of the tested compound 13. The t ½ for compound 13 was about 103 min with AUClast approximately 18746 (min*ng/ml) at a dose of 3 mg/kg. Moreover, the bioavailability was around 2% at a 10 mg/kg dosage. Hence, these data demonstrate that compound 13 may possess elevated PK profiles compared to the lead compound 24.
Discussion
In the current report, we show that the synthetic 2-methyl-8-(4-(pyrrolidin-1-yl)phenyl)chromeno [7,8-d]imidazol-6(1H)-one (compound 13) possesses improved in vitro ADMET features and is active in mouse models of depression. Moreover, in the mouse brain hippocampus, this compound activates the TrkB receptor and exerts robust antidepressant effects in both FST and TST assays. Therefore, our study demonstrates that this compound is a potential candidate for in-depth drug development. Molecular modeling also supports that 7,8-DHF and compound 13 might bind to the LRR motif on TrkB ECD (Supplemental Figure 1), fitting with our previous results found with in vitro binding assay 10, 11. This approach may shed light on our future drug design for further improving the lead compound, if the co-crystal (agonist/TrkB ECD) structure is experimentally resolved.
Our previous structure-activity relationship (SAR) study demonstrates that the 7,8-dihydroxy groups on the A ring and the middle heteroatomic Chromen-4-one C ring are essential for the TrkB stimulatory effect. Additionally, the 4′-position on the B ring is also critical for the agonistic effect. An electron-withdrawing group, such as F, or an electron-donating OH at this position suppresses the activity. Nonetheless, replacement with a dimethylamino- or pyrrolidino- group yields the desired activity. Based on the lead compound 4′-DMA-7,8-DHF, we initiated a lead optimization campaign by synthesizing a series of new compounds. Since the lead compound exhibits robust agonistic effect and potent anti-depressant efficacy, we employed the bioisosteric strategy to enhance the desired biological or physical properties of a compound without making significant changes in chemical structure. We added a fluoride group at different positions, and replaced the 7,8-dihydroxy groups with an imidazole ring or changed the dimethylamino group into a pyrrolidino ring. From compounds 31 and 32, we found that addition of a fluoride group barely affects TrkB stimulatory activity (Figure 2). Replacing the 7,8-dihydroxy groups with an imidazole ring in compound 11 elevated its agonistic activity compared to the lead compound 24, though the in vivo TrkB stimulatory activity remained comparable for these two compounds (Figure 2B & C). Although compound 11 strongly activated TrkB in mouse brain and decreased the immobility in FST, this compound highly augmented the locomotor activity as well (Figure 3), suggesting that it might reduce the immobility in FST by increasing the locomotor activity. Since dimethylamino group may be prone to metabolic demethylation, we replaced the dimethylamino group with a pyrrolidino group. Remarkably, compound 13 not only displayed higher agonistic activity than compound 11 but it also attenuated the locomotor enhancement effect by compound 11 (Figure 5). As expected, compound 13 was active in both FST and TST depression behavioral assays. It remains unclear why compound 23 exhibits potent TrkB stimulatory activity after oral administration but fails to show significant immobility reduction activity in either antidepressant behavioral assay. Conceivably, its high reactivity and poor microsomal stability account for its lack of an efficacious antidepressant effect (Table 2 & 3).
Catechol-related compounds usually possess poor pharmacokinetic profiles due to oxidation, glucuronidation, sulfation or methylation. For instance, catechol-containing Apomorphine, is a non-narcotic morphine derivative that acts as a potent dopaminergic agonist. Apomorphine metabolism occurs through several enzymatic pathways, including N-demethylation, sulfation, glucuronidation, and catechol-O-methylation as well as nonenzymatic oxidation.17 L-DOPA is the primary component of Parkinson’s disease (PD) therapy; this drug is usually administered orally, but it is extensively metabolized in the gastrointestinal tract, so relatively little circulates in the bloodstream as intact L-DOPA. To minimize the conversion to dopamine outside the central nervous system, L-DOPA is usually given in combination with peripheral inhibitors of Aromatic L-Amino Acid Decarboxylase and COMT (catechol methyltransferase) inhibitor 18. Our preliminary in vivo metabolism study shows that 7,8-DHF is also subjected to oxidation, glucuronidation, sulfation and methylation (Supplemental Figure 2). Among the modifications, glucuronidation and sulfation are mainly responsible for the in vivo clearance of the flavonoids. In addition, we have detected the O-methylated metabolites in both the plasma and brain samples after oral administration. Conceivably, these modification pathways may explain the relatively short half-life of 7,8-DHF and its synthetic derivatives. Alteration of this labile group into the bioisosteric imidazole derivatives escalates the PK profiles in compound 13 (Table 1). Though compound 13 displays an improved ADMET profile than the lead compound 24, its oral bioavailability remains poor and the in vivo half-life is relatively short. Additional medicinal chemistry is needed to further optimize the lead compound and improve the PK parameters.
Among the leading causes of drug candidate failure (~60%) are poor pharmacokinetics (PK)/ADME, toxicological properties and adverse effects, which contribute more significantly than ‘lack of efficacy’ (~30%). In order to optimize the ADME properties and also foresee liabilities of drug candidates, in vitro and in vivo ADMET (ADME plus toxicity) filters are being developed and implemented in various stages of the drug discovery and development process to alert to potential ADMET issues in the clinic.23 Toxicology- and pharmacology-related safety has become a leading cause for compound failure during preclinical development and clinical trials. The most withdrawals of marketed drugs were associated with hERG inhibition 24, 25 and hepatotoxicity 26. Accordingly, we have examined our compounds’ in vitro ADMET profiles. Remarkably, compound 12, 13 and 23 exhibited negligible cytotoxicity or genotoxicity (Figure 6). The hERG inhibition index is also trifling for compound 13. Moreover, the microsomal stability and reactive metabolic screening assays support that compound 13 is more stable than compound 23 in vitro. Though compound 13’s in vivo half-life is about 103 min in mice, its oral bioavailability is only 2%. Nonetheless, it exhibited a much improved in vivo PK profile than the lead compound 24 (Table 1 & 6), demonstrating our progress in the optimization mission. It is worth noting that there is significant discrepancy between the remarkable therapeutic efficacy in the mouse models of antidepression and poor in vivo oral bioavailability. The potential explanation for this difference might be the compound’s extreme potency, so that a low concentration in the circulation system is sufficient for provoking the physiological effects. It is also possible that the metabolites of the compound are active as well. For instance, the B ring in compound 13 can be metabolized via hydroxylation in the lead compound 24 and compound 13. Our previous study shows that hydroxylation on 2′ or 3′ position escalates 7,8-DHF’s agonistic activity 12. On the other hand, it remains unclear how 4′-DMA-7,8-DHF, which has a short half-life, exerts its physiological activities in animals. Conceivably, its O-methylated or B-ring hydroxylated metabolites in mouse brain might contribute to these actions. Clearly, further medicinal chemistry is necessary to continue developing compound 13 to meet the preclinical standards for a promising candidate. Specifically, we will determine the potential metabolites, which may shed light on how to metabolically stabilize this compound. Further, we will reduce the candidate’s plasma protein binding affinity, so that we can improve its brain bioavailability. Plausibly, the re-synthesized novel compounds will possess longer t1/2 and much-improved oral bioavailability with low toxicity. Together, our data support that 7,8-imidazole and 4′-pyrrolidone-substituted flavones are excellent lead compounds justifying further medicinal modification. These compounds not only provide a novel tool to dissect the biological functions of BDNF/TrkB signaling but also act as good lead compounds with great potential for future drug development for various neurological diseases, including depression.
Experimental Procedures
Cells, Reagents, and Mice
Anti-p-TrkB 817 was from Epitomics. Anti-p-TrkB 706 was from Santa Cruz. Anti-TrkB antibody was from Cell signaling. The wild-type C57BL/6 mice were bred in a pathogen-free environment in accordance with Emory Medical School guidelines. All chemicals not included above were purchased from Sigma. 7,8-DHF was purchased from TCI. The synthesis of compound 24–27 were reported in our previous study 12. The fluoro-substituted flavonoids in Figure 2A (compound 25, 30, 31, 32, 33, 34 and 35) were from Sundia (Shanghai, China). Compound 28 and 29 in Figure 2A were provided by NIMH, Chemical Synthesis and Drug Supply Program at RTI. NMR spectrum (Bruker AV300K, 300 MHz), MS spectrum (Shimadzu LCMS), HPLC (PE, dual pumper, SPD detector, ODS-C18 reverse phase, 254 nm, CH3CN-H2O-0.1%TFA). Phospho-TrkB Y816 antibody was described before 12. This phospho-TrkB was utilized for immunostaining the brain sections. Anti-TrkB (Cell Signaling, which recognizes both full-length and truncated TrkB) was used for immunoblotting. P-Akt 473 Sandwich ELISA was from Cell Signaling. BDNF was from Peprotech. Anti-phospho-TrkA 794, anti-TrkA, Phospho-Akt-473, anti-Akt, and Anti-phospho-Erk1/2 antibodies were from Cell Signaling.
Synthesis of compounds 11, 12, 13 and 23
4-Aminomethyl benzoate (2)
To a solution of compound 1 (6.857 g, 50 mmol) in MeOH (120 mL) was treated with SOCl2 (7 g, 59 mmol, 1.2 eq.) at −10°C and the mixture was stirred at room temperature overnight. LC-MS and TLC showed the reaction was over and the desired product was found. The mixture was concentrated and the residue was washed with Et2O to give compound 2 as a pale-yellow solid (with purity ~ 95% by HPLC, 6.0 g, used for the next step without 1HNMR confirmed).
Methyl 4-Pyrrolidin-1-ylbenzoate (3)
A mixture of compound 2 (3.02 g, 20 mmol), K2CO3 (5.52g, 40 mmol) and 1,4-dibromobutane (5.18 g, 24 mmol) in dioxane (30 mL) and water (30 mL) was treated with TBAB (t-Butylamine Borane) and the mixture was refluxed for 48 h. LC-MS and TLC showed the reaction was not over and the desired product was found. The mixture was concentrated and the residue was diluted with EA (ethyl acetate) and washed with water and brine, dried over Na2SO4. Concentrated and purified by silica gel (PE (petroleum ether)~PE:EA=5:1~3:1) to give compound 3 as a pale-yellow solid (with purity > 95% by HPLC, 1.12 g, confirmed by 1HNMR). 1HNMR (400 MHz, DMSO-d6): δ 7.82 (d, J = 8.8 Hz, 2H), 6.61 (d, J = 8.8 Hz, 2H), 3.81 (s, 3H), 3.36 (m, 4H), 2.02 (m, 4H).
4-(Pyrrolidin-1-yl)benzoic acid (4)
A mixture of compound 3 (1.12 g, 5.46 mmol) and NaOH (1.1g, 27.3 mmol) in MeOH (15 mL) and water (15 mL) was refluxed overnight. LC-MS and TLC showed the reaction was over and the desired product was found. The mixture was concentrated and the residue was acidified with 6 N HCl to pH~3 and the precipitate was collected by filtration and dried with vacuum to give compound 4 as a white solid (with purity >95% by HPLC, 600 mg, confirmed by 1HNMR). 1HNMR (400 MHz, DMSO-d6): δ 12.03 (br s, 1H), 7.74 (d, J = 8.8 Hz, 2H), 6.54 (d, J = 8.4 Hz, 2H), 3.30 (m, 4H), 1.97 (m, 4H).
4-(Pyrrolidin-1-yl)benzoyl chloride (5)
To a suspension of compound 4 (115 mg, 0.6 mmol) in DCM (2 mL) at 0°C was added DMF, followed by (COCl)2 (91 mg, 0.72 mmol) and the mixture was stirred at room temperature for 2 h and got a clear solution. TLC (quenched by MeOH) showed the reaction was over. The mixture was concentrated and the residue was added with toluene and then concentrated twice to give compound 5 as a pale-yellow solid, which was used for the next step without further purification.
8-(4′-(Dimethylamino)phenyl)chromeno[7,8-d]imidazol-6(1H)-one (11) 3-acetamido-6-acetyl-2-nitrophenyl 4-(dimethylamino)benzoate (8)
To a mixture of N-(4-acetyl-3-hydroxy-2-nitrophenyl)acetamide (compound 6, 1g, 4.1 mmol, 1.0eq) and triethylamine(1.5mL) was added 4-(dimethylamino)benzoyl chloride (compound 7 with R: dimethylamino-; 6.3 mmol) in 3 portions at 0°C. Then the mixture was stirred at room temperature for 3 h. Diluted with dichloromethane (100 mL), washed with 1N HCl (100 mL) and water (50 mL). The organic phase was separated, dried with sodium sulfate, filtered and concentrated to afford gray solid, which was purified (PE/EA = 1/1) to afford (compound 8, 1.2g, yield: 75% with purity > 95% by HPLC).
7-Amino-2-(4′-(dimethylamino)phenyl)-8-nitro-4H-chromen-4-one (9)
A mixture of 3-acetamido-6-acetyl-2-nitrophenyl 4-(dimethylamino)benzoate (compound 8, 2 g,) and potassium hydroxide (8 g) in pyridine (20 mL) was heated to 60°C for 1 h and poured into icy 1N HCl (100 mL). The yellow solid was collected and dissolved in acetic acid (20 mL) and concentrated sulfuric acid. The resulting mixture was heated to 110°C for 30 min. The mixture was cooled to rt and poured into saturated sodium carbonate. The yellow solid was filtered and dried in vacuo to afford 7-amino-2-(4-(dimethylamino)phenyl)-8-nitro-4H-chromen-4-one (compound 9 with R: dimethylamino-) (1.5 g, yield: 89% with purity > 95% by HPLC)
7,8-Diamino-2-(4′-(dimethylamino)phenyl)-4H-chromen-4-one hydrochloride (10)
A solution of 7-amino-2-(4-(dimethylamino)phenyl)-8-nitro-4H-chromen-4-one (compound 9, 900 mg, 2.77 mmol) and 10% Pd/C (450 mg)in methanol (9 mL) and concentrated hydrochloride (aqeous, 9 mL) was stirred at the atmosphere of hydrogen overnight. The solid was filtered and the filtrate was evaporated at reduced pressure to afford 7,8-diamino-2-(4′-(dimethylamino)phenyl)-4H-chromen-4-one hydrochloride (compound 10) as a light yellow solid (810 mg, yield: 88% with purity > 95% by HPLC).
8-(4′-(Dimethylamino)phenyl)chromeno[7,8-d]imidazol-6(1H)-one (11)
A solution of 7,8-diamino-2-(4′-(dimethylamino)phenyl)-4H-chromen-4-one hydrochloride (compound 10, 500 mg) in HCO2H (5 mL) was heated to reflux for 1 h. The volatiles were evaporated in reduced pressure and the residue partitioned between EA/isopropanol = 20/1 (50 mL) and saturated sodium carbonate (25 mL). The organic phase was separated, dried with sodium sulfate, filtered and concentrated to afford yellow solid, which was recrystallized from EA (25 mL) to afford light yellow solid (compound 11 with R: dimethylamino-, 111 mg, yield: 24%). 1H NMR (300 MHz, DMSO-d6) δ 8.46(m,1H),10.09 (br s,1H), 8.01(m, 2H), 7.82 (m, 1H),7.61 (m, 2H), 6.84~6.88(m, 3H), MS-ESI: calculated: 305; found: 306(M+H)+. HPLC: 99.23%
8-(4′-(Pyrrolidin-1-yl)phenyl)chromeno[7,8-d]imidazol-6(1H)-one (12 and 13)
To a solution of compound N-(4-acetyl-3-hydroxy-2-nitrophenyl) acetamide (compound 6, 700 mg, 2.94 mmol) in dry DCM (dichloromethane,10 mL) was added DIPEA (N,N-Diisopropylethylamine, 0.8 mL) and followed by compound 7 4-(pyrrolidin-1-yl)benzoyl chloride (950 mg) at 0°C and the resulting mixture was stirred at room temperature overnight. The mixture was quenched with water and extracted with dichloromethane, the combined extracts were washed with water and brine, dried over Na2SO4. Concentrated and purified by silica gel (PE~ PE:EA = 10:1 ~ 7:1~5:1~3:1) to give the coupled ester compound as an orange solid (750 mg), which was then followed the similar procedures as described for compound 11. After cyclization with KOH/pyridine, followed by H2SO4/AcOH reflux, the nitro group in intermediate was reduced with Fe (300 mg, 5.36 mmol) and NH4Cl (154 mg, 2.85 mmol) to yield the reduced 7,8-diamino- compound 10 with R: pyrrolidin-1-yl-. A mixture of compound 7,8-diamino-2-(4′-(N-pyrrolidino-)phenyl)-4H-chromen-4-one hydrochloride (1.1 g) in HCOOH (10 mL) was refluxed for 2 h. TLC showed the reaction was over. Combined with other batches, the mixture was basified to pH~8 by 1 N NaOH, the mixture was then concentrated and purified by silica gel (dichloromethane~dichloromethane:MeOH = 100 : 1 ~ 50 : 1~ 20 : 1) to give a mixture of compound 12 and 2-methylated compound 13 (80 mg), which was purified by pre-HPLC to give compound 12 as orange solid (50 mg, confirmed by 1HNMR); 1HNMR (400 MHz, CD3OD): δ 8.81 (s, 1H), 8.04 (m, 3H), 7.68 (d, J = 8.8 Hz, 1H), 6.78 (s, 1H), 6.66 (d, J = 8.8 Hz, 2H), 3.37 (m, 4H), 2.07 (m, 4H); HPLC: 96%; MS-ESI: calculated 331.4; found 332.1 (M+1)+, and compound 13 as dark-orange solid (25 mg, confirmed by 1HNMR). 1HNMR (400 MHz, CD3OD): δ 8.09 (d, J = 8.4 Hz, 1H), 8.01(d, J = 8.4 Hz, 2H), 7.64 (d, J=8.4 Hz, 1H), 6.78 (s, 1H), 6.69(d, J = 8.8 Hz, 2H), 3.41 (m, 4H), 2.92 (s, 3H), 2.10 (m, 4H); HPLC: 94%; MS-ESI: calculated: 345.4; found: 345.9 (M+1)+.
8-(4′-(Pyrrolidino-)phenyl)chromeno[7,8-d]indoloyl-6(1H)-one (23)
Methyl 2-methoxy-4-methylbenzoate (15)
A mixture of compound 14 (10 g, 65.73 mmol), CH3I (37.32 g, 262.9 mmol) and K2CO3 (45.4 g, 329 mmol) in dry DMF (100 mL) was stirred at room temperature overnight. TLC showed the reaction was over. The mixture was filtered and the filtrated was concentrated, the residue was diluted with EA, washed with water and brine, dried over Na2SO4. Concentrated to give compound 15 as yellow oil (with purity >95% by HPLC, 12 g, confirmed by 1HNMR, 100% yield). 1HNMR (400 MHz, CDCl3): δ 7.71 (d, J = 8.4 Hz, 1H), 6.78 (m, 2H), 3.89 (s, 3H), 3.87 (s, 3H), 2.38 (s, 3H).
Methyl 2-methoxy-4,5-dimethylbenzoate (16)
To a solution of compound 15 (10.0 g, 55.5 mmol) in dry DMF (80 mL) was added NCS (8.15 g, 61 mmol) in portions and the resulting mixture was stirred at room temperature overnight. The mixture was concentrated and the residue was dissolved in EA, washed with water and brine, dried over Na2SO4. Concentrated to give compound 16 as a yellow solid (with purity >95% by HPLC, 11.0 g, confirmed by 1HNMR). 1HNMR (400 MHz, CDCl3): δ 7.78 (s, 1H), 6.83 (s, 1H), 3.88 (m, 6H), 2.39 (s, 3H).
Methyl 2-methoxy-4,5-dimethyl-3-nitrobenzoate (17)
To a solution of compound 16 (5 g, 23.3 mmol) in concentrated H2SO4 (23 mL) at 0°C was added HNO3 (2.3 mL) in portions and the resulting mixture was stirred at 0°C for 2 h. TLC showed the reaction was over. The mixture was poured into ice and extracted with Et2O, the combined extracts were washed with water and brine, dried and concentrated to give compound 17 as a yellow oil(with purity >95%, 4.5 g, confirmed by 1HNMR). 1HNMR (400 MHz, CDCl3): δ 7.99 (s, 1H), 3.92 (m, 6H), 2.33 (s, 3H).
(E)-Methyl 4-(2-(dimethylamino)vinyl)-2-methoxy-5-methyl-3-nitrobenzoate (18)
A mixture of compound 17 (2.6 g, 10 mmol) in dry DMF(10 mL) was treated with Dimethylformamide Dimethylacetal (DMF-DMA) (3.58 g, 30 mmol) and the mixture was stirred at 120°C overnight. TLC showed the reaction was over. The mixture was cooled and concentrated, the residue was dissolved in EA, washed with water and brine, dried over Na2SO4. Concentrated to give crude as a brown semi-solid (compound 18 with purity >95%, 3 g, used for the next step without further purification).
Methyl 7-methoxy-1H-indole-6-carboxylate (19)
A mixture of compound 18 (3.1 g, 11.5 mmol) and Pd(OH)2 (160 mg) in MeOH (20 mL) was stirred at room temperature under H2 overnight. TLC showed the reaction was over. The mixture was filtered and concentrated and purified by silica gel (PE~PE:EA=10:1~5:1) to give compound 19 as a white solid (with purity > 95%, 1. 66 g, confirmed by 1HNMR). 1HNMR (400 MHz, CDCl3): δ 8.61 (br s, 1H), 7.64 (d, J = 8.4 Hz, 1H), 7.38 (d, J = 8.4 Hz, 1H), 7.34 (m, 1H), 6.59 (m, 1H), 4.04 (s, 3H), 3.94 (s, 3H).
7-Methoxy-1H-indole-6-carboxylic acid (20)
A mixture of compound 19 (1.66 g, 8.09 mmol) and NaOH (1.62 g, 40 .5 mmol) in MeOH (15 mL), THF (5 mL) and water (15 mL) was refluxed for 3 h. TLC showed the reaction was over. The mixture was cooled and concentrated, the residue was acidified to pH~2 by 2N HCl and the precipitate was collected by filtration and dried to give compound 20 as a off-white solid (with purity >95% by HPLC, 980 mg, confirmed by 1HNMR). 1HNMR (400 MHz, DMSO-d6): δ 12.41 (br s, 1H), 11.59 (s, 1H), 7.48 (s, 3H), 7.40 (d, J = 8.0 Hz, 1H), 7.31 (d, J = 8.4 Hz, 1H), 6.50 (s, 1H), 3.92 (s, 3H).
7-Methoxy-1H-indole-6-carbonyl chloride (21)
To a mixture of compound 20 (100 mg, 0.523 mmol) in dry THF (5 mL) was added DMF, followed by (COCl)2 (77 mg, 0.61 mmol) at 0°C and the resulting mixture was stirred at 0°C for 1 h. TLC showed the reaction was almost over. The mixture was concentrated to give compound 21 as a yellow solid, which was used for the next step without further purification.
2-(4-(Pyrrolidin-1-yl)phenyl)pyrano[3,2-g]indol-4(9H)-one (23)
To a solution of compound 4′-N-pyrrolidino-benzoyl methyl ketone (154 mg, 0.813 mmol) in dry THF(10 mL) at −20°C was added LiHMDS (2 mL, 2 mmol) and the mixture was stirred at −20°C for 1 h. Compound 21 7-methoxy-6-indoloyl chloride (200 mg, 0.976 mmol) with (COCl)2) in dry THF was added and the mixture was stirred at −20°C for 1 h then room temperature overnight. TLC and LC-MS showed the desired product was found. The mixture was quenched with NH4Cl aqueous and extracted with ethyl acetate (EA), the combined extracts were washed with water and brine, dried over Na2SO4. Concentrated and purified by silica gel (PE~PE:EA = 5:1~2:1) to give intermediate compound 22, which was refluxed in HBr (48%, 6 mL) for 3 h. TLC showed the reaction was over. Combined with other batches and cooled to room temperature, water was added and the mixture was basified to pH~8 by NaOH (1 N) and concentrated and purified by silica gel (dichloromethane~ dichloromethane:MeOH =20:1 ~10:1) to give crude compound 23 as a brown solid. This crude product was purified by pre-HPLC to give compound 23 as a brown solid. 1HNMR (400 MHz, DMSO-d6): δ 12.40 (br s, 1H), 8.16 (d, J = 8.8 Hz, 2H), 7.70 (s, 1H), 7.57 (s, 2H), 6.80 (s, 1H), 6.68 (m, 3H), 3.36 (m, 4H), 2.00(m, 4H); HPLC: 99%; MS-ESI: calculated: 330.4; found: 331.1 (M+1)+
Phospho-Akt S473 ELISA
PathScan Phospho-Akt1 (Ser473) Sandwich ELISA kit was purchased from Cell Signaling (Cat.# 7160). 100 μl sample in sample diluent (supplied in the kit) was added to each well and incubated overnight at 4°C. After 4x wash with 200ul wash buffer (supplied in the kit), 100ul/well detection antibody was added and incubated for 1 hour at 37°C. After 4x wash, 100 μl of HRP-linked secondary antibody (supplied in the kit) was added and incubated for 30 minutes at 37°C. After a final wash, 100 μl of TMB substrate was added to each well and incubated for 10 minutes at 37°C. The reaction was stopped by adding 100 μl/well stop solution. Record the values of each well using microplate reader at 450 nm and 650 nm. The optical density was determined by the subtraction of the reading at 650 nm from the reading’s at 450 nm.
TrkB Agonists Drug Administration
Male C57BL/6 mice aged of two months were administrated orally with 4′-DMA-7,8-DHF derivatives at a single dose of 1 mg/kg for 4 h. The control mice were injected with saline. The mice were sacrificed and brains were homogenated and ultracentrafuged. The supernatant (40 μg) was employed for SDS-PAGE and immunoblotting analysis with indicated antibodies, respectively. Male C57BL/6 mice aged of two months were administrated orally with compound (32) or (11) at the dose of 5 mg/kg/day each and compound 13 or 23 at the dose of 2.5 mg/kg/day each for 21 days. BrdU (100 mg/kg) was i. p. injected two hours before the TrkB agonist treated animals were sacrificed and the hippocampal section lysates were analyzed by immunoblotting with p-TrkB and total TrkB antibodies, p-AKT ELISA.
Immunohistochemistry Staining
Brain tissues were fixed in 4% paraformaldehyde overnight followed by paraffin embedding. Sections of 6 μm were cut. For immunohistochemical staining, brain sections were deparaffinized in xylene and rehydratedm in graded alcohols. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 5 min and all slides were boiled in 10 mM sodium citrate buffer (pH 6.0) for 10 min. Phosphorylated TrkB 816 and TrkB were detected using specific antibodies. Paraffin section were deparaffinized in xylene and rehydrated gradient ethanol solution. Samples were boiled in 10 mM sodium citrate buffer for 20 min for antigen retrieval purpose. Brain sections were incubated with anti-TrkB (BD Biosciences, San Jose, CA) 1:50, p-TrkB 1:300 dilution. Secondary antibody was applied using anti-rabbit-Alexa 594 (red), anti-mouse-fluorescein isothiocyanate (FITC) (green). DAPI (blue) was used for nuclear staining.
Forced Swim Test
Adult male mice (2–3 months old) were randomly submitted to a forced swim test without a preswim. Saline, TrkB agonists were orally administrated by gavage for 21 days. The mice were allowed to adapt to the test room for 2 days. The mice were placed in a clear glass cylinder with a diameter of 16 cm, half-filled with clear water at 24 °C (water depth of 14 cm did not allow the mice to reach the bottom of the cylinder) for a total of 6 min, and immobility was recorded during the last 4 min by an investigator blind to the treatment.
Tail suspension test
Mice will be individually suspended by the tail to a horizontal ring stand bar 30 cm above the floor using adhesive tape for 6 minutes and videotaped. Latency to immobility and time spent immobile will be scored for each mouse. Following the test, mice will be returned to their home cage. Immobility scores were compared by unpaired t test.
Locomotor activity
Locomotor activity was assessed using an automated system (San Diego Instruments, La Jolla, CA, USA) with photobeams that recorded ambulations (consecutive beam breaks). Drug-treated mice were placed in the locomotor chambers, and their activity was recorded for 2 h with 30 min interval.
Neurogenesis Analysis in TrkB Agonists-Treated Hippocampus
Adult male mice (2–3 months old) were orally administrated with saline, compound 13 and 23 (2.5 mg/kg) for 21 days. Then BrdU (100 mg/kg) was i.p. injected. In 2 h, the mice were perfused with 4% paraformaldehyde. Immunohistochemical staining was performed on formalin-fixed paraffinembedded sections. Sections from brain were cut, deparaffinized in xylene, and rehydrated in graded alcohols. The slides were boiled in 10 mM citric acid (pH 6.0) for 10 min, followed by an incubation in 2 N HCl for 10 min in room temperature. The slides were then permeabilized and blocked with 1% BSA in 0.2% PBS Tween-20 (PBST). The incorporated BrdU were stained using anti-BrdU-FITC (Abcam, USA) at 4 °C for 16 h. After three times of washing in PBS, the cells were then stained with DAPI for another 10 min at room temperature. The slides were finally mounted with AquaMount (Lerner Laboratories, USA) containing 0.01% 1,4-diazobicyclo(2,2,2)octane and examined under a fluorescence microscope.
Micronucleus assay
The cells treated with 7,8-DHF derivatives at 50 μM for 24 hrs were washed with PBS, incubated in mild hypotonic solution (0.075 M KCL/0.9% NaCl, 1:19) for 10 min at 37°C, and fixed with methanol-glacial acetic acid (3:1) for 15 min at 37°C, rinsed with distilled water and air dried. Fixed cells were stained with DAPI (2 μg/ml) for 30 min in the dark at room temperature. Cells were rinsed with PBS and distilled water, and mounted with Fluoromount-G (Southern Biotech). Micronuclei were identified based on the criteria specified by Miller at al. 27. One thousand cells per treatment were analyzed using the fluorescence microscope. Data are mean ± SEM of three independent experiments.
Single cell gel electrophoresis (SCGE, the comet assay)
The treated and control HepG2 cells embedded in 0.75% LMP (low melting point) agarose and spread on a base layer of 1% NMP (normal melting point) agarose in PBS buffer were placed in a lysis solution (2.5M NaCl, 200 mM Na2EDTA, 10 mM Tris-HCl, pH10 and 1% Triton X-100) at 4 °C for 2 h. Slides were transferred to an electrophoresic box and immersed in an alkaline solution (300 mM NaOH, 1 mM Na2EDTA, pH >13). After 40 min unwinding time a voltage of 25 V (300 mA) was applied for 30min at 4 °C. Slides were neutralized with 3×5 min washes with Tris-HCl (0.4 M, pH7.4), and stained with ethidium bromide (EtBr, 10 μg/ml). EtBr stained nucleotides were examined with fluorescence microscope. Total cell numbers in a field (>100) were counted and the number of nucleoids exhibiting comet tail formation was identified 22, 28. Results were quantified as the number of comet nuclei out of the total number of nuclei observed from 3 independent experiments.
In vitro ADMET and In vivo Pharmacokinetic studies
Twenty to thirty grams of mice with age of 6–8 weeks old were administrated with the indicated compounds i.v. or orally. At different time points (3, 10, 30, 60, 120, 240, 360 and 480 min), blood aliquots (300–400 ml) were collected in tubes coated with lithium heparin, mixed gently, then kept on ice and centrifuged at 2500 × g for 15 min at 4°C, within 1 h of collection. The plasma was then harvested and kept frozen at −20°C until further processing. For each time point, 3 mice/group were employed. The plasma samples were analyzed by HPLC. The in vitro ADMET assays were conducted in Apredica, Inc. The experimental conditions for the ADMET are available at www.apredica.com. The standard and control compounds for ADMET assays are included in Supplemental Table 1–4.
Homology modeling and docking of 7,8-DHF to TrkB ECD
A structure-guided sequence alignment between human TrkA ECD and human TrkB ECD was edited to accommodate the short deletions/insertions at the tips of the connecting loops between secondary structural elements. A monomeric structure of TrkA was selected from the crystal structure of the TrkA-Nerve growth factor complex (PDB ID: 2IFG) 29 as the template. Comparative modeling was carried out with the MODELLER program by satisfaction of spatial restraints 30. Docking of 7,8-DHF to the modeled TrkB ECD structure was carried out with MEDock based on a maximum entropy optimization algorithm 31. The top five probable solutions resulted from random seed searches, with lowest docked energies ranging from −9.3 to −8.7 kcal/mol, were selected for graphical analysis.
Supplementary Material
Acknowledgments
This work is supported by grant from National Institute of Health, NICCD (RO1 DC010204) to K. Ye. The authors wish to thank Andrei Halavaty for the help in molecular modeling. The authors are thankful to Dr. Obianyo at Ye laboratory for proof reading of the manuscript.
Abbreviation Used
- 7,8-DHF
7,8-dihydroxyflavone
- 4′-DMA-7
8-DHF, 4′-dimethylamino-7,8-dihydroxyflavone
- ECD
extracellular domain
- FITC
Fluorescein isothiocyanate
- DAPI
4′,6-diamidino-2-phenylindole
- NT-3
Neurotrophic factor 3
- FST
Forced Swim Test
- COMT
Catechol methyltransferase
- BDNF
Brain-derived neurotrophic factor
- NGF
Nerve growth factor
- TrkB
Tropomyosin-related kinase B
- MeCP2
methyl CpG binding protein 2
- TST
Tail suspension test
- LDH
Lactate dehydrogenase
Footnotes
Supporting Information Available: Two supplemental figures about 7,8-DHF metabolism and molecular modeling Four Supplemental Tables for the positive controls in the ADMET profiling. This material is available free of charge via the internet at http://pubs.acs.org
References
- 1.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 7.Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6:311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay RM. Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview. Philos Trans R Soc Lond B Biol Sci. 1996;351:365–373. doi: 10.1098/rstb.1996.0030. [DOI] [PubMed] [Google Scholar]
- 9.Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 10.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-DHF. Proc Natl Acad Sci U S A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang SW, Liu X, Chan CB, France SA, Sayeed I, Tang W, Lin X, Xiao G, Andero R, Chang Q, Ressler KJ, Ye K. Deoxygedunin, a natural product with potent neurotrophic activity in mice. PLoS One. 2010;5:e11528. doi: 10.1371/journal.pone.0011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Chan CB, Jang SW, Pradoldej S, Huang J, He K, Phun LH, France S, Xiao G, Jia Y, Luo HR, Ye K. A Synthetic 7,8-DHF Derivative Promotes Neurogenesis and Exhibits Potent Antidepressant Effect. J Med Chem. 2010;53:8274–8286. doi: 10.1021/jm101206p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devi L, Ohno M. 7,8-DHF, a Small-Molecule TrkB Agonist, Reverses Memory Deficits and BACE1 Elevation in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology. 2012;37:434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blugeot A, Rivat C, Bouvier E, Molet J, Mouchard A, Zeau B, Bernard C, Benoliel JJ, Becker C. Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J Neurosci. 2011;31:12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, Xie CW. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31:17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Johnson RA, Lam M, Punzo AM, Li H, Lin BR, Ye K, Mitchell GS, Chang Q. 7,8-DHF exhibits therapeutic efficacy in a mouse model of Rett syndrome. J Appl Physiol. 2012;112:704–710. doi: 10.1152/japplphysiol.01361.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeWitt PA. Subcutaneously administered apomorphine: pharmacokinetics and metabolism. Neurology. 2004;62:S8–11. doi: 10.1212/wnl.62.6_suppl_4.s8. [DOI] [PubMed] [Google Scholar]
- 18.Di Stefano A, Sozio P, Cerasa LS, Iannitelli A. L-Dopa prodrugs: an overview of trends for improving Parkinson’s disease treatment. Curr Pharm Des. 2011;17:3482–3493. doi: 10.2174/138161211798194495. [DOI] [PubMed] [Google Scholar]
- 19.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 20.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–55. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 21.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Kozics K, Valovicova Z, Slamenova D. Structure of flavonoids influences the degree inhibition of Benzo(a)pyrene - induced DNA damage and micronuclei in HepG2 cells. Neoplasma. 2011;58:516–524. doi: 10.4149/neo_2011_06_516. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Urban L, Bojanic D. Maximising use of in vitro ADMET tools to predict in vivo bioavailability and safety. Expert Opin Drug Metab Toxicol. 2007;3:641–665. doi: 10.1517/17425255.3.5.641. [DOI] [PubMed] [Google Scholar]
- 24.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 25.Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 26.Goldkind L, Laine L. A systematic review of NSAIDs withdrawn from the market due to hepatotoxicity: lessons learned from the bromfenac experience. Pharmacoepidemiol Drug Saf. 2006;15:213–220. doi: 10.1002/pds.1207. [DOI] [PubMed] [Google Scholar]
- 27.Miller BM, Pujadas E, Gocke E. Evaluation of the micronucleus test in vitro using Chinese hamster cells: results of four chemicals weakly positive in the in vivo micronucleus test. Environ Mol Mutagen. 1995;26:240–247. doi: 10.1002/em.2850260309. [DOI] [PubMed] [Google Scholar]
- 28.Kivovich V, Gilbert L, Vuento M, Naides SJ. The putative metal coordination motif in the endonuclease domain of human Parvovirus B19 NS1 is critical for NS1 induced S phase arrest and DNA damage. Int J Biol Sci. 2012;8:79–92. doi: 10.7150/ijbs.8.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53:25–38. doi: 10.1016/j.neuron.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 30.Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, Pieper U, Sali A. Comparative protein structure modeling using Modeller. Curr Protoc Bioinformatics. 2006;Chapter 5(Unit 5):6. doi: 10.1002/0471250953.bi0506s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang DT, Oyang YJ, Lin JH. MEDock: a web server for efficient prediction of ligand binding sites based on a novel optimization algorithm. Nucleic Acids Res. 2005;33:W233–238. doi: 10.1093/nar/gki586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.