Abstract
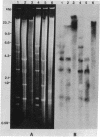
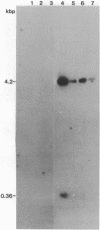
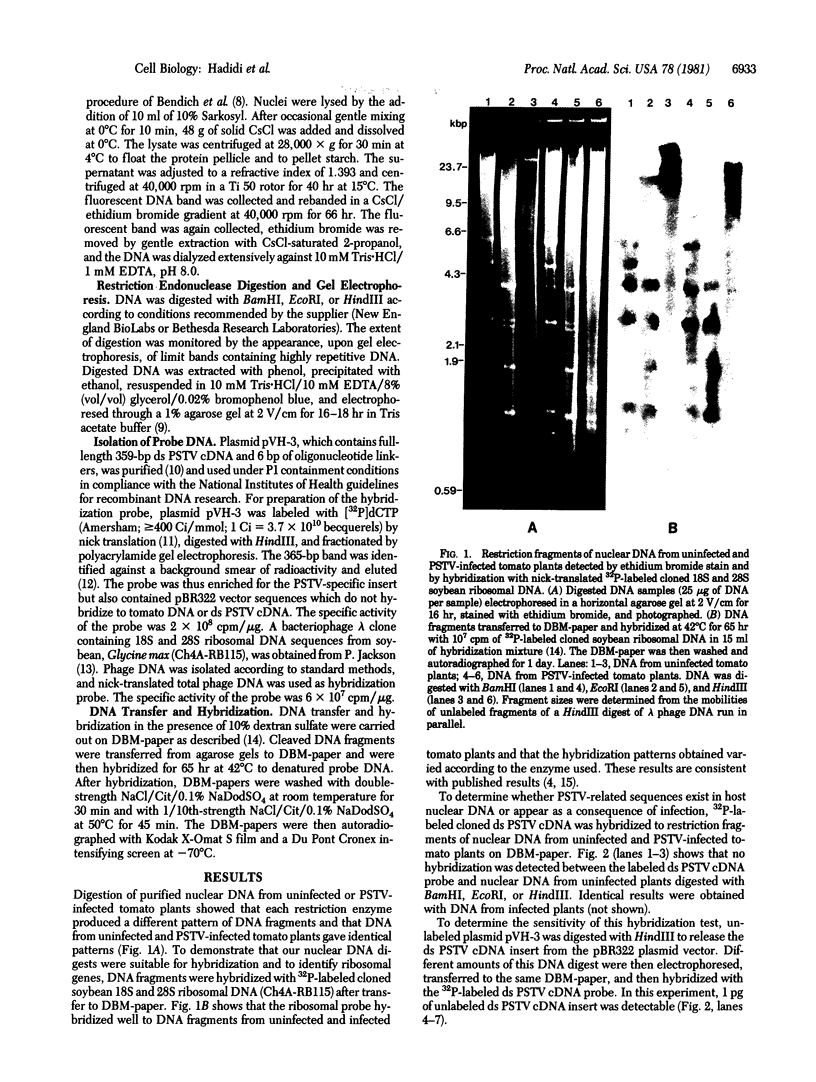
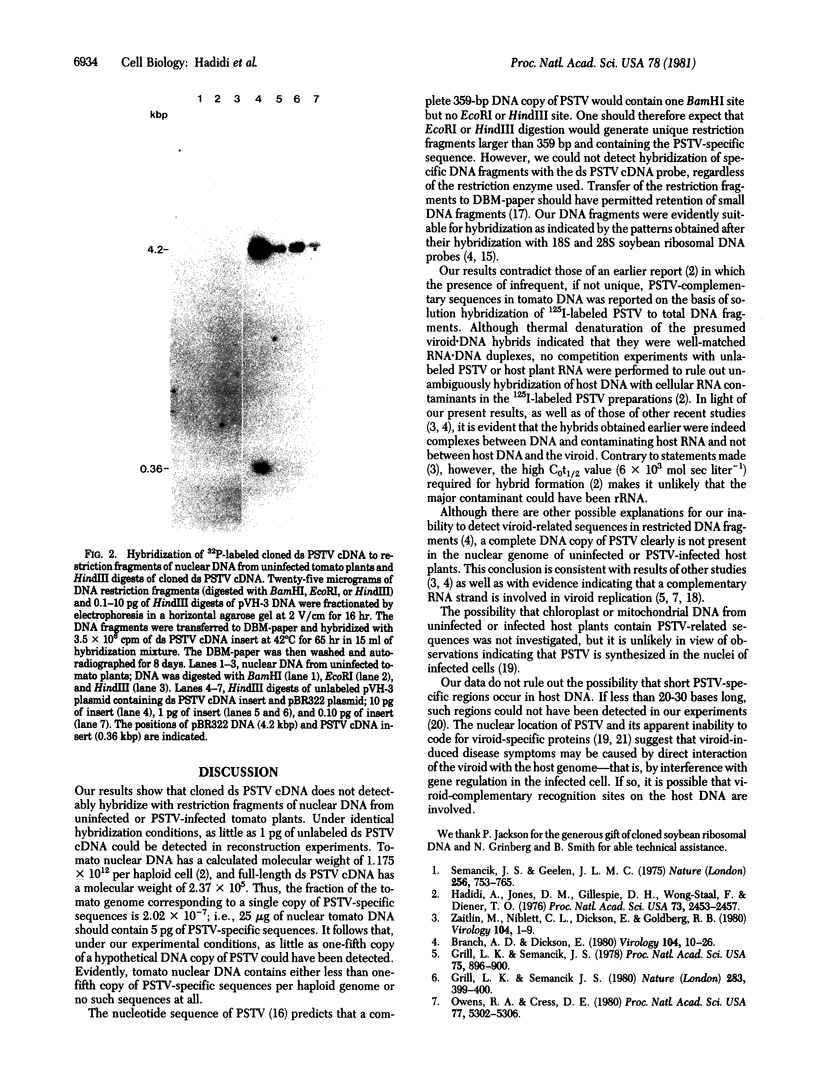
High molecular weight tomato nuclear DNA was isolated from uninfected and potato spindle tuber viroid (PSTV)-infected tomato leaves. Restriction digests were fractionated on agarose gels, denatured and transferred to diazobenzyloxymethylpaper, and hybridized to 32P-labeled cloned double-stranded PSTV cDNA. No hybridization to DNA from either uninfected or infected tissue could be detected under conditions that permitted detection of cloned double-stranded PSTV cDNA at a concentration equivalent to one-fifth copy of PSTV-related DNA per haploid tomato genome. Hybridization of tomato DNA to 32P-labeled cloned soybean 18S and 28S ribosomal DNA sequences showed that the restricted nuclear DNA was suitable for hybridization to probes containing homologous sequences. Our results indicate that neither PSTV nor its complementary strand is transcribed from nuclear DNA but do not rule out the possibility of sequence homology between host DNA and a small portion of PSTV or its complement.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Caboche M., Lark K. G. Preferential replication of repeated DNA sequences in nuclei isolated from soybean cells grown in suspension culture. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1731–1735. doi: 10.1073/pnas.78.3.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. Viroids: structure and function. Science. 1979 Aug 31;205(4409):859–866. doi: 10.1126/science.472709. [DOI] [PubMed] [Google Scholar]
- Grill L. K., Semancik J. S. RNA sequences complementary to citrus exocortis viroid in nucleic acid preparations from infected Gynura aurantiaca. Proc Natl Acad Sci U S A. 1978 Feb;75(2):896–900. doi: 10.1073/pnas.75.2.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Hadidi A., Jones D. M., Gillespie D. H., Wong-Staal F., Diener T. O. Hybridization of potato spindle tuber viroid to cellular DNA of normal plants. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2453–2457. doi: 10.1073/pnas.73.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Cress D. E. Molecular cloning and characterization of potato spindle tuber viroid cDNA sequences. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5302–5306. doi: 10.1073/pnas.77.9.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Geelen J. L. Detection of DNA complementary to pathogenic viroid RNA in exocortis disease. Nature. 1975 Aug 28;256(5520):753–756. doi: 10.1038/256753a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]