Abstract
Previous studies in rat and mouse documented that a subpopulation of dorsal root ganglion (DRG) neurons innervating non-visceral tissues express tyrosine hydroxylase (TH). Here we studied whether or not mouse DRG neurons retrogradely traced with Fast Blue (FB) from colorectal or urinary bladder also express immunohistochemically detectable TH. The lumbar sympathetic chain (LSC) and major pelvic ganglion (MPG) were included in the analysis. Previously characterized antibodies against TH, norepinephrine transporter type 1 (NET-1) and calcitonin gene-related peptide (CGRP) were used. On average, 14% of colorectal and 17 % of urinary bladder DRG neurons expressed TH and spanned virtually all neuronal sizes, although more often in the medium-sized to small ranges. Also, they were more abundant in lumbosacral than thoracolumbar DRGs, and often coexpressed CGRP. We also detected several TH-immunoreactive (IR) colorectal and urinary bladder neurons in the LSC and the MPG, more frequently in the former. No NET-1-IR neurons were detected in DRGs, whereas the majority of FB-labeled, TH-IR neurons in the LSC and MPG coexpressed this marker (as did most other THIR neurons not labeled from the target organs). TH-IR nerve fibers were detected in all layers of the colorectum and the urinary bladder, with some also reaching the basal mucosal cells. Most TH-IR fibers in these organs lacked CGRP. Taken together, we show: 1) that a previously undescribed population of colorectal and urinary bladder DRG neurons expresses TH, often CGRP but not NET-1, suggesting absence of a noradrenergic phenotype; and 2) that TH-IR axons/terminals in colon or urinary bladder, naturally expected to derive from autonomic sources, could also originate from sensory neurons.
Keywords: autonomic neurons, catecholamines, colorectum, DRG, neuropeptides, urinary bladder
Visceral organs such as the colorectum and the urinary bladder are innervated both by sensory and autonomic neurons (see Robinson and Gebhart, 2008), classically grouped as either “intrinsic” or “extrinsic”. The former are found along the full extent of the gut, including the colorectum, and comprise enteric sensory and motor neurons residing within ganglionic layers of the gut wall, creating an “intrinsic” neuronal network (Furness et al., 2004). “Extrinsic” neurons in rodents (as well as in humans) belong to a variety of neuronal systems: 1) peripheral projections of thoracolumbar (TL) (from the 8th thoracic to the 1st lumbar) and lumbosacral (LS) (from the 6th lumbar to the 2nd sacral) DRG neurons (see Robinson and Gebhart, 2008); 2) postganglionic projections of sympathetic neurons in the lumbar sympathetic chain (LSC), or 3) sympathetic and parasympathetic neurons present in the ‘mixed’ major pelvic ganglion (MPG) (Furness, 2006; Keast, 2006). Fibers from the afferent sensory and efferent autonomic nervous systems travel together in the pelvic (LS) and lumbar splanchnic/hypogastric (TL) nerves. In recent studies, afferent fibers in these two nerves have been characterized in mouse colorectum (Brierley et al., 2004; Brierley et al., 2005) and urinary bladder (Xu and Gebhart, 2008) with respect to mechanosensitivity, and differentiated into mucosal, muscular/mucosal, muscular, mesenteric and serosal classes.
As shown both in rat (De Groat, 1987; Keast and De Groat, 1992; Callsen-Cencic and Mense, 1997; Wang et al., 1998; Keast and Stephensen, 2000; Christianson et al., 2006; Olsson et al., 2006) and mouse (Robinson et al., 2004; Christianson et al., 2006; Spencer et al., 2008; Brumovsky et al., 2011), colorectal and urinary bladder sensory neurons synthesize a variety of neurotransmitters and associated molecules. These include excitatory neurotransmitters such as glutamate and aspartate (Keast and Stephensen, 2000), the related vesicular glutamate transporters (VGLUTs) (Olsson et al., 2006; Brumovsky et al., 2011), neuropeptides such as the calcitonin generelated peptide (CGRP) (De Groat, 1987; Keast and De Groat, 1992; Callsen-Cencic and Mense, 1997; Wang et al., 1998; Robinson et al., 2004; Hwang et al., 2005), pituitary adenylate cyclase-activating peptide (Wang et al., 1998), substance P and somatostatin (Wang et al., 1998) or galanin (Callsen-Cencic and Mense, 1997; Wang et al., 1998). Among several receptors involved in pain mechanisms, many colorectal and urinary bladder DRG neurons also express the transient receptor potential cation channel, subfamily V, member 1 (TRPV1) (Christianson et al., 2006; Spencer et al., 2008; La et al., 2011), a nonselective cation channel activated by pH, heat and capsaicin (Caterina et al., 1997).
Tyrosine hydroxylase (TH), the rate-limiting enzyme for the catecholamine (CA) synthesis (Nagatsu et al., 1964; Levitt et al., 1965), has been traditionally utilized to detect catecholaminergic neurons, both in the central and the peripheral nervous systems. In addition to TH, the majority of sympathetic neurons in the autonomic nervous system contain aromatic aminoacid decarboxylase (AADC) and dopamine (DA) β-hydroxylase (DβH) which are sequential in the synthesis of DA to norepinephrine (NE), the principal neurotransmitter of the sympathetic nervous system (see von Euler, 1971). Some sensory neurons also express TH, as demonstrated in rat nodose and petrosal ganglia (Katz and Black, 1986; Ichikawa et al., 1991; Kummer et al., 1993; Matsumoto et al., 2003) and non-visceral DRG neurons (Price and Mudge, 1983; Jonakait et al., 1984; Price, 1985; Vega et al., 1991; Herradon et al., 2008; Kobayashi et al., 2010). The presence of TH has also been confirmed in mouse embryonic (Forgie et al., 2000; Ichikawa et al., 2005) and adult lumbar DRG neurons innervating non-visceral structures such as the glabrous (Brumovsky et al., 2006) and hairy hindpaw skin (Brumovsky et al., 2006; Li et al., 2011).
In the present study we investigated whether or not mouse visceral sensory neurons, identified by retrograde tracing with Fast Blue from the colon or the urinary bladder, also express TH. Thus, by means of immunohistochemistry, we explored the presence of this enzyme in visceral DRG, LSC and MPG neurons. We also examined colocalization of TH with CGRP in these neurons, as well as with the NE transporter type 1 (NET-1), the latter involved in the re-uptake of norepinephrine into presynaptic nerve terminals (Pacholczyk et al., 1991). Finally, we studied the distribution of axons/terminals containing TH in all layers of the colorectum and urinary bladder, including the mucosal layer.
EXPERIMENTAL PROCEDURES
Animals
Male BALB/c mice (Taconic, Germantown, NJ, USA) were used in all experiments. All research protocols followed the Uniform Requirements for Manuscripts Submitted to Biomedical Journals, were reviewed and approved by the Institutional Animal Care and Use Committee (University of Pittsburgh), and adhered to the United States Public Health Service policies regarding the care and use of animals in research.
Retrograde tracing
The colon (n=5) or the urinary bladder (n=5) of 6-week-old BALB/c male mice was injected with the fluorescent retrograde neuronal tracer Fast Blue (FB, 2% in saline; EMS-Chemie, Gross Umstadt, Germany). Under aseptic conditions, animals were anesthetized with isoflurane (Hospira Inc, Lake Forest, IL). A laparotomy exposed the target organ, and three to five injections (~ 5 µl total, using a Hamilton syringe with a 25-gauge needle) of FB were made at different sites into the wall of the descending colon or the urinary bladder. Care was taken not to allow the dye to spread to areas other than the injection site, both by containing any spillage using a cotton tip, and by rinsing the peritoneal cavity with abundant sterile saline before suturing muscle and skin. After treating the skin incision with dibucaine ointment (1%; Perrigo, Allegan, MI) and treating with buprenorphine (0.1 mg/kg; Bedford Labs, Bedford, OH) for postoperative analgesia, mice were allowed to recover in a warm environment under close observation.
Immunohistochemistry
Twelve days after injection of FB, and including 6 naïve BALB/C mice, animals were deeply anaesthetized with sodium pentobarbital (60 mg/kg, i.p.; Ovation Pharmaceuticals, Deerfield, IL) and perfused via the ascending aorta with 20 ml of Tyrode’s buffer (37°C), followed by 20 ml of a mixture of 4% paraformaldehyde and 0.2% picric acid dissolved in 0.16 M phosphate buffer (pH 6.9; 37°C) and 50 ml of the same mixture at 4°C, the latter for approximately 5–7 min. TL (T8-L1) and LS (L6-S2) DRGs, the MPG, the LSC, the colorectum and the urinary bladder were dissected out and post-fixed for 90 min at 4°C in the same fixative and immersed in 10–20% sucrose in phosphate-buffered saline (PBS) (pH 7.4) containing 0.01% sodium azide and 0.02% bacitracin (both from Sigma, St. Louis, MO) (4°C) for 48 h. After embedding in Tissue-Tek O.C.T. compound (Sakura, Torrence, CA) and freezing on dry ice, tissue was sectioned in a cryostat (Leica, Heidelberg, Germany) at 12-µm (DRG, LSC) or 20-µm thicknesses (MPG, colorectum, urinary bladder).
For single-staining of TH and NET-1, sections were washed twice in PBS and incubated for 24 h at 4°C with sheep anti-TH (1:4,000; cat. n°: AB1542, Chemicon, MA) or rabbit anti-NET-1 (1:2,000; cat. n°: HPA004057, Sigma) antibodies, diluted in 0.01M PBS containing 0.3% Triton X-100 and 0.5% bovine serum albumin (BSA). To visualize immunoreactivity, sections were processed using a commercial kit based on tyramide signal amplification (Adams, 1992) (TSA Plus, NEN Life Science Products, Inc., Boston, MA). Briefly, the sections were washed in TNT buffer (0.1 M Tris-HCl, pH 7.5; 0.15 M NaCl; 0.05% Tween 20) for 10 min, incubated with TNB buffer (kit; 0.1 M Tris-HCl, pH 7.5; 0.15 M NaCl; 0.5% Dupont Blocking Reagent; NEN) for 30 min at room temperature (RT) and incubated for 60 min with either a donkey antisheep or anti-rabbit/horseradish peroxidase (HRP) conjugate (cat. n°: 711-035-152 and 713-035-003, respectively, Jackson Immuno-Research, West Grove, PA) diluted 1:200 in TNB buffer. Sections were washed twice in TNT buffer and incubated in biotinyl tyramide-fluoride thiocyanate (BT-FITC) conjugate (kit; cat. n°: NEL741, NEN) diluted 1:700 in amplification diluent (kit; NEN) for 30 min at RT.
For double-staining (TSA plus combined with indirect immunofluorescence) of NET-1 plus TH or TH plus CGRP, sections were first incubated with rabbit anti-NET-1, sheep or rabbit (1:4000; cat. n°: AB152, Millipore, Temecula, CA) anti-TH antibodies, followed by the TSA plus technique for their visualization (as above), and then processed according to Coons (1958). Thus, after two washes in PBS, incubation for 24 h at 4°C with sheep anti-TH (1:400) or rabbit anti-CGRP (1:8,000; cat. n°: C8198, Sigma) antibodies, and two washes in PBS, sections were incubated for 30 min at RT using either a donkey anti-sheep or anti-rabbit/ tetramethyl rhodamine isothiocyanate (TRITC) conjugate (1:400; cat. n°: 713-025-003 and 711-025-152, respectively, Jackson Immuno-Research), for visualization of TH or CGRP.
For double-staining (double indirect immunofluorescence) of CGRP plus TH, some sections were first incubated with the rabbit CGRP antiserum, followed by visualization using a donkey anti-rabbit/FITC conjugate (1:200; cat. n°: 711-095-152, Jackson Immuno-Research). Later, sections were incubated for 24 h at 4°C with the sheep anti-TH antibody (1:400), followed by incubation with a donkey anti-sheep/ TRITC conjugate (1:400; Jackson Immuno-Research) for 30 min at RT, for visualization.
The antibodies used throughout the study have been validated, either in previous studies or as specified by the manufacturer. For visualization of TH, we used two different antibodies. The one raised in sheep, and used throughout most of the present study, is a purified polyclonal antibody against TH (Haycock and Waymire, 1982), specifically binds to tyrosine hydroxylase and it reacts with the ~60 kDa TH protein in PC12 cells stimulated with Okadaic acid (Chemicon). This antibody also cross-reacts with all mammalian forms tested (including mouse and rat). The rabbit TH antibody is also purified and shows, by western blot, a single band at approximately 62 kDA corresponding to tyrosine hydroxylase. It also shows cross-reactivity with mouse and rat (Millipore). Finally, when tested in the same slides, both sheep and rabbit TH antibodies stained the same DRG neurons (present study; data not shown). The CGRP antibody reacts with CGRP (rat) conjugated to BSA in dot blot immunoassay, and its specific staining is inhibited by pre-incubation with 10 µM CGRP (rat) (Sigma). The antibody does not cross-react with substance P, vasoactive intestinal peptide, neuropeptide Y, calcitonin or somatostatin, conjugated to BSA (Sigma). Finally, the specificity of the NET-1 antibody has been validated (via western blot) in a recent article (Mulder et al., 2009). Nonspecific staining by the secondary antibody was tested by omission of the primary antibody in some sections. Also, single-stained control sections were processed for comparison with double-stained sections.
Microscopy and image processing
All sections were cover-slipped using 2.5% DABCO in glycerol (Sigma) and examined on a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan) provided with appropriate filters and a Retiga 2000 R Fast CCD camera (Q-Imaging, Surrey, British Columbia, BC, Canada) using IPLab software (Scanalytics Inc., Vancouver, BC, Canada). For colocalization analysis, a Fluoview FV 1000 confocal laser scanning biological microscope equipped with 10× (0.45 N.A.), 20× (0.75 N.A.) and 60× oil (1.40 N.A.) objectives was used (Olympus, Tokyo, Japan). The FITC labeling was excited using a 547–514 nm argon multi-line laser. For the detection of TRITC and TMR, a 543 nm HeNe laser was used.
Resolution, brightness and contrast of the images were optimized using the Adobe Photoshop CS3 software (Adobe Systems Inc., San Jose, CA). Because confocal imaging of FB was not possible due to lack of appropriate filters, whenever it was necessary (the MPG), images were composed by merging separate optical (FB) and confocal (other markers of interest) photomicrographs. Care was taken to match the position of these images perfectly, based on the shape of the contour of ganglia and NPs, for production of the merged micrographs.
Quantification and statistical analysis
Retrogradely traced colorectal and urinary bladder neurons, identified by their content of FB, were quantified in T8-T13 and L6-S2 DRGs. Every fifth DRG section was used to quantify the number of FB-positive (+) neuron profiles (NPs) present in each section. TH-immunoreactive (IR) colorectal or urinary bladder NPs were also counted. In total, 5–8 sections per ganglion were used for quantification. Percentages of TH-expressing colorectal or urinary bladder neurons were obtained by counting, within the total number of FB+ neurons, those expressing TH. Throughout these immunohistochemical experiments, background was low, allowing for the easy detection of a positive signal.
We included in the quantification all possible FB+ NPs per section, regardless of the presence of a visible nucleus. This was done for two main reasons: 1) the discrete number of FB+ colorectal neurons in TL and LS DRGs, which at least in the rat, represent only ~4% of all DRG neurons (see Christianson et al., 2006; Brumovsky and Gebhart, 2010), and 2) the number of sections per DRG to assess the colocalization of TH with other markers such as CGRP or NET-1 are few (~25–30 at 12 µm each), thus limiting application of quantification methods such as neurostereology (Mayhew and Gundersen, 1996). The current quantification approach could have led to the overestimation of large diameter neurons, and also to a reduction in opportunity for small neurons to be counted (see Guillery, 2002). In such a scenario, and considering that most TH-IR NPs were medium and small-sized, it is possible that the percentages presented here are underestimated. These methodological short comings, however, do not violate the intention of the present study, which is to provide an estimate of the expression of TH in visceral sensory neurons and of the differential distribution between colorectal vs. urinary bladder and LS vs. TL sensory neurons. Establishing the exact number of visceral DRG neurons in the mouse would require neurostereology.
For the assessment of neuronal size distribution, the area of FB+/TH+ (colorectal or urinary bladder) and FB-negative/TH+ (TH-only) DRG NPs with identifiable nucleus was measured using the public domain NIH program ImageJ 1.36B (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/) and plotted into histograms. A total of 670 NPs, including FB+TH+ (colorectal or urinary bladder) and TH-only NPs were measured (n of mice = 4), and frequencies were plotted as percentage of the total number of neurons in each group. The average size of DRG NPs in both groups was also obtained in order to establish potential statistically significant differences.
Data are presented as mean ± SEM. Statistical significance among groups was tested using the one-way ANOVA test, followed by the Tukey HSD post hoc test; P≤0.05 was considered significant.
RESULTS
TH expression in colorectal and urinary bladder DRG neurons
In previous studies it was established that most neurons projecting to the colorectum or the urinary bladder are distributed in two main groups, the thoracolumbar (TL, T8-L1) and lumbosacral (LS, L6-S2) DRGs (Robinson et al., 2004; Christianson et al., 2006). In the present study, retrograde tracing from the colorectum or the urinary bladder revealed a discrete number of FB+ NPs per DRG section, both at TL and LS levels, and easily differentiated from FB− negative NPs (Fig. 1). Throughout this section, DRG NPs will be described as “colorectal” or “urinary bladder” when containing FB. These NPs will be further subdivided into FB+TH+ when expressing the noradrenergic marker. One additional category will be TH-only NPs, being those expressing TH and lacking FB.
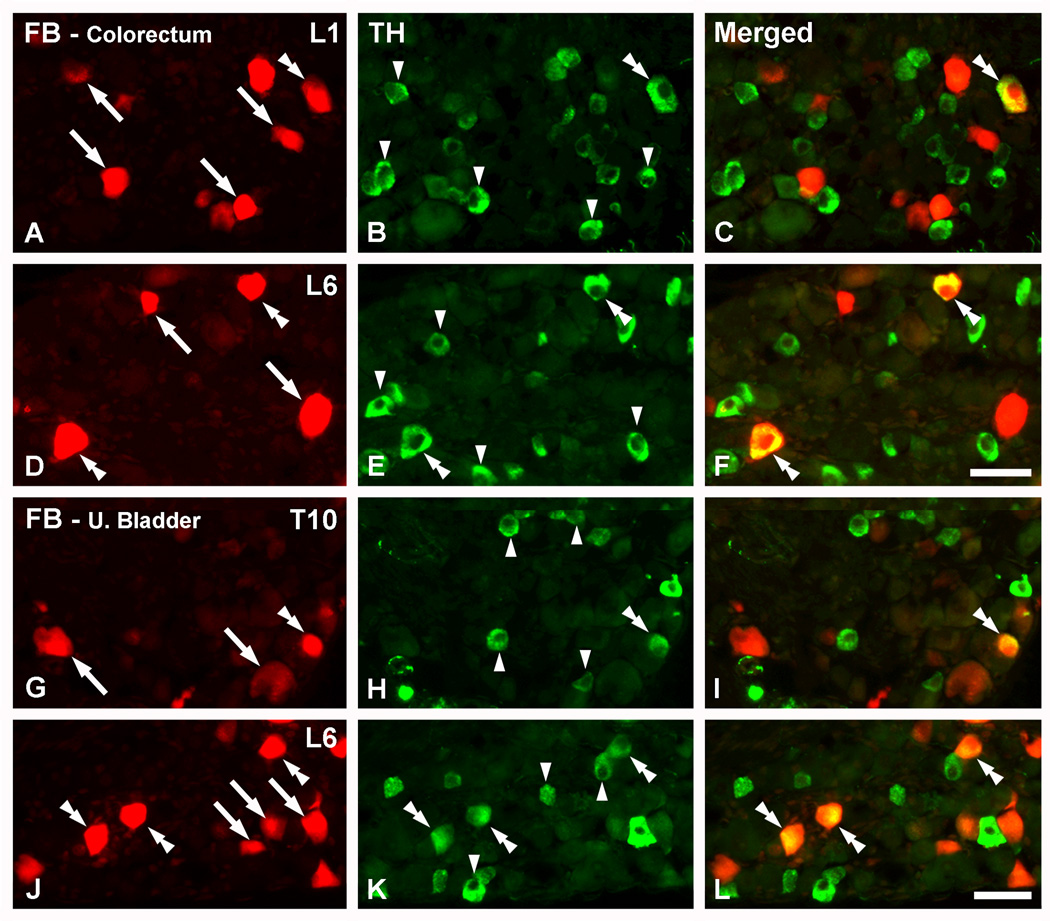
Figure 1.
TH is expressed in colorectal and urinary bladder DRG neurons. Optical immunofluorescence photomicrographs of sections of L1 (A–C), L6 (D–F; J–L) or T10 (G–I) DRGs incubated with antiserum to TH. Retrogradely labeled colorectal (A–F) or urinary bladder (G–L) neurons containing FB (A, D, G, J) are shown in red. (C, F, I, L) show merged micrographs. (A–F) A number of colorectal NPs, as evidenced by the presence of FB, express TH in L1 and L6 DRGs (doble arrowheads in A–F). FB+ colorectal NPs lacking the enzyme are also present (arrows in A, D), as well as several TH-only NPs (arrowheads in B, E). (G–L) FB+TH+ urinary bladder NPs are seen in T10 (double arrowheads in G–I) and L6 (double arrrowheads in J–L) DRGs. Also here, FB+ urinary bladder (arrows in G, J) or TH-only (arrowheads in H, K) DRG NPs are detected. Scale bar: 50 µm (F=A–E; L=G–K).
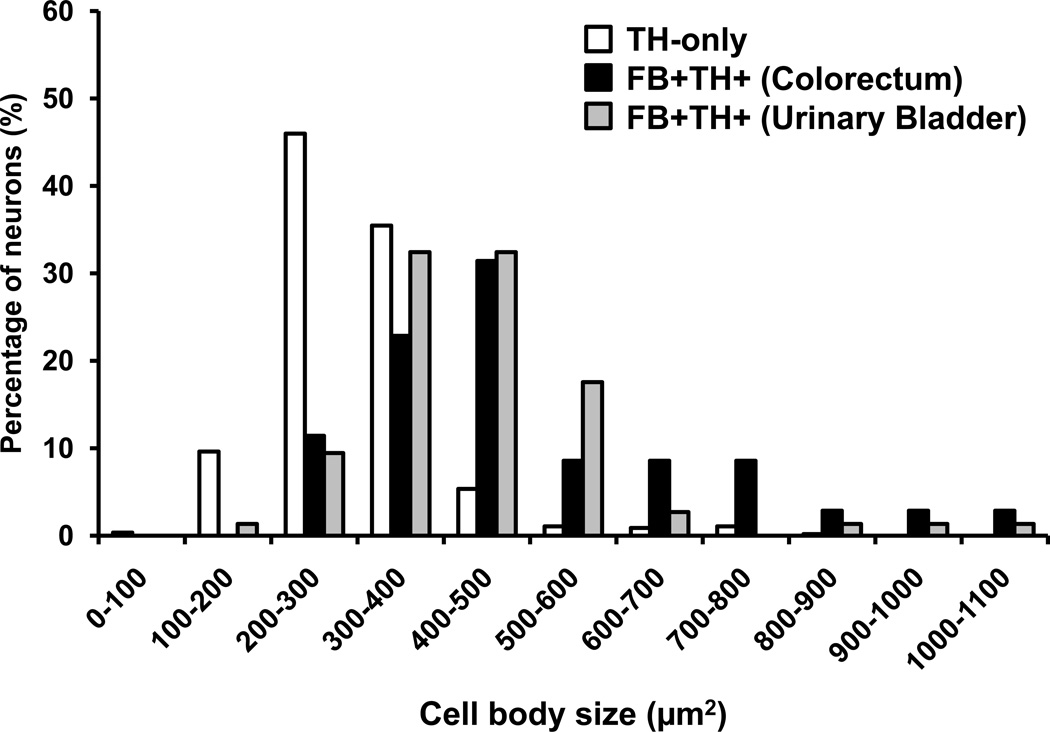
TH was expressed in 14.4 ± 1.1% and 17.2 ± 0.9% of mouse FB+ DRG NPs traced from the colorectum or urinary bladder, respectively (Figs. 1, 2). FB+TH+ colorectal and urinary bladder NPs were mostly medium and small-sized, ranging from 100 to over 1000 µm2 and showing a peak between 300 and 500 µm2 (Fig. 3). In contrast, TH-only neurons were usually small, ranging from less than 100 to a maximum of around 700 µm2 and with a peak between 200 and 400 µm2. Seldom had these neurons larger areas than 600 µm2 (Fig. 3). The average sizes of TH-only NPs was 296.93 ± 4.1 µm2 (combined data from DRGs traced either from the colorectum or the urinary bladder, n=561), whereas for FB+TH+ NPs these were 503.3 ± 31.8 µm2 (colorectum, n=35) and 441.4 ± 16.7 µm2 (urinary bladder, n=74). Comparison among groups showed statistical differences between TH-only vs. FB+TH+ colorectal or urinary bladder DRG NPs (P<0.0001). Finally, FB+ colorectal or urinary bladder DRG NPs lacking TH were often detected (Fig. 1).
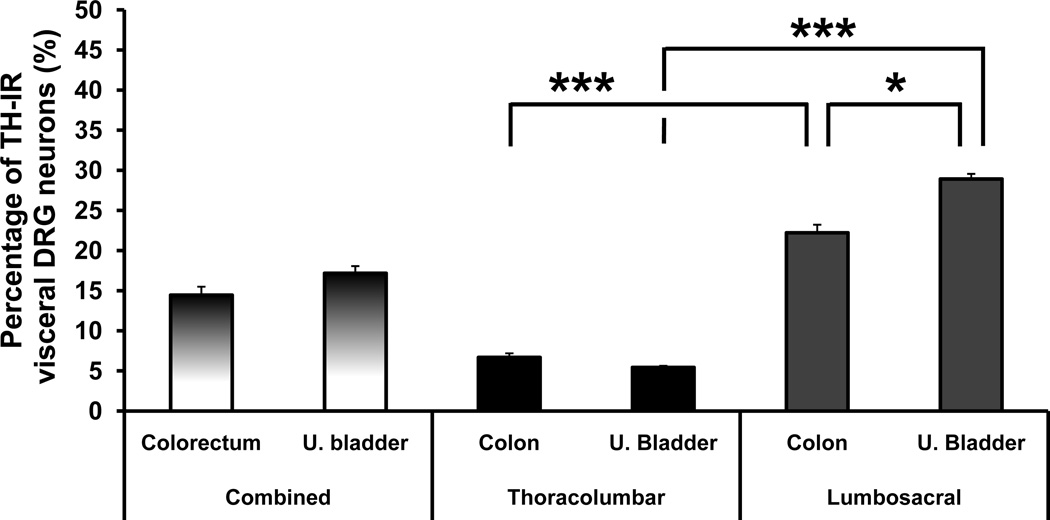
Figure 2.
TH is expressed more abundantly in lumbosacral than in thoracolumbar visceral DRG neurons. Percentage of FB+ colorectal or urinary bladder NPs expressing TH, shown as combined (thoracolumbar plus lumbosacral DRGs) or independent values. *, P<0.05; ***, P<0.001.
Figure 3.
FB+TH+ DRG neurons tend to be larger than TH-only ones. Graphs showing the size-distribution of TH-only (white bars), FB+TH+ colorectal (black bars) and FB+TH+ urinary bladder (gray bars) DRG neurons in retrogradely traced mice (n = 4). Data are expressed in square µm and include the measurement of 670 DRG NPs (TH-only, 561; FB+TH+ colorectal, 35 neurons; FB+TH+ urinary bladder, 74).
When comparing the number of FB+TH+ colorectal or urinary bladder NPs in TL vs. LS DRGs, statistical significance was also found (P<0.0001). Thus, TH was present in 6.7 ± 0.5% of colorectal TL versus 22.2 ± 1.0% of LS NPs (P<0.001; Figs. 1A–F; 2). Similarly, TH was detected in 5.4 ± 0.2% of TL versus 28.9 ± 0.6% of LS urinary bladder DRG NPs (P<0.001; Figs. 1G–L; 2). Finally, a significantly higher percentage of FB+TH+ LS urinary bladder DRG NPs than colorectal NPs was observed (P<0.05; Fig. 2). No statistically significant differences in the average size of TH-only or FB+TH+ DRG NPs were observed between TL and LS DRG NPs (data not shown).
TH and CGRP coexpression in colorectal and urinary bladder DRG neurons
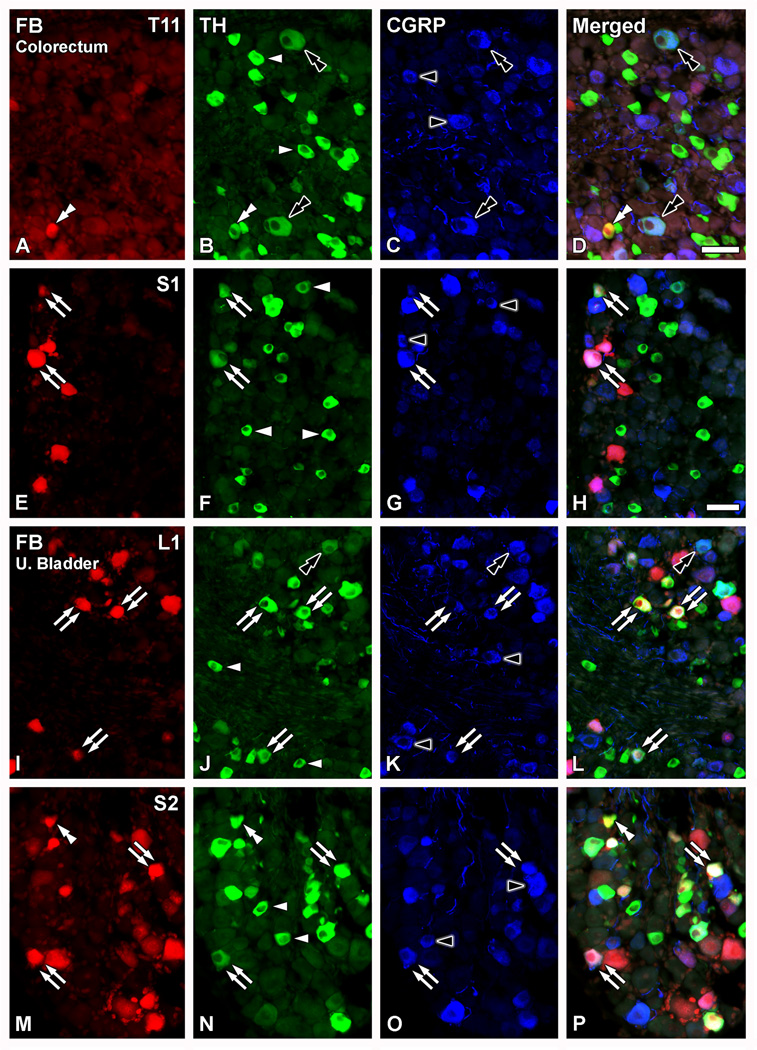
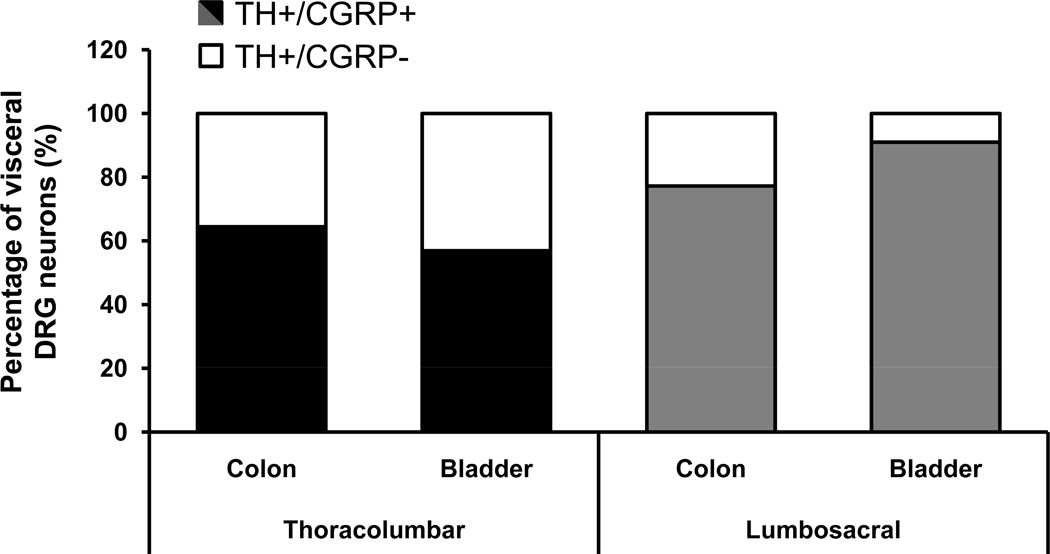
More than half of the FB+TH+ colorectal and urinary bladder DRG neurons coexpressed CGRP (Figs. 4, 5). Thus, 64.5 ± 8.6% of TL and 77.3 ± 7.4% of LS colorectal DRG NPs coexpressed TH and CGRP (Figs. 4A–H; 5). Likewise, 57.0 ± 4.9% of TL and 91 ± 3.2% of LS urinary bladder DRG NPs coexpressed TH and CGRP (Figs. 4I–P; 5). A number of CGRP- or TH-only NPs were present throughout the ganglia (Fig. 4A–P). Occasional TH-only NPs coexpressing CGRP were also detected (Fig. 4A–D; I–L).
Figure 4.
TH-expressing colorectal and urinary bladder DRG neurons colocalize with CGRP. Optical immunofluorescence photomicrographs of sections of T11 (A–D), S1 (E–H), L1 (I–L) or S2 (M–P) DRGs after co-incubation with TH (B, F, J, N) and CGRP antisera (A, E, I, M). Retrogradely labeled colorectal (A–H) or urinary bladder (I–P) neurons containing FB (A, E, I, M) are shown in red. (D, H, L, P) show merged micrographs. (A–P) Several TH-IR NPs are detected (arrowheads in B, F, J, N). Most of the TH-only DRG NPs lack CGRP-LI, with the exception of occasional neurons coexpressing both, the enzyme and the peptide (black double arrowhead in A–D; I–L). Additional non-traced CGRP-IR NPs are also detected (black arrowheads in C, G, K, O). A number of FB+TH+ colorectal (double arrows in E–H) or urinary bladder (double arrows in I–P) NPs are detected, often coexpressing with CGRP. However, some FB+TH+ colorectal (white double arrowhead in A–D) or urinary bladder (white double-arrowhead in M–P) NPs lacking CGRP-LI are also present. Scale bars: 50 µm (D=A-C, M–P; H=E–G; I–L).
Figure 5.
A large proportion of TH-expressing colorectal and urinary bladder DRG neurons are peptidergic. Percentages of FB+TH+ colorectal or urinary bladder NPs lacking (white bar segments) or coexpressing (black bar segments) CGRP in thoracolumbar and lumbosacral DRGs.
TH expression in colorectal or urinary bladder LSC and MPG neurons
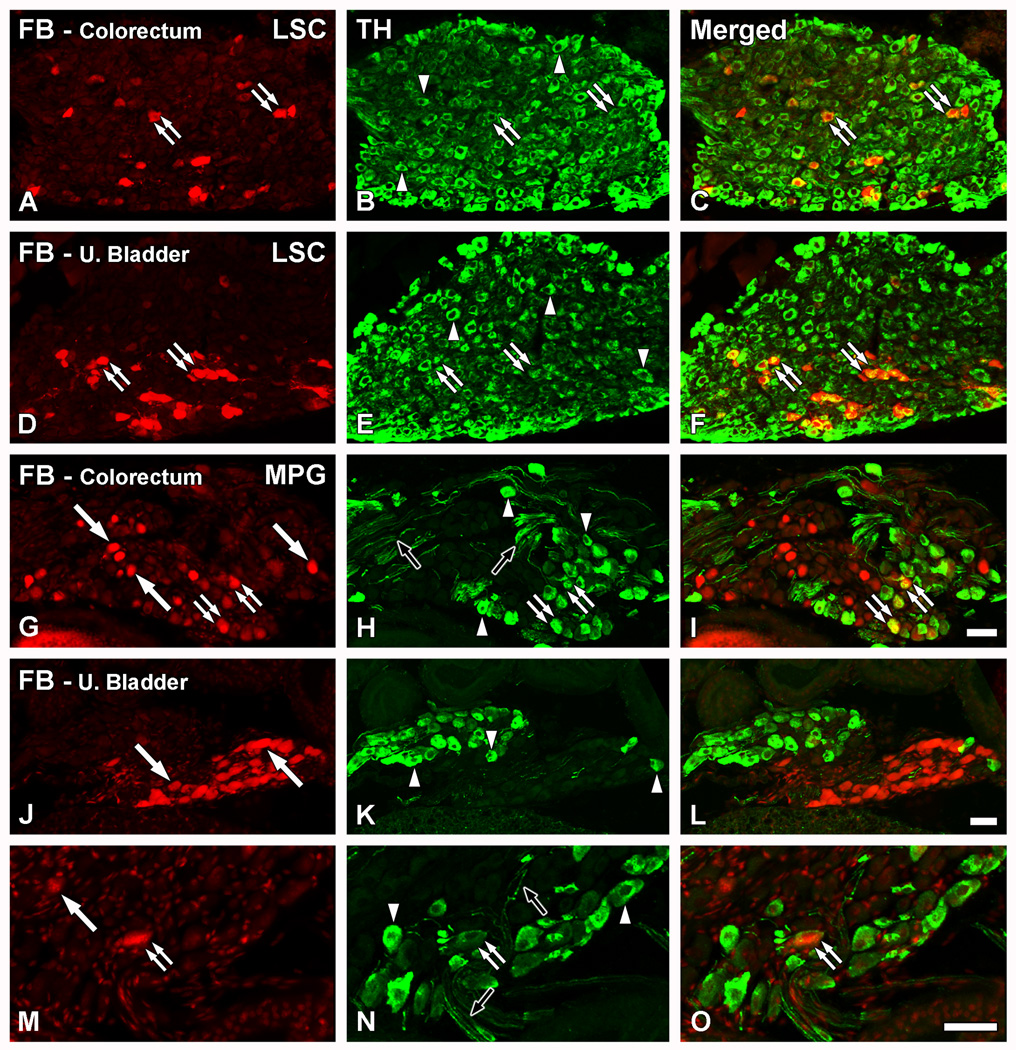
Virtually all LSC neurons expressed TH, including those retrogradely labeled with FB from the colorectum (Fig. 6A–C) or the urinary bladder (Fig. 6D–F).
Figure 6.
The main source of autonomic TH fibers in the colorectum and the urinary bladder is the LSC. Optical (A–F; G, J, M) and confocal (H, K, N) immunofluorescence photomicrographs of sections of the LSC (A–F) or MPG (G–O) after incubation with TH (B, E, H, K, N) antiserum. Retrogradely labeled colorectal (A–C; G–I) or urinary bladder (D–F; J–O) neurons containing FB (A, D, G, J, M) are shown in red. (C, F, I, L, O) show merged micrographs. (A–F) A number of FB+TH+ colorectal (A–C) or urinary bladder (D–F) NPs is detected in the LSC (double arrows). In addition, numerous TH-only NPs are also present (arrowheads in B, E). (G–O) In most cases, FB+ colorectal (arrows in G) or urinary bladder (arrows in J, M) NPs lacked TH-LI in the MPG. Likewise, several TH-only NPs could be detected (arrowheads in H, K, N). However, a few TH-IR colorectal (double arrows in G–I) and occasional urinary bladder (double arrows in M–O) NPs are detected. TH-IR fibers were abundant in the MPG (black arrows in H, N). Scale bars: 50 µm (I=A–H; L=J, K; O=M, N).
In the MPG, many FB+ colorectal (Fig. 6G), and urinary bladder NPs (Fig. 6J), were found. In the latter, these neurons formed distinct clusters (Figs. 6J; 7Q). However, and in contrast to the LSC, only a few of these neurons expressed TH (Fig. 6G–I; M–O). TH-only NPs and TH-IR fibers were detected throughout the ganglia (Fig. 6G–O).
Figure 7.
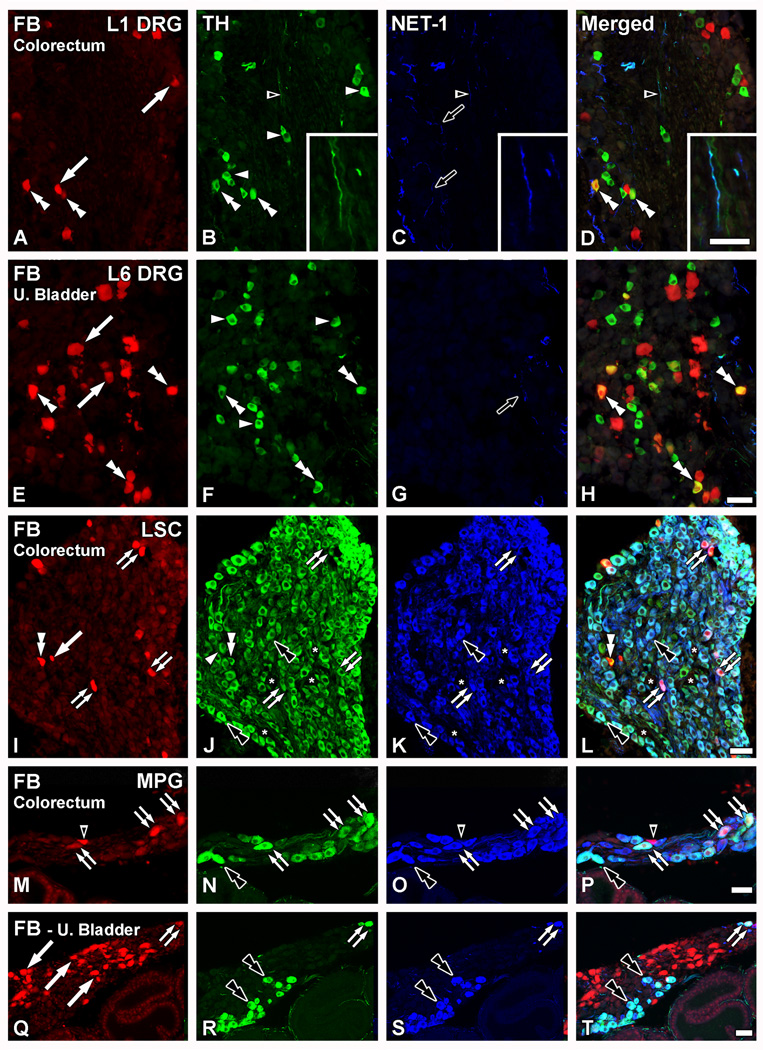
NET-1 and TH coexpression is observed in the LSC and MPG neurons, but not in DRG neurons. Optical (A–H; I–L; M, Q) and confocal (N, O, R, S) immunofluorescence photomicrographs of sections of L1 (A–D) or L6 (EH) DRGs, the LSC (I–L) or the MPG (M–T) after incubation with TH (B, F, J, N, R) and NET-1 antisera (A, E, I, M, Q). Retrogradely labeled colorectal (A–D; I–P) or urinary bladder (E–H; Q–T) neurons containing FB (A, E, I, M, Q) are shown in red. (D, H, L, P, T) show merged micrographs. (A–H) Neither TH-only (white arrowheads in B, F) nor FB+TH+ (double arrowheads in A–D, E–H) colorectal or urinary bladder NPs in L1 and L6 DRGs coexpressed with NET-1, the latter only present in fiber profiles (black arrows in C, G). Occasional TH-/NET-1-IR fiber profiles were found (black arrowhead in A–D; magnified view in insets B through D). Additional FB+ colorectal (arrows in A) or urinary bladder (arrows in E) NPs lacking TH are also present. (I–L) Virtually all FB+TH+ colorectal (double arrows in I–L), and the vast majority of TH-only LSC NPs (black double arrowheads in I33 L) coexpressed with NET-1. Note, however, a rare FB+TH+ colorectal LSC NP lacking NET-1-LI (white double arrowhead in I–L). Additional TH-only LSC NPs lacking NET-1-LI were also detected (arrowhead in J), as well as a few NPs lacking both markers (asterisks in J–L). A FB+TH+ colorectal neuron lacking both TH and NET-1 is also shown (arrow in I). (M–T) In the MPG, virtually all TH-IR NPs show NET-LI, including colorectal (double arrows in M–P), urinary bladder (double arrows in Q–T) and TH-only NPs (black double arrowheads in M–T). Occasional NET-1-only colorectal NPs could be found (black arrowhead in M–P). Also, several FB+ urinary bladder NPs lacking both TH and NET-1 were often detected (arrows in Q). Scale Bar: 100 µm (H=A–G); 50 µm (L=I–K; P=M–O; T=Q–S); 25 µm (inset in B–D).
NET-1 expression in colorectal or urinary bladder DRG, LSC and MPG neurons – Coexpression with TH
FB+TH+ colorectal (Fig. 7A–D) or urinary bladder DRG NPs (Fig. 7E–H) virtually never colocalized with NET-1. In fact, no NET-1-IR NPs were detected in TL (Fig. 7C) or LS (Fig. 7G) DRGs. However, TH-only NPs and TH-IR fibers (Fig. 7B, F) as well as NET-1-IR fibers (Fig. 7C, G) were observed in TL or LS DRGs. A few TH-IR fibers coexpressing NET-1 could be detected (Fig. 7A–D).
The great majority of the TH-IR NPs present in LSC (Fig. 7I–L) and MPG (Fig. 7M–T), however, exhibited a high degree of coexpression with NET-1. This also included most FB+TH+ colorectal (Fig. 7I–P) or urinary bladder NPs (Fig. 7Q–T; urinary bladder LSC is not shown for coexpression between TH and NET-1, as the results replicated those obtained with LSCs from colorectum traced ganglia). However, occasional FB+TH+ LSC colorectal neurons (Fig. 7I–P) and a few FB-negative NPs (Fig. 7J, N) showing TH-LI but lacking NET-LI could be found in LSC (Fig. 7I–L) and MPG (Fig. 7M–P). Finally, a number of FB-negative NPs lacking both TH- or NET-1-LIs in the LSC (Fig. 7J–L) were observed.
TH expression in fibers innervating the colorectum or the urinary bladder – Coexpression with CGRP
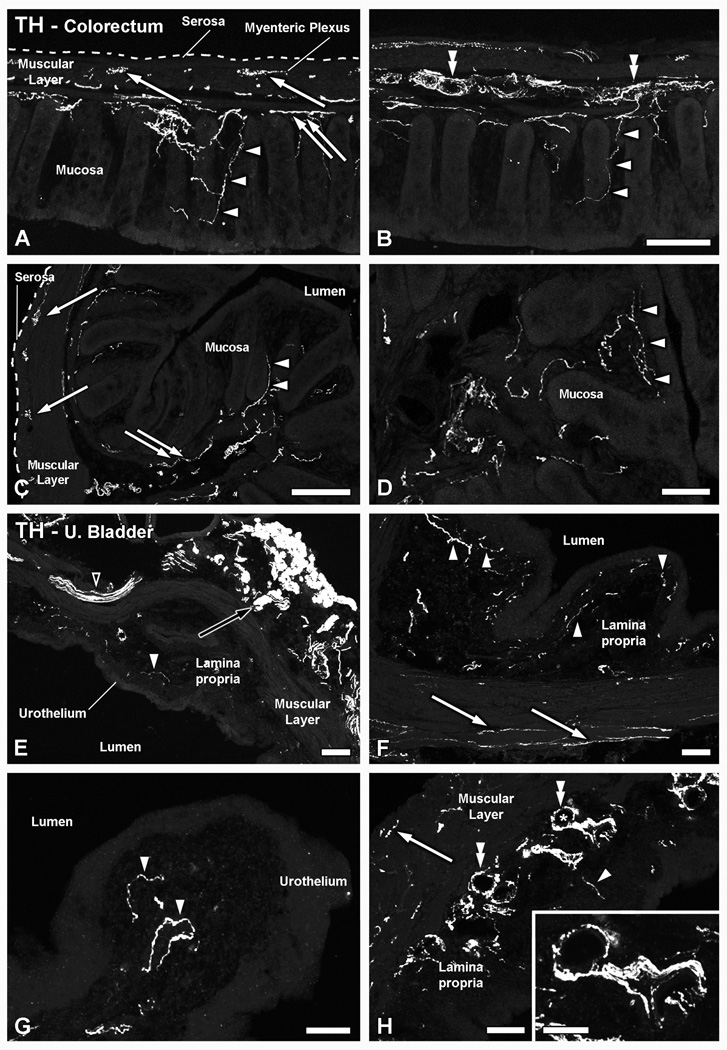
In the colorectum, TH-LI spanned all layers of the organ (Fig. 8A–D). Thus, THIR fibers were detected in the muscular layers of the colorectum, associated, whenever visible, with the myenteric plexus (Fig. 8A, C). Abundant TH-LI was detected in the submucosal layer (Fig. 8A–C), often arranged in thick bundles and also surrounding blood vessels in the area (Fig. 8B). TH-IR nerve fibers were also seen penetrating the colorectal villi and reaching the basal epithelial cells of the mucosal layer (Fig. 8A–D).
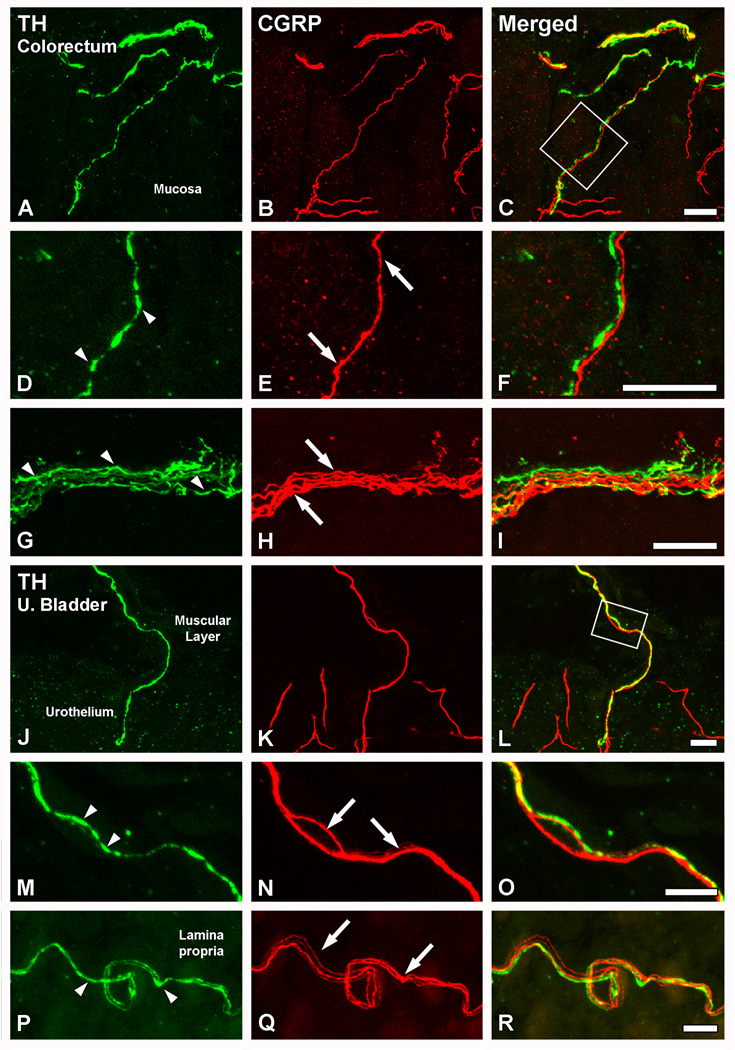
Figure 8.
Patterns of distribution of TH-IR fibers in the colorectum and the urinary bladder. Confocal immunofluorescence photomicrographs of colorectal (A–D) and urinary bladder (E–H) sections, after incubation with TH antiserum. (A–D) In longitudinal (A, B) as well as coronal (C, D) sections of the colorectum, TH-IR fibers were detected in all layers. Thus, TH-IR fibers could be seen in the muscular layers, in association with the myenteric plexus (arrows in A and C), or in the submucosal layer, either around blood vessels (double arrowheads in B) or in small nerve bundles (double arrows in A, C). A number of TH-IR fibers could also be seen in the colorectal villi, occasionally reaching the basal mucosal cells (arrowheads in A–D). (E–H) In the urinary bladder, thick TH-IR nerve bundles were found penetrating the organ (black arrowhead in E), traveling within the muscular layer (arrows in F, H) or distributed through the lamina propria (white arrowheads in E–H). In the muscular layer, TH-IR nerve profiles appeared sparsely distributed (arrows in F, H). A few of the lamina propria TH-IR fibers were detected in the vicinity of the urothelium (white arrowheads in G). In addition, TH-IR fibers could be observed arranged in thick bundles around blood vessels (asterisk in H, shown at higher magnification in inset) present in the lamina propria (double arrowheads in H). Note the presence of the major pelvic ganglion, strongly immunoreactive for TH (black arrow in E) Scale Bars: 100 µm (B=A; C; E; H); 50 µm (D; F; G; inset in G).
In the urinary bladder, TH-LI was observed in all layers of the organ, although apparently being more abundant in the lamina propria (submucosal layer) (Fig. 8E–H). Thus, TH-IR fiber bundles penetrating the organ from the muscular layer of the urinary bladder (Fig. 8E, F), as well as individual fibers reaching basal urothelial (mucosal) cells (Fig. 8E–G), were often found. In the lamina propria, thick TH-IR bundles could be observed, also surrounding the profiles of blood vessels present in their vicinity (Fig. 8H).
Colocalization analysis of nerve fibers innervating the colorectum or the urinary bladder revealed that TH and CGRP were virtually never coexpressed (Fig. 9). In the colorectum, a number of TH- (Fig. 9A, D, G) and CGRP-IR (Fig. 9B, E, H) fibers were observed in all layers of the organ. However, the analysis of fibers in the mucosal layer (Fig. 9A–F) or the myenteric plexus (Fig. 9G–I) showed two different populations of TH- and CGRP-IR nerve fibers. Furthermore, the two nerve populations often appeared to be closely juxtaposed (Fig. 9D–F). In the urinary bladder, and as observed in the colorectum, signs of colocalization between TH and CGRP were virtually absent (Fig. 9J–R), and the same close juxtaposition between the two types of nerve fibers was apparent, often winding with each other (Fig. 9M–R). In addition, analysis of sections processed with sheep or rabbit TH, or NET-1 antibodies, and co-stained with CGRP also failed to show colocalization between the peptide and the noradrenergic markers in nerve fibers innervating colorectum or the urinary bladder (data not shown).
Figure 9.
TH and CGRP appear to be present in different populations of nerve fibers innervating the colorectum or the urinary bladder. Confocal immunofluorescence photomicrographs of parasagittal (A–I) and transverse (JR) sections of the colorectum (A–I) or the urinary bladder (J–R), after coincubation with TH (A, D, G, J, M, P) and CGRP (B, E, H, K, N, Q) antisera. (C, F, I, L, O, R) show merged figures. (A–I) In the colorectum, TH- or CGRP-IR fibers were seen in close apposition (A–C). However, closer examination showed that the two markers were present in different nerve populations. This was seen, both in the mucosal layer (inset in C, magnified in D–F), as well as at the level of the myenteric plexus in the muscular layers (G–I). (J–R) In the urinary bladder, and as for the colorectum, TH- (arrowheads in M, P) and CGRP-IR fibers (arrows in N, Q) were found closely juxtaposed with each other (J–L), and virtually always present in different fiber populations. Inset in L is shown at higher magnification in M–L. Scale bars: 20 µm (C=A, B; F=D, E; I= G, H; L=J, K); 10 µm (R=P, Q; O=M, N).
DISCUSSION
In this study on male mice, we document the presence of previously undescribed populations of colorectal and urinary bladder DRG neurons that express the catecholaminergic marker TH. In the following sections, we will further analyze the characteristics of TH-expressing sensory neurons, focusing on those targeting visceral organs, and discuss their potential physiological significance.
Presence of TH in visceral DRG neurons
Expression of TH in non-visceral peripheral sensory neurons was first reported in the 1980´s. Price and Mudge (Price and Mudge, 1983) showed that some adult rat DRG neurons express TH, an observation that was confirmed in subsequent studies (Jonakait et al., 1984; Vega et al., 1991; Herradon et al., 2008). More recently, the presence of TH protein (Brumovsky et al., 2006; Li et al., 2011) and transcript (Brumovsky et al., 2006) was shown in adult mouse DRG neurons innervating non-visceral tissue. TH expression has also been extensively documented in visceral sensory neurons in nodose, petrosal (Katz and Black, 1986; Ichikawa et al., 1991; Matsumoto et al., 2003) and glossopharyngeal (Fukuda et al., 2006) ganglia of adult rats. Our present study is the first showing that a population of mouse sensory DRG neurons projecting to the colorectum or the urinary bladder expresses TH. We show that these neurons are of different sizes, but usually medium to small-sized, are often peptidergic, and apparently lack an uptake mechanism. In contrast, we (Brumovsky et al., 2006) and others (Li et al., 2011) have shown that nonvisceral TH-expressing DRG neurons are mostly small and non-peptidergic, suggesting potential differences in sensory processing between visceral and non-visceral TH-expressing DRG neurons.
We also show that the proportion of TH-expressing visceral DRG neurons is not equal when comparing their numbers in LS relative to TL DRGs, presenting with threefold (colorectum) and fivefold (urinary bladder) higher percentages at LS levels. Differences in neurochemical expression between TL and LS DRGs innervating the mouse colorectum and urinary bladder have been previously described. For example, in DRGs innervating the colorectum, the protein expression of TRPV1 (Brierley et al., 2005; Christianson et al., 2006) and the P2X purinoceptor 3 (Brierley et al., 2005) was higher in TL vs. LS DRGs. Similarly mRNA expression of the transient receptor potential cation channel, member A1 was higher in TL vs. LS DRGs innervating the urinary bladder (La et al., 2011). In contrast, the expression of the outward rectifying potassium channel protein type-1 mRNA (La et al., 2011) and the VGLUT1 protein (Brumovsky et al., 2011; Brumovsky et al., unpublished) was found to be higher in LS vs. TL DRG neurons innervating the colorectum or the urinary bladder. Interestingly, at least some of these neurochemical differences appear to have a positive correlation with neuronal function (e.g., higher expression of TRPV1 equal stronger response to applied capsaicin) (Brierley et al., 2005; La et al., 2011). If differences in the expression of TH in visceral TL vs. LS DRG neurons influence their physiology, it remains to be established.
Expression of TH in colorectal and urinary bladder afferent nerve terminals
The immunohistochemical detection of TH in peripheral nerves/tissues is commonly utilized to identify sympathetic nerve fibers, and to differentiate them from sensory nerve fibers, including those innervating visceral organs. Thus, it is known that in the gut, the major contributor of mesenteric and submucosal blood vessel innervations is the sympathetic nervous system (see Brookes et al., 2009; Lomax et al., 2010). In the urinary bladder of the adult rat, few nerve fibers expressing TH innervate the detrusor muscle (Keast and De Groat, 1989) contrasting with the profuse parasympathetic innervations of the muscle and associated mucosa (Dickson et al., 2006; Biallosterski et al., 2010). As in the rat, we here show a sparse innervation of TH-IR nerve fibers in the detrusor muscle of the adult mouse urinary bladder, in contrast to their abundance in the adjacent lamina propria and mucosa. Our present results also confirm that the LSC is the origin of a large proportion of TH-IR fibers in the colorectum and the urinary bladder, with smaller contributions from the MPG. In the latter, however, most of the several retrogradely traced neurons from the urinary bladder lacked TH and NET-1, possibly representing cholinergic input, as previously shown in studies on rat (Keast et al., 1995) and guinea pig (Elfvin et al., 1997).
However, in a recent study in mouse, Tan and cols. (Tan et al., 2010) suggested that some TH-IR fibers apparently contacting jejunal myenteric plexus neurons, and with a confirmed extrinsic origin, could have a sensory origin. Our present results in mouse support the hypothesis of TH-expressing visceral sensory neurons as being the origin of at least a fraction of nerve fibers innervating the colorectum or the urinary bladder. However, additional sources of TH-IR nerve fibers should be taken into account. Thus, a small number of intrinsic neurons in the gut of mouse and guinea pig (but not the rat, see Phillips and Powley, 2007) express TH (Li et al., 2004; Qu et al., 2008). Also, it has been suggested that the vagus nerve in the rat reaches the distal colon (De Groat et al., 1996; Gschossmann et al., 2002; Tong et al., 2010), and since a number of vagal sensory neurons express TH (Ichikawa et al., 1991; Kummer et al., 1993; Matsumoto et al., 2003), they could contribute a fraction of TH-IR afferent fibers in the colorectum.
Most TH-IR colorectal and urinary bladder DRG neurons are peptidergic
CGRP is a peptidergic marker typically expressed in rodent visceral (Robinson and Gebhart, 2008) and non-visceral sensory neurons (McMahon and Priestley, 2005), normally used to differentiate sensory vs. autonomic fibers/neurons. In fact, the majority of colorectal (Robinson and Gebhart, 2008) or urinary bladder (De Groat, 2006) rodent DRG neurons are peptidergic. Accordingly, we found considerable colocalization of TH and CGRP in the soma of colorectal or urinary bladder (more in the latter than in the former) DRG neurons in the mouse. Additional colorectal or urinary bladder DRG neurons, as well as many other presumably non-visceral TH-IR sensory neurons lacking CGRP were also found, in agreement with studies in rat petrosal ganglia (Finley et al., 1992) and mouse non-visceral DRGs (Brumovsky et al., 2006; Li et al., 2011), where TH neurons are non-peptidergic.
However, and despite the clear colocalization of peptidergic and catecholaminergic markers in the cell body of several DRG neurons projecting to the colorectum or urinary bladder, TH- and CGRP-IR nerve fibers in both organs virtually always appeared as independent structures, often intertwining very closely. A similar neuroanatomical pattern has been reported for CGRPand ChAT-IR nerve fibers in the guinea pig bladder (Gillespie et al., 2006), CGRP- and NET-1-IR nerve fibers in the pelvic wall of the rat kidney (Kopp et al., 2007), and CGRP- and TH-IR nerve fibers in the mouse jejunum (Tan et al., 2010). Is then TH transported by the axons of DRG neurons? Some evidence in non-visceral neurons suggests that at least central DRG axons do not, as shown by the virtual absence of TH-LI fibers in dorsal roots and by the lack of effect of dorsal rhizotomy on TH-IR fibers in the dorsal horn (Brumovsky et al., 2006). In such a scenario, it could be speculated that TH was only a “somatic” enzyme with functions at the DRG cell soma. In fact, DA release from the somatodendritic structures of neurons in the substantia nigra has been previously demonstrated (Geffen et al., 1976; Sarre et al., 2004).
Expression of TH in visceral sensory neurons and catecholamine synthesis
The presence of TH-expressing visceral, as well as non-visceral (see Brumovsky et al., 2006; Li et al., 2011) DRG neurons is intriguing. Although a growing body of evidence suggests that some cranial and DRG sensory neurons are catecholaminergic and participate in neurotransmission, it remains controversial whether DA and/or NE, or even the DA precursor L-DOPA (Misu and Goshima, 1993), is the actual neurotransmitter(s) involved. Moreover, it has been suggested that TH in guinea pig cranial and DRG neurons is not functional, and therefore incapable of participating in the synthesis of dopamine (Kummer et al., 1990). In support, only weakly stained AADC, TH-negative DRG neurons were observed in the mouse (Brumovsky et al., 2006). In our present study in mouse, we also show that colorectal and urinary bladder DRG neurons in the mouse do not synthesize NET-1. Moreover, in non-visceral DRG neurons, DβH is reportedly absent in rat (Price, 1985; Vega et al., 1991) and mouse (Brumovsky et al., 2006). Altogether, the evidence suggests that neither dopamine nor noradrenaline are synthesized or taken up by DRG sensory neurons.
However, synthesis (Philippe et al., 1993; Weil-Fugazza et al., 1993) and release (Hertzberg et al., 1995; Iturriaga et al., 2003) of DA has been described in chick (Philippe et al., 1993) and rat (Weil-Fugazza et al., 1993) DRGs, and cat petrosal ganglion neurons (Hertzberg et al., 1995; Iturriaga et al., 2003). In support, coexpression of TH and AADC, paralleled by lack of DβH and phenyletanolamine N-methyl-transferase (PNMT), the latter enzyme responsible for the synthesis of epinephrine from NE (Katz and Black, 1986) was shown in rat petrosal ganglion neurons (Finley et al., 1992). Finally, in a recent work in adult rats, a noradrenergic phenotype is proposed for DRG neurons that respond to capsaicin (Dina et al., 2008).
Supporting an active role for TH in non-visceral DRG neurons, modulation of its synthesis in different conditions has been described. Thus, in rats after chronic constriction injury, the TH transcript (and also proenkephalin) is decreased (Herradon et al., 2008). A similar tendency was observed for TH transcript and protein in mice after sciatic nerve axotomy (Brumovsky et al., 2006). Furthermore, in transgenic mice where TH-expressing neurons and nerve fibers are identified by their content of enhanced green fluorescent protein (EGFP), the number of TH-expressing DRG NPs decreased after spinal nerve ligation (Xie et al., 2011).
Potential significance of TH/catecholaminergic sensory neurons in visceral sensation and pain
Information on the role of CAs in visceral pain is modest. At the spinal cord level, binding to the DA receptor 2 (DA2R) results on inhibition of spinal presynaptic N-type calcium currents of chemosensory visceral afferents and in inhibition of neurotransmitter release (Kline et al., 2009). Furthermore, the activation of DA1- or DA2Rs depresses the activity of rat DRG neurons (Li et al., 2005). The likely sources of DA in these cases are supraspinal descending dopaminergic projections (Millan, 2002). The question remains, if other peripheral sources such as sympathetic neurons (Rubi and Maechler, 2010) or even the sensory neurons described here and elsewhere (Brumovsky et al., 2006), could also participate. In the periphery, increases in the number of TH-IR nerve fibers innervating the urinary bladder have been shown in patients presenting with classic and non-ulcer interstitial cystitis (Peeker et al., 2000), and in NGF-overexpressing mice an important increase of TH-IR nerve fibers in the urinary bladder was observed (Schnegelsberg et al., 2010). The running hypothesis in these studies is that the increased catecholaminergic input to the urinary bladder depends on sympathetic neurons, and that it could be associated to pain signs and symptoms (Peeker et al., 2000; Schnegelsberg et al., 2010). However, the expression and potential role of TH in DRG neurons under the conditions described above remain to be analyzed.
Finally, recent studies highlight the importance of TH-expressing sensory neurons. It has been shown that the vesicular glutamate transporter type 2 (VGLUT2) is essential for normal perception of acute pain and heat hyperalgesia (Scherrer et al., 2010). In transgenic mice with deletion of VGLUT2 selectively from neurons expressing TH experience a decreased response to radiant heat (Lagerström et al., 2010). Therefore, the effect reported in TH-expressing sensory neurons with deleted VGLUT2, which also express TRPV1, suggest their relevancy to the physiology of thermal sensation (Lagerström et al., 2010). Unfortunately, a visceral pain phenotype was not assessed in these transgenic mice, and thus remains to be established. More recently, Li and Cols. (Li et al., 2011) showed that the population of THexpressing small diameter non-visceral sensory DRG neurons identified in the mouse (Brumovsky et al., 2011) is molecularly unique, produce C-low threshold mechanoreceptors (LTMRs) and only innervate hairy areas of the mouse skin. Moreover, the authors also demonstrated that different types of LTMRs, including those expressing TH, are functionally distinct mechanosensory end organs, and that their central projections are integrated within discrete dorsal horn LTMR columns (Li et al., 2011). More research is necessary to establish if similar molecular, morphological and physiological peculiarities also apply to TH-expressing DRG neurons innervating visceral organs.
CONCLUSION
We show that not only many colorectal or urinary bladder neurons in the LSC and some in the MPG, but also a number of DRG neurons retrogradely traced from these organs express TH and may contribute an additional source of THIR nerve fibers in the target organs. While the functional significance of the expression of this enzyme in visceral and non-visceral sensory neurons awaits further clarification, increasing evidence suggests that neurons expressing TH comprise a subpopulation serving important roles on the peripheral processing of sensation and pain.
> A fraction of colorectal or urinary bladder DRG neurons in the mouse express TH > A large proportion of these TH-positive DRG neurons is peptidergic > THpositive DRG neurons lack the norepinephrine transporter NET-1 > THexpressing nerve terminals in pelvic organs may also originate in sensory neurons> TH-expressing visceral DRG neurons may be relevant to sensation and pain.
Acknowledgments
We would like to thank Mr. Tim McMurray for his excellent technical assistance. We also thank Dr. Dave Robinson for valuable advice and assistance in tracing visceral sensory neurons. This study was supported by NIH awards R01 NS035790 and DK093525, an Austral University grant, and the Swedish Research Council.
Abbreviations
- AADC
aromatic aminoacid decarboxylase
- CGRP
calcitonin gene-related peptide
- ChAT
CA, catecholamine; choline acetyl transferase
- DA
dopamine
- DβH
DA beta-hydroxylase
- DRG
dorsal root ganglia
- FB
fast blue
- IB4
isolectin B4
- IR
immunoreactive
- LI
like-immunoreactivity
- LS
lumbosacral
- LSC
lumbar sympathetic chain
- LTMRs
low-threshold mechanoreceptors
- MPG
major pelvic ganglion
- NE
norepinephrine
- NET
NE transporter type 1
- NP
neuron profile
- RT-PCR
Reverse transcription polymerase chain reaction
- RT
room temperature
- TH
tyrosine hydroxylase
- TL
thoracolumbar
- TRPV1
transient receptor potential cation channel type-1
- VGLUT
vesicular glutamate transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adams JC. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem. 1992;40:1457–1463. doi: 10.1177/40.10.1527370. [DOI] [PubMed] [Google Scholar]
- Biallosterski BT, de Wachter SG, van Koeveringe GA, van Kerrebroeck PE, DE VJ, Mulder MT, Gillespie JI. Changes in bladder innervation in a mouse model of Alzheimer's disease. J. Chem. Neuroanat. 2010;39:204–210. doi: 10.1016/j.jchemneu.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W, III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol. 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, III, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Dinning PG, Gladman MA. Neuroanatomy and physiology of colorectal function and defaecation: from basic science to human clinical studies. Neurogastroenterol. Motil. 2009;21(Suppl 2):9–19. doi: 10.1111/j.1365-2982.2009.01400.x. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Villar MJ, Hokfelt T. Tyrosine hydroxylase is expressed in a subpopulation of small dorsal root ganglion neurons in the adult mouse. Exp. Neurol. 2006;200:153–165. doi: 10.1016/j.expneurol.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization - An integrated perspective. Auton Neurosci. 2010 doi: 10.1016/j.autneu.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky PR, Robinson DR, La JH, Seroogy KB, Lundgren KH, Albers KM, Kiyatkin ME, Seal RP, Edwards RH, Watanabe M, Hokfelt T, Gebhart GF. Expression of vesicular glutamate transporters type 1 and 2 in sensory and autonomic neurons innervating the mouse colorectum. J. Comp Neurol. 2011;519:3346–3366. doi: 10.1002/cne.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callsen-Cencic P, Mense S. Expression of neuropeptides and nitric oxide synthase in neurones innervating the inflamed rat urinary bladder. J. Auton. Nerv. Syst. 1997;65:33–44. doi: 10.1016/s0165-1838(97)00032-5. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- Coons AH. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- De Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br. J. Pharmacol. 2006;147(Suppl 2):S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC. Neuropeptides in pelvic afferent pathways. Experientia. 1987;43:801–813. doi: 10.1007/BF01945358. [DOI] [PubMed] [Google Scholar]
- De Groat WC, Card JP, Vizzard MA. Central nervous system sites labeled following injection of pseudorabies virus (PRV) into the distal colon or bladder of adult rats. Soc Neurosci Abst. 1996 [Google Scholar]
- Dickson A, Avelino A, Cruz F, Ribeiro-da-Silva A. Peptidergic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience. 2006;141:1633–1647. doi: 10.1016/j.neuroscience.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Dina OA, Khasar SG, essandri-Haber N, Bogen O, Chen X, Green PG, Reichling DB, Messing RO, Levine JD. Neurotoxic catecholamine metabolite in nociceptors contributes to painful peripheral neuropathy. Eur. J. Neurosci. 2008;28:1180–1190. doi: 10.1111/j.1460-9568.2008.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfvin LG, Holmberg K, Emson P, Schemann M, Hokfelt T. Nitric oxide synthase, choline acetyltransferase, catecholamine enzymes and neuropeptides and their colocalization in the anterior pelvic ganglion, the inferior mesenteric ganglion and the hypogastric nerve of the male guinea pig. J Chem. Neuroanat. 1997;14:33–49. doi: 10.1016/s0891-0618(97)10010-2. [DOI] [PubMed] [Google Scholar]
- Finley JC, Polak J, Katz DM. Transmitter diversity in carotid body afferent neurons: dopaminergic and peptidergic phenotypes. Neuroscience. 1992;51:973–987. doi: 10.1016/0306-4522(92)90534-9. [DOI] [PubMed] [Google Scholar]
- Forgie A, Kuehnel F, Wyatt S, Davies AM. In vivo survival requirement of a subset of nodose ganglion neurons for nerve growth factor. Eur. J. Neurosci. 2000;12:670–676. doi: 10.1046/j.1460-9568.2000.00951.x. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Ichikawa H, Terayama R, Yamaai T, Kuboki T, Sugimoto T. ASIC3- immunoreactive neurons in the rat vagal and glossopharyngeal sensory ganglia. Brain Res. 2006;1081:150–155. doi: 10.1016/j.brainres.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Furness JB. The organisation of the autonomic nervous system: peripheral connections. Auton Neurosci. 2006;130:1–5. doi: 10.1016/j.autneu.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol. 2004;72:143–164. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Gillespie JI, Markerink-VAN IM, DE VJ. Sensory collaterals, intramural ganglia and motor nerves in the guinea-pig bladder: evidence for intramural neural circuits. Cell Tissue Res. 2006;325:33–45. doi: 10.1007/s00441-006-0166-8. [DOI] [PubMed] [Google Scholar]
- Gschossmann JM, Mayer EA, Miller JC, Raybould HE. Subdiaphragmatic vagal afferent innervation in activation of an opioidergic antinociceptive system in response to colorectal distension in rats. Neurogastroenterol. Motil. 2002;14:403–408. doi: 10.1046/j.1365-2982.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J. Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Waymire JC. Activating antibodies to tyrosine hydroxylase. J. Biol. Chem. 1982;257:9416–9423. [PubMed] [Google Scholar]
- Herradon G, Ezquerra L, Nguyen T, Wang C, Siso A, Franklin B, Dilorenzo L, Rossenfeld J, Silos-Santiago I, Alguacil LF. Noradrenergic and opioidergic alterations in neuropathy in different rat strains. Neurosci. Lett. 2008;438:186–189. doi: 10.1016/j.neulet.2008.03.095. [DOI] [PubMed] [Google Scholar]
- Hertzberg T, Brosenitsch T, Katz DM. Depolarizing stimuli induce high levels of dopamine synthesis in fetal rat sensory neurons. Neuroreport. 1995;7:233–237. [PubMed] [Google Scholar]
- Hwang SJ, Oh JM, Valtschanoff JG. The majority of bladder sensory afferents to the rat lumbosacral spinal cord are both IB4- and CGRP-positive. Brain Res. 2005;1062:86–91. doi: 10.1016/j.brainres.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Jacobowitz DM, Winsky L, Helke CJ. Calretinin-immunoreactivity in vagal and glossopharyngeal sensory neurons of the rat: distribution and coexistence with putative transmitter agents. Brain Res. 1991;557:316–321. doi: 10.1016/0006-8993(91)90152-l. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Mo Z, Xiang M, Sugimoto T. Brn-3a deficiency increases tyrosine hydroxylase-immunoreactive neurons in the dorsal root ganglion. Brain Res. 2005;1036:192–195. doi: 10.1016/j.brainres.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Cerpa V, Zapata P, Alcayaga J. Catecholamine release from isolated sensory neurons of cat petrosal ganglia in tissue culture. Brain Res. 2003;984:104–110. doi: 10.1016/s0006-8993(03)03118-4. [DOI] [PubMed] [Google Scholar]
- Jonakait GM, Markey KA, Goldstein M, Black IB. Transient expression of selected catecholaminergic traits in cranial sensory and dorsal root ganglia of the embryonic rat. Dev. Biol. 1984;101:51–60. doi: 10.1016/0012-1606(84)90116-7. [DOI] [PubMed] [Google Scholar]
- Katz DM, Black IB. Expression and regulation of catecholaminergic traits in primary sensory neurons: relationship to target innervation in vivo. J. Neurosci. 1986;6:983–989. doi: 10.1523/JNEUROSCI.06-04-00983.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int. Rev. Cytol. 2006;248:141–208. doi: 10.1016/S0074-7696(06)48003-7. [DOI] [PubMed] [Google Scholar]
- Keast JR, De Groat WC. Immunohistochemical characterization of pelvic neurons which project to the bladder, colon, or penis in rats. J. Comp Neurol. 1989;288:387–400. doi: 10.1002/cne.902880303. [DOI] [PubMed] [Google Scholar]
- Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Keast JR, Luckensmeyer GB, Schemann M. All pelvic neurons in male rats contain immunoreactivity for the synthetic enzymes of either noradrenaline or acetylcholine. Neurosci. Lett. 1995;196:209–212. doi: 10.1016/0304-3940(95)11874-v. [DOI] [PubMed] [Google Scholar]
- Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J. Comp Neurol. 2000;424:577–587. [PubMed] [Google Scholar]
- Kline DD, Hendricks G, Hermann G, Rogers RC, Kunze DL. Dopamine inhibits N-type channels in visceral afferents to reduce synaptic transmitter release under normoxic and chronic intermittent hypoxic conditions. J. Neurophysiol. 2009;101:2270–2278. doi: 10.1152/jn.91304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Mwaka ES, Baba H, Kokubo Y, Yayama T, Kubota M, Nakajima H, Meir A. Microvascular system of the lumbar dorsal root ganglia in rats. Part II: neurogenic control of intraganglionic blood flow. J. Neurosurg. Spine. 2010;12:203–209. doi: 10.3171/2009.8.SPINE08895. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA, Mulder J, Hokfelt T. Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2-dependent activation of alpha1- and alpha2-adrenoceptors on renal sensory nerve fibers. Am. J. Physiol Regul. Integr. Comp Physiol. 2007;293:R1561–R1572. doi: 10.1152/ajpregu.00485.2007. [DOI] [PubMed] [Google Scholar]
- Kummer W, Bachmann S, Neuhuber WL, Hanze J, Lang RE. Tyrosine-hydroxylase-containing vagal afferent neurons in the rat nodose ganglion are independent from neuropeptide-Y-containing populations and project to esophagus and stomach. Cell Tissue Res. 1993;271:135–144. doi: 10.1007/BF00297551. [DOI] [PubMed] [Google Scholar]
- Kummer W, Gibbins IL, Stefan P, Kapoor V. Catecholamines and catecholamine-synthesizing enzymes in guinea-pig sensory ganglia. Cell Tissue Res. 1990;261:595–606. doi: 10.1007/BF00313540. [DOI] [PubMed] [Google Scholar]
- La JH, Schwartz ES, Gebhart GF. Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore-domain K(+) channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience. 2011;186:179–187. doi: 10.1016/j.neuroscience.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerström MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, Wood JN, Wallen-Mackenzie A, Kullander K. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M, Spector S, Sjoerdsma A, Udenfriend S. Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused guinea-pig heart. J. Pharmacol. Exp. Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- Li GH, Guan BC, Li ZW. Effects of dopamine, SKF-38393 and R(−)-NPA on ATP-activated currents in rat DRG neurons. Can. J. Physiol Pharmacol. 2005;83:267–277. doi: 10.1139/y05-010. [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J. Neurosci. 2004;24:1330–1339. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol. Motil. 2010;22:7–18. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Emori Y, Nakamura S, Shimizu K, Arai S, Abe K. DNA microarray cluster analysis reveals tissue similarity and potential neuron-specific genes expressed in cranial sensory ganglia. J. Neurosci. Res. 2003;74:818–828. doi: 10.1002/jnr.10814. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJ. If you assume, you can make an ass out of u and me': a decade of the disector for stereological counting of particles in 3D space. J. Anat. 1996;188(Pt 1):1–15. [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Priestley JV. Nociceptor Plasticity. In: Hunt SP, Koltzenburg M, editors. The Neurobiology of Pain. Oxford: Oxford Univ. Press; 2005. pp. 35–64. [Google Scholar]
- Millan MJ. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mulder J, Bjorling E, Jonasson K, Wernerus H, Hober S, Hokfelt T, Uhlen M. Tissue profiling of the mammalian central nervous system using human antibody-based proteomics. Mol. Cell Proteomics. 2009;8:1612–1622. doi: 10.1074/mcp.M800539-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Levitt M, Udenfriend S. Tyronsine hydroxylase: the initial step in norepinephrine biosynthesis. J. Biol. Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- Olsson C, Chen BN, Jones S, Chataway TK, Costa M, Brookes SJ. Comparison of extrinsic efferent innervation of guinea pig distal colon and rectum. J. Comp Neurol. 2006;496:787–801. doi: 10.1002/cne.20965. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Peeker R, Aldenborg F, Dahlstrom A, Johansson SL, Li JY, Fall M. Increased tyrosine hydroxylase immunoreactivity in bladder tissue from patients with classic and nonulcer interstitial cystitis. J. Urol. 2000;163:1112–1115. [PubMed] [Google Scholar]
- Philippe E, Zhou C, Audet G, Geffard M, Gaulin F. Expression of dopamine by chick primary sensory neurons and their related targets. Brain Res Bull. 1993;30:227–230. doi: 10.1016/0361-9230(93)90248-a. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. An immunohistochemical and quantitative examination of dorsal root ganglion neuronal subpopulations. J. Neurosci. 1985;5:2051–2059. doi: 10.1523/JNEUROSCI.05-08-02051.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Mudge AW. A subpopulation of rat dorsal root ganglion neurones is catecholaminergic. Nature. 1983;301:241–243. doi: 10.1038/301241a0. [DOI] [PubMed] [Google Scholar]
- Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv. 2008;8:242–253. doi: 10.1124/mi.8.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Rubi B, Maechler P. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let's seek the balance. Endocrinology. 2010;151:5570–5581. doi: 10.1210/en.2010-0745. [DOI] [PubMed] [Google Scholar]
- Sarre S, Yuan H, Jonkers N, Van HA, Ebinger G, Michotte Y. In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. J Neurochem. 2004;90:29–39. doi: 10.1111/j.1471-4159.2004.02471.x. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:22296–22301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. AmJ. Physiol Regul. Integr. Comp Physiol. 2010;298:R534–R547. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Kerrin A, Singer CA, Hennig GW, Gerthoffer WT, McDonnell O. Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience. 2008;153:518–534. doi: 10.1016/j.neuroscience.2008.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LL, Bornstein JC, Anderson CR. The neurochemistry and innervation patterns of extrinsic sensory and sympathetic nerves in the myenteric plexus of the C57Bl6 mouse jejunum. Neuroscience. 2010;166:564–579. doi: 10.1016/j.neuroscience.2009.12.034. [DOI] [PubMed] [Google Scholar]
- Tong WD, Ridolfi TJ, Kosinski L, Ludwig K, Takahashi T. Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol. Motil. 2010;22:688–693. doi: 10.1111/j.1365-2982.2009.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega JA, Amenta F, Hernandez LC, del Valle ME. Presence of catecholamine-related enzymes in a subpopulation of primary sensory neurons in dorsal root ganglia of the rat. Cell Mol. Biol. 1991;37:519–530. [PubMed] [Google Scholar]
- von Euler US. Adrenergic neurotransmitter functions. Science. 1971;173:202–206. doi: 10.1126/science.173.3993.202. [DOI] [PubMed] [Google Scholar]
- Wang HF, Shortland P, Park MJ, Grant G. Retrograde and transganglionic transport of horseradish peroxidase-conjugated cholera toxin B subunit, wheatgerm agglutinin and isolectin B4 from Griffonia simplicifolia I in primary afferent neurons innervating the rat urinary bladder. Neuroscience. 1998;87:275–288. doi: 10.1016/s0306-4522(98)00061-x. [DOI] [PubMed] [Google Scholar]
- Weil-Fugazza J, Onteniente B, Audet G, Philippe E. Dopamine as trace amine in the dorsal root ganglia. Neurochem Res. 1993;18:965–969. doi: 10.1007/BF00966754. [DOI] [PubMed] [Google Scholar]
- Xie W, Strong JA, Mao J, Zhang JM. Highly localized interactions between sensory neurons and sprouting sympathetic fibers observed in a transgenic tyrosine hydroxylase reporter mouse. Mol. Pain. 2011;7:53. doi: 10.1186/1744-8069-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244–253. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]