Irritable bowel syndrome: what is in a name?
In a paper recently published online and included in this issue of Therapeutic Advances in Gastroenterology [Camilleri, 2012], Dr Michael Camilleri challenged the validity of irritable bowel syndrome (IBS) both as a term and as a clinical diagnosis, coming to the conclusion that it is ‘time to avoid the diagnosis of “IBS” in gastroenterological practice and to replace it with more meaningful, pathophysiology-based diagnoses’. To emphasize the point he placed the term ‘diagnosis’ in parentheses in the title of the paper, thereby questioning its legitimacy.
In support of his objection to the name ‘irritable bowel syndrome’ Dr Camilleri cites quotations from two books by Lewis Carroll on the significance of names, implying that the term irritable bowel syndrome is neither precise nor meaningful. We agree; the name is lacking in many respects. The term ‘irritable’, which has an historic basis, does not faithfully reflect the disorder as we are coming to understand it today. In fact, it may do a disservice to patients who see it as a judgmental term that does not describe their symptoms and to physicians who are trying to explain the nature of the disorder to their patients. Is there an alternative term that might be better? A better term might be, for example, ‘sensitive bowel syndrome’, but that name is also too limited and does not reflect the range of factors involved. In any event, the name IBS has become so ingrained and common throughout the world that there is little chance or logic in trying to change it.
Irritable bowel syndrome: a diagnosis that has outlived its usefulness?
Of course, the more important issue raised by Dr Camilleri is whether IBS should be discarded as a diagnostic entity. We would suggest approaching this question by breaking it down into two parts: is the stage set for such a change now, and would abandoning the concept of IBS as a functional gastrointestinal (GI) disorder be advantageous to research and clinical practice at any time in the foreseeable future? Who might benefit from abandoning this diagnostic category? Patients, physicians, researchers, designers of clinical trials? Or none of the above?
In 2007 Kellow wrote in a paper in support of the Rome III criteria that ‘Other potential biomarkers in the functional GI disorders include mucosal histology, cardiovascular reactivity, gut permeability and blood, stool, and genetic markers. Unfortunately, none of these have as yet proved reliable or accurate enough to supplant, or form part of, the symptom-based criteria’ [Kellow, 2007]. Five years later we see no new support for a change to this statement.
How about the foreseeable future? Over recent decades research into the pathophysiology and treatment of IBS and publication of scientific papers on it in the medical literature have increased exponentially (Figure 1) and with it our understanding of the disorder. However, a satisfactory picture of its etiology has not yet emerged, and in fact, finding a single etiology is not likely. The main obstacle to a precise understanding of this disorder is its complex multidimensional nature. Multiple putative etiological factors have been proposed and studied (Table 1). Many have been implicated, in varying combinations, in the clinical presentation of IBS. In addition, psychosocial variables such as generalized and gut-specific anxiety, somatization, abuse history, poor coping skills, and inadequate social support have been implicated as modulating factors.
Figure 1.
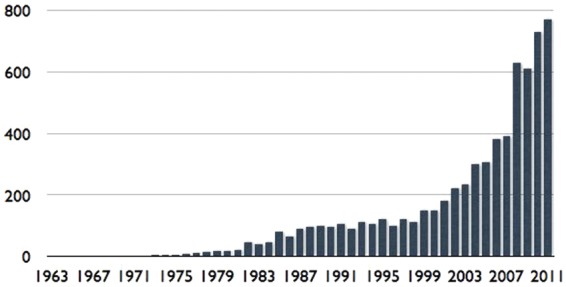
The exponential growth of scientific papers on IBS and the relationship to the publication of the Rome I, II, and III diagnostic criteria. Rome I was published in 1994, Rome II in 2000, and Rome III in 2006.
Table 1.
Factors implicated in the pathogenesis of irritable bowel syndrome indicating its complex, multidimensional nature.
| Disrupted motility |
| Stress |
| Food intolerance/allergy |
| Intestinal infection |
| Injury, e.g. abdominal or pelvic surgery |
| Intestinal immune disruption and/or inflammation |
| Changes in the intestinal microbiota/bacterial overgrowth |
| Genetic transmission |
| Abuse |
| Early life learning |
| Visceral hypersensitivity |
| Disrupted communication along the bidirectional brain–gut axis |
Further complicating our understanding of IBS is the question of whether IBS is a universal disorder with a similar genotype and phenotype in various geographical regions, as well as cultural and ethnic groups, a question that has generated increasing research interest over the last few years [Sperber, 2009; Sperber et al. 2012]. Yet, do all these unanswered questions mean that IBS is not a clinically useful or parsimonious diagnostic entity?
The rationale for symptom-based diagnosis
There is a basic rationale for the use of symptom-based criteria. Patients present to physicians with symptoms, not physiological dysfunction [Talley et al. 1998; Kellow, 2007] and these symptoms comprise symptom clusters that are consistent across clinical samples and in population studies [Tytgat, 2006]. The Rome criteria are modeled after the DSM system in psychiatry and provide diagnostic standards for clinical practice and clinical trials. Symptoms are the means by which patients communicate their problems to physicians at first presentation. In epidemiological terms symptoms have similar frequencies across populations and factor analysis has enabled the delineation of symptom-based subgroups. The characterization of these subgroups provides the basis for more specific physiological investigations. It was the use of symptom-based Rome diagnostic criteria that allowed for the explosion of research in testing for visceral sensation, brain–gut interactions, and microflora, taking the field significantly beyond the narrow confines of motility studies.
The search for biomarkers
The symptom-based approach to diagnosis stands in sharp contrast to attempts to reduce and condense IBS or transform it into individual ‘organic’ medical entities with specific biomarkers. The latter are believed to be not only necessary but also sufficient to explain pathophysiology, to serve as a clinical diagnostic criterion, and to guide patient management. While biomedical investigations may be of great value in explaining the basic mechanisms of ‘disease’, they do not encompass ‘illness’, the patient’s personal experience of ill health. The rationale is that there can be no ‘illness without a disease’ [Drossman, 1998, 1999]. Yet, the concept integrating the relationship of illness and disease is at the basis of the bio-psycho-social approach to patient care and is relevant to the treatment of a majority of patients who come to physicians with ‘unexplained’ medical symptoms [Sharpe and Carson, 2001]. However, this perspective contradicts the medical education that most of us have received. Since we are trained to look for diagnostic biomarkers to reach clear-cut diagnoses and these patients have none, we may relentlessly seek a diagnosis with further tests, since the all too familiar symptom pattern does not seem sufficient for a ‘real’ diagnosis. Eventually we feel confused and ‘drained’ [Drossman, 2001]. After giving up, we may then come to view these patients as ‘difficult’ [Schwenk et al. 1989; Guthrie and Creed, 1994] rather than ‘challenging’ and often discharge them from our care with the statement that ‘nothing is wrong with you’ or ‘it’s all in your head’. Interestingly, accepting the symptom-based diagnosis allows both physician and patient to move forward from the diagnostic process toward working together on care [Drossman, 2005].
In his paper, Dr Camilleri quotes Dr Walter C. Alvarez, who is credited with coining the term ‘irritable colon’ [Alvarez, 1915], as saying that ‘The great thing in handling these persons is not to reinforce their fear that there is something seriously wrong with the colon’ [Alvarez, 1947]. This statement speaks, to our mind, to the minimizing of testing and the importance of a bio-psycho-social approach to treatment [Drossman, 1998]. The extensive search for a positive test often serves to reinforce the fear that something is being missed and limits the development of a therapeutic partnership.
Thus, the search for a pathophysiological-based diagnosis can lead in the short term to a return to ‘diagnosis by exclusion’, which the symptom-based Rome criteria (together with ‘alarm features’) has replaced, and in the long run to the ‘organification’ or ‘medicalization’ of disorders that have interacting structural, functional and psychosocial components [Drossman, 2003].
Specific physiological examples
Dr Camilleri favors the use of diagnostic testing in patients with chronic constipation to differentiate between IBS constipation and other problems, such as evacuation disorders and slow transit constipation. This would include tests of transit time and tests of pelvic floor function. While these tests are justified in some patients, are they really justified or necessary in all patients with chronic constipation, especially those who are not seen in the tertiary setting?
One example that Dr Camilleri refers to is physiological testing for orocecal and colonic transit time (CTT), including radio-opaque markers, scintigraphy, and wireless motility capsule (WMC). While we agree that tests of transit time are useful in selected patients, the results may be approximated by subjective patient reporting, such as the Bristol Stool Form Scale (BSFS). For example, Manabe and colleagues, in a study of CTT in patients with lower functional gastrointestinal disorders (FGIDs), found evidence of abnormal transit in only 30% of the patients [Manabe et al. 2010]. Although the authors suggest that scintigraphic CTT fulfills many qualifications as a biomarker in lower FGID, no data on sensitivity or specificity are presented. In addition, Tornblom and colleagues found that 80% of patients with IBS had normal CTT [Tornblom et al. 2012]. Saad and colleagues did a post hoc analysis of data on subjects with chronic constipation and healthy controls who underwent the three types of transit time tests cited above, and completed the BSFS [Saad et al. 2010]. They found that the sensitivity and specificity of the BSFS (average stool form cutoff value of 2.5) were 82% and 83%, respectively, compared with CTT by WMC, and 80% and 81%, respectively compared with radio-opaque markers. The authors concluded that stool form, as reported by the patient, is a simple and useful office-based surrogate for CTT tests.
Dr Camilleri presents pelvic floor dyssenergia (PFD) as an alternative diagnosis in patients with constipation. We agree that a proportion of patients with chronic constipation suffer from PFD and can benefit from specific treatment, such as biofeedback [Chiarioni et al. 2005; Shim et al. 2011]. Since many of these patients have symptoms that can be related to obstructive defecation, such as straining, a feeling of incomplete evacuation, a sensation of anorectal obstruction, and/or the need for manual maneuvers to facilitate defecation such as digital evacuation or support of the pelvic floor [Wald et al. 2006], a high level of suspicion for PFD can be reached by means of an appropriate history supported by a properly conducted rectal examination, an office-based balloon expulsion test, and a radio-opaque marker test result that is consistent with an evacuation disorder. We agree that these patients should have an appropriate workup of pelvic floor anatomy and function. However, even in patients with a manometric, defecographic, and/or ultrasonic diagnosis of PFD there are often other elements of IBS that need to be treated together with the obstructive defecation, such as abdominal pain, bloating and flatulence, and psychological comorbidity, such as anxiety and somatization. In these cases, which are similar to the cases of IBS-celiac and IBS-inflammatory bowel disease (IBD) discussed below and might be termed PFD-IBS, monotherapy such as biofeedback may not be sufficient in all patients and psychosocial factors should be addressed. Interestingly, a major study showing the efficacy of biofeedback for patients with PFD used global satisfaction with therapy as the outcome variable [Shim et al. 2011]. Satisfaction with treatment is a multidimensional variable that includes clinical and psychosocial elements. In the same study the variable ‘willingness to participate in treatment’, another multidimensional variable, was found to predict treatment success, again defined as satisfaction with treatment, in the multilinear regression analysis, although not in the final logistic regression analysis model, because the data were skewed with most of the patients having an extremely high score on this variable. Willingness to participate has been demonstrated to predict successful biofeedback outcomes for chronic constipation in previous reports as well [Gilliland et al. 1997].
Can the diagnosis of irritable bowel syndrome be made with confidence using symptom-based diagnostic criteria or are we missing other diagnoses?
In an area that lacks gold standards for diagnosis it is logical to use these symptoms as the basis for diagnosis together with a patient-tailored workup of tests and procedures. Cash and colleagues investigated the tests that are reasonable to conduct in the presence and absence of ‘red flags’, such as age over 50, unexplained weight loss, unexplained rectal bleeding, and unexplained anemia [Cash et al. 2002]. The result is a surprisingly minimal number of recommended tests.
Accumulating evidence has shown that the diagnosis of IBS can be made with reasonable confidence in patients who fulfill the symptom-based criteria and who either do not have alarm symptoms or who have a minimal extension of the workup because of an alarm symptom, for example age or rectal bleeding.
Irritable bowel syndrome and its association with other comorbid functional disorders
The use of biomarkers, even if available, cannot explain other characteristics of IBS such as comorbidity with other structural and functional disorders, the waxing and waning of symptoms, and the relation between disease severity and psychosocial factors.
For example, we are becoming increasingly aware of the comorbidity between IBS and structural disorders such as celiac disease and IBD. In a recent study, Dorn and colleagues showed that psychosocial factors are more important than disease activity in determining GI symptoms and health status in adults referred to a major medical center with a diagnosis of celiac disease confirmed by biopsy [Dorn et al. 2010]. These patients actually have concomitant IBS and celiac disease so that the degree of activity of the celiac disease (as determined by Marsh classification or biomarkers) does not necessarily explain the symptoms of pain and diarrhea experienced by the patients. Physicians treating these patients should be aware of the possibility of overlapping IBS.
In fact, in the referral-based population reported by Dorn and colleagues over half of the patients met Rome III criteria for IBS, and yet, almost 80% were considered to have ‘classic’ celiac disease as the explanation for their IBS-like symptoms. We might speculate that patients with celiac disease who report diarrhea and abdominal pain are more likely to have ‘IBS-celiac’, a comorbid association that is similar to the increasingly recognized clinical entity ‘IBS-IBD’ [Grover et al. 2009]. Furthermore, as in postinfectious IBS [Gwee et al. 1996] as well as IBS-IBD [Simren et al. 2002], these symptoms and behaviors are strongly influenced by psychosocial factors [Levy et al. 2006].
This being the case, the examples given by Dr Camilleri, such as celiac disease, not only comprise a small proportion of patients who might otherwise have been diagnosed with IBS, but they actually may have overlapping disorders. Many of these patients do not respond to gluten-free diets.
The most characterized comorbid association of IBS with functional disorders in other organ systems is with fibromyalgia, but multiple associations with other comorbid conditions have been identified (Table 2). Patients with more than one comorbid condition have greater symptom severity, more impaired health-related quality of life, lower coping skills, and reduced social support compared with those with only one functional syndrome [Sperber et al. 1999a, 1999b]. The various functional disorders have important characteristics in common, including epidemiology (female predominance), pathophysiology (inflammation, hypersensitivity, impaired central processing of afferent sensory information, role of serotonin, psychological distress and somatization, the role of stress and life events), diagnosis (symptom based), the central role of the patient–physician relationship in therapy, and common therapeutic modalities (antidepressants, hypnosis, cognitive-behavioral therapy, etc.) [Sperber and Dekel, 2010].
Table 2.
Overlapping functional syndromes. Multiple comorbid conditions in the same patients cause increased disease severity, impaired quality of life, and increased psychopathology. These aspects of functional disorders, which have a strong bearing on the therapeutic approach, cannot be diagnosed or assessed by biomarkers.
| Functional dyspepsia and other functional GI disorders |
| Fibromyalgia |
| Chronic fatigue syndrome |
| Multiple chemical sensitivity syndrome |
| Post-traumatic stress disorder |
| Chronic pelvic pain |
| Psychological comorbidity |
| Somatization disorder |
| Depression |
| Anxiety |
| Panic disorder |
| Interstitial cystitis and dysuria |
| Migraine and tension headaches |
| Temperomandibular joint disorder |
| Sexual-related disorders |
| Dyspareunia |
| Exacerbation of IBS during menses |
| Decreased libido |
GI, gastrointestinal; IBS, irritable bowel syndrome.
These overlapping conditions all share a central sensitization component and have been called central sensitivity syndromes [Yunus, 2007, 2008, 2012]. They are best understood from a bio-psycho-social perspective, which is also the basis for optimal treatment. They cannot be understood, diagnosed or assessed by means of biomarkers alone.
The multidimensional approach
Some might call the search for biomarkers the ‘science of medicine’ while treatment of patients with unexplained medical symptoms and disorders might be called the ‘art of medicine’. We believe the two to be inseparable; taken together they provide the optimal approach to patient care, that is, the ‘science of the art of medicine’ [Sperber, 2006].
To advance this science of the art of medicine the next step in the evolution of the Rome process for characterizing IBS will be the development of a multidimensional clinical approach to help in the understanding of clinical subsets of patients with IBS. This strategy will profile patients through several dimensions over and above the categorical diagnostic criteria primarily for the purpose of treatment planning. This could include clinical subsets (e.g. IBS constipation, IBS diarrhea or IBS mixed, or postinfectious), psychosocial descriptors, and biomarkers/physiological descriptors, as well as the overall impact (overall severity), all of which together may lead to more patient-specific treatments [Drossman et al. 2011]. Thus, for example, a patient may be diagnosed as having diarrhea-predominant post-infectious IBS of high-grade severity and with fecal incontinence, comorbid chronic fatigue, anxiety, and severely impaired quality of life. This description might also help identify the need for physiological assessment such as ano-rectal motility for better characterization of the fecal incontinence, or bile malabsorption as an element of the diarrhea, or a psychological consultation for treatment. In comparison, a patient who meets criteria for IBS-constipation having abdominal pain with infrequent bowel movements and severe straining and no psychosocial difficulties may also undergo a colonic transit time study or an ano-rectal motility assessment for physiological characterization, which could indicate focused treatment with medication and biofeedback for PFD. This clinical profiling may address some of Dr Camilleri’s concerns (shared by us) by including in the characterization of patients IBS biomarkers and physiological descriptors. These descriptors would not affect the diagnosis of IBS per se, but could have a salutary effect by setting the stage for a more individualized, targeted treatment. This approach would also allow for the example discussed above in which a patient can have concomitant IBS and PFD, perhaps modulated by psychosocial distress.
Conclusions
In summary, IBS is a complex, multidimensional disorder. It is an illness that fits the bio-psycho-social model and is best diagnosed, characterized, and treated from that perspective. The fact that the historic name ‘irritable bowel syndrome’ may not express this condition does not mean that the diagnosis itself should be abandoned. We believe that the diagnosis of IBS plays a major facilitating and fostering role in clinical practice, basic and clinical research, and clinical drug trials. Thus it is incumbent upon us, together with the search for a more profound pathophysiological understanding of this disorder, to increase our knowledge of its bio-psycho-social aspects and to improve our symptom-based diagnostic criteria in an ongoing process.
Biomarkers (as they become available) and physiological descriptors can be integrated into a multidimensional profile of patients with IBS. This integrated approach will lead to more optimal treatment of patients, a goal that is shared by all.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
References
- Alvarez W. (1915) The motor functions of the intestine from a new point of view. JAMA 65: 388–394 [Google Scholar]
- Alvarez W. (1947) Indigestion and abdominal pain with negative findings. Canadian Medical Association Journal 57: 425–432 [PubMed] [Google Scholar]
- Camilleri M. (2012) Irritable bowel syndrome: how useful is the term and the ‘diagnosis’? Therapeutic Advances in Gastroenterology 27 March (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash B., Schoenfeld P., Chey W. (2002) The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. American Journal of Gastroenterology 97: 2812–2819 [DOI] [PubMed] [Google Scholar]
- Chiarioni G., Salandini L., Whitehead W. (2005) Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 129: 86–97 [DOI] [PubMed] [Google Scholar]
- Dorn S., Hernandez L., Minaya M., Morris C., Hu Y., Lewis S., et al. (2010) Psychosocial factors are more important than disease activity in determining gastrointestinal symptoms and health status in adults at a celiac disease referral center. Digestive Diseases and Sciences 55: 3154–3163 [DOI] [PubMed] [Google Scholar]
- Drossman D. (1998) Presidential address: gastrointestinal illness and the biopsychosocial model. Psychosom Med 60: 258–267 [DOI] [PubMed] [Google Scholar]
- Drossman D. (1999) The Rome criteria process: diagnosis and legitimization of irritable bowel syndrome. Am J Gastroenterol 94: 2803–2807 [DOI] [PubMed] [Google Scholar]
- Drossman D. (2001) Challenges in the physician–patient relationship: feeling ‘drained’. Gastroenterology 121: 1037–1038 [DOI] [PubMed] [Google Scholar]
- Drossman D. (2003) The ‘organification’ of functional GI disorders: implications for research. Gastroenterology 124: 6–7 [DOI] [PubMed] [Google Scholar]
- Drossman D. (2005) Functional GI disorders: what’s in a name? Gastroenterology 128: 1771–1772 [DOI] [PubMed] [Google Scholar]
- Drossman D., Chang L., Bellamy N., Gallo-Torres H., Lembo A., Mearin F., et al. (2011) Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol 106: 1749–1759 [DOI] [PubMed] [Google Scholar]
- Gilliland R., Heymen S., Altomare D., Park U., Vickers D., Wexner S. (1997) Outcome and predictors of success of biofeedback for constipation. Br J Surg 84: 1123–1126 [PubMed] [Google Scholar]
- Grover M., Herfarth H., Drossman D. (2009) The functional–organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease–irritable bowel syndrome. Clin Gastroenterol Hepatol 7: 48–53 [DOI] [PubMed] [Google Scholar]
- Guthrie E., Creed F. (1994) The difficult patient: treating the mind and the gut. Eur J Gastroenterol Hepatol 6: 489–494 [Google Scholar]
- Gwee K., Graham J., Mckendrick M., Collins S., Marshall J., Read N. (1996) Psychological scores and persistence of irritable bowel syndrome after infectious diarrhea. Lancet 347: 150–153 [DOI] [PubMed] [Google Scholar]
- Kellow J. (2007) The ‘pro’ case. The Rome III criteria. Neurogastroenterol Motil 19: 787–792 [DOI] [PubMed] [Google Scholar]
- Levy R., Olden K., Naliboff B., Bradley L., Francisconi C., Drossman D., et al. (2006) Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology 130: 1447–1458 [DOI] [PubMed] [Google Scholar]
- Manabe N., Wong B., Camilleri M., Burton D., Mckinzie S., Zinsmeister A. (2010) Lower functional gastrointestinal disorders: evidence of abnormal colonic trasnsit in a 287 patient cohort. Neurogastroenterol Motil 22: 293–e282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad R., Rao S., Koch K., Kuo B., Parkman H., Mccallum R., et al. (2010) Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol 105: 403–411 [DOI] [PubMed] [Google Scholar]
- Schwenk T., Marquez J., Lefever R., Cohen M. (1989) Physician and patient determinants of difficult physician–patient relationships. J Fam Pract 28: 59–63 [PubMed] [Google Scholar]
- Sharpe M., Carson A. (2001) ‘Unexplained’ somatic symptoms, functional syndromes, and somatization: do we need a paradigm shift? Ann Intern Med 134: 926–930 [DOI] [PubMed] [Google Scholar]
- Shim L., Jones M., Prott G., Morris L., Kellow J., Malcolm A. (2011) Predictors of outcome of anorectal biofeedback therapy in patients with constipation. Aliment Pharmacol Ther 33: 1245–1251 [DOI] [PubMed] [Google Scholar]
- Simren M., Axelsson J., Gillberg R., Abrahamsson H., Svedlund J., Bjornsson E. (2002) Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol 97: 389–396 [DOI] [PubMed] [Google Scholar]
- Sperber A. (2006) Treating IBS patients: the science of the art of medicine. US Gastroenterol Rev (2): 73–75 [Google Scholar]
- Sperber A. (2009) The challenge of cross-cultural, multi-national research: potential benefits in the functional gastrointestinal disorders. Neurogastroenterol Motil 21: 351–360 [DOI] [PubMed] [Google Scholar]
- Sperber A., Atzmon Y., Neumann L., Weisberg I., Shalit Y., Abu-Shakrah M., et al. (1999a) Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. Am J Gastroenterol 94: 3541–3546 [DOI] [PubMed] [Google Scholar]
- Sperber A., Carmel S., Atzmon Y., Weisberg I., Shalit Y., Neumann L., et al. (1999b) The Sense of coherence index and the irritable bowel syndrome. A cross-sectional comparison among irritable bowel syndrome patients with and without coexisting fibromyalgia, irritable bowel syndrome non-patients, and controls. Scand J Gastroenterol 34: 259–263 [DOI] [PubMed] [Google Scholar]
- Sperber A., Dekel R. (2010) Irritable bowel syndrome and co-morbid gastrointestinal and extra-gastrointestinal functional syndromes. J Neurogastroenterol Motil 16: 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber A., Drossman D., Quigley E. (2012) IBS – the global perspective (a Rome Foundation-WGO Symposium) Am J Gastroenterol (in press): [Google Scholar]
- Talley N., Boyce P., Jones M. (1998) Identification of distinct upper and lower gastrointestinal symptom groupings in an urban population. Gut 42: 690–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornblom H., Van Oudenhove L., Sadik R., Abrahamsson H., Tack J., Simren M. (2012) Colonic transit time and IBS symptoms: what’s the link? Am J Gastroenterol 107: 754–760 [DOI] [PubMed] [Google Scholar]
- Tytgat G. (2006) Foreword II. In: Drossman D., Corazziari E., Delvaux M., Spiller R., Talley N., Thompson W., et al. (eds), Rome III. The Functional Gastrointestinal Disorders, 2nd ed McLean, VA: Degnon Associates, Inc, pp. xxiii–xxv [Google Scholar]
- Wald A., Bharucha A., Enck P., Rao S. (2006) Functional anorectal disorders. In: Drossman S., Corazziari E., Delvaux M., Spiller R., Talley N., Thompson W., et al. (eds), Rome III. The Functional Gastrointestinal Disorders. McLean, VA: Degnon Associates, Inc, pp. 639–685 [Google Scholar]
- Yunus M. (2007) Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum 36: 339–356 [DOI] [PubMed] [Google Scholar]
- Yunus M. (2008) Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum 37: 339–352 [DOI] [PubMed] [Google Scholar]
- Yunus M. (2012) The prevalence of fibromyalgia in other chronic pain conditions. Pain Res Treat 2012: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]


