Abstract
Clostridium difficile-associated diarrhea (CDAD) is the most common cause of healthcare-associated diarrhea. The current recommended treatment regimens of metronidazole and vancomycin have not changed in nearly 25 years. Fidaxomicin, an exceedingly narrow spectrum macrolide antibiotic, was recently approved for the treatment of CDAD. In phase III clinical trials, fidaxomicin was noninferior to vancomycin in achieving clinical cure of CDAD. Furthermore, fidaxomicin was associated with fewer recurrences of CDAD compared with vancomycin in clinical trials. These results, combined with the ease of administration and a good safety profile, make fidaxomicin an attractive treatment option for treating CDAD.
Keywords: Clostridium difficile, fidaxomicin, vancomycin
Background
Clostridium difficile, a spore-forming gram-positive anaerobic bacillus, is a major cause of healthcare-and antibiotic-associated diarrhea [Cohen et al. 2010]. Hospital discharges with C. difficile-associated diarrhea (CDAD) listed as a diagnosis increased from 31 to 61/100,000 between 1998 and 2003 [McDonald et al. 2006]. The most common symptoms of infection include diarrhea, abdominal cramping and peripheral leukocytosis [Cohen et al. 2010]. Risk factors for CDAD include prolonged hospitalizations or extensive exposure to healthcare, advanced age, prolonged antibiotic exposure, history of nonsurgical gastrointestinal (GI) procedures, use of proton-pump inhibitors and feeding tubes [Bignardi, 1998]. C. difficile produces toxin A and B which cause mucosal damage and fluid secretion leading to diarrhea [Kelly and LaMont, 2008]. A third toxin, binary toxin, has also been discovered but the significance and virulence of this toxin are unknown [McDonald et al. 2005]. However, it has been suggested that binary toxin may improve the adhesion of bacteria to target cells [Schwan et al. 2009].
More virulent strains of C. difficile, such as the North American Pulse-field type 1 pattern (on gel electrophoresis) and a BI pattern (on restriction endonuclease analysis), and type 027 (on ribotyping) (BI/NAP1/027) strain, have emerged which now threaten a larger, healthier population [Kelly and LaMont, 2008; McDonald et al. 2005; Pepin et al. 2004; Centers for Disease Control and Prevention, 2005; Abrahamian et al. 2006]. Outbreaks of BI/NAP1/027 in Canada and the United States have been associated with high rates of morbidity and mortality. The increased virulence of this strain is likely multifactorial, related to increased sporulation activity, increased toxin production as well as increased fluoroquinolone resistance [Kelly and LaMont, 2008; McDonald et al. 2005; Dubberke and Wertheimer, 2009].
Current treatment strategies
The treatment options for CDAD have not changed in almost 25 years and include metronidazole and vancomycin depending on disease severity [Cohen et al. 2010]. Metronidazole (500 mg orally, three times per day) is recommended as initial treatment for mild to moderate disease. Vancomycin is the only medication licensed by the US Food and Drug Administration for the treatment of CDAD and is generally reserved for more severe cases of C. difficile, or if metronidazole is ineffective at curing disease.
There are a number of challenges associated with these therapies. First, recurrent CDAD has become a significant problem linked to prolonged hospitalizations and high medical costs as well as future recurrences [Ghantoji et al. 2010; Johnson, 2009]. The mechanism for recurrent infection is likely related to inadequate immune response and disruption of the gut flora [Johnson, 2009]. Approximately 25% of patients adequately treated with either metronidazole or vancomycin experience recurrences [Kelly and LaMont, 2008]. Both metronidazole and vancomycin have been shown to alter the gut flora, which may contribute to recurrent disease [Kelly and LaMont, 2008; Al-Nassir et al. 2008; Louie et al. 2009a]. Furthermore, C. difficile spores can persist despite adequate therapy with either metronidazole or vancomycin. Approximately 20–30% of hospitalized patients are colonized with C. difficile versus 3% of outpatients [Bartlett, 2002]. The repercussions of asymptomatic colonization with C. difficile are unknown. In addition, there have been multiple reports of growing resistance and poor outcomes associated with metronidazole [Zar et al. 2007; Bartlett, 2008; Louie et al. 2011; Musher et al. 2005]. Finally, it has also been shown that treatment with vancomycin or metronidazole promotes overgrowth of vancomycin-resistant enterococci [Al-Nassir et al. 2008].
A number of other therapies are currently being studied as alternative treatments for C. difficile. Nitazoxanide is a 5-nitrothiazole compound used for treatment of helminthic and protozoal parasites. In vitro, nitazoxanide has been shown to be active against C. difficile [Freeman et al. 2011]. Teicoplanin, a glycopeptide similar to vancomycin, has been studied internationally as a treatment for CDAD and shown to be effective [Venuto et al., 2010]. Rifaximin, a nonabsorbable oral antibiotic that achieves high colonic concentrations, has been studied in prevention of recurrent disease. In a recent clinical trial, patients with CDAD who received a course of rifaximin after treatment with metronidazole or vancomycin experienced few recurrences [Garey et al. 2011]. However, there have been studies which show that C. difficile can easily develop resistance against rifaximin [Goldstein et al. 2011]. More recently, LFF571, a novel antimicrobial agent which targets the protein synthesis elongation factor Tu, was found to have potent activity against C. difficile and other anaerobes in the gut flora in vitro [Citron et al. 2012]. Fidaxomicin (developed by Optimer Pharmaceuticals, San Diego, CA, USA) was recently approved for the treatment of CDAD. This review will focus on fidaxomicin as an alternative therapy for treatment of CDAD infections.
Mechanism of action
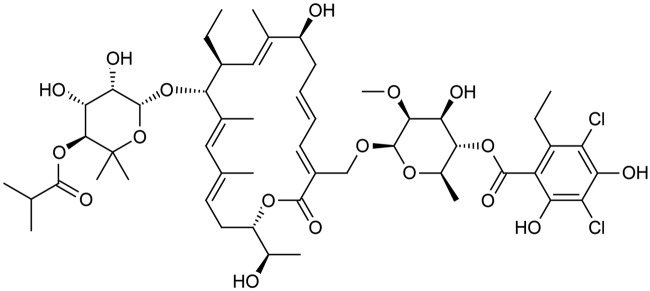
Fidaxomicin is a narrow spectrum 18-ring macrolide antibiotic [Gerber and Ackermann, 2008]. Originally known as lipiarmycin A4, this compound was first discovered in 1975 produced by Actinoplanes deccanensis [Coronelli et al. 1975]. The mechanism of action is inhibition of RNA transcription at the sigma subunit and mobile-clamp domain β’ of the RNA polymerase [Gualtieri et al. 2006; Tupin et al. 2010]. The major metabolite of fidaxomicin, OP-1118, is also active against C. difficile and formed by hydrolysis of the isobutyryl ester located at the 4″ position of fidaxomicin [Babakhani et al. 2011]. The molecular structure of fidaxomicin is shown in Figure 1.
Figure 1.
Molecular structure of fidaxomicin.
In vitro, fidaxomicin is bacteriocidal against many species of Clostridia and all types of C. difficile compared with vancomycin, which is bacteriostatic [Ackermann et al. 2004; Credito and Appelbaum, 2004; Finegold et al. 2004; Karlowsky et al. 2008; Hecht et al. 2007]. In vitro, fidaxomicin kills organisms more rapidly with a prolonged postantibiotic effect (5.5–10 h) compared with vancomycin (0–1.5 h) [Babakhani et al. 2011; Biedenbach et al. 2010]. Fidaxomicin is also active against virulent forms of C. difficile, including BI/NAP1/027 strain. [Louie et al. 2011]. The Minimum Inhibitory Concentration required to inhibit the growth of 90% of organisms (MIC90) of fidaxomicin against C. difficile ranges between 0.0078 and 0.25 µg/ml [Ackermann et al. 2004; Credito and Appelbaum, 2004; Finegold et al. 2004]. Fidaxomicin is poorly active against gram-negative organisms, Bacteroides spp. and Candida spp. [Finegold et al. 2004]. In vitro, fidaxomicin was tested against gram-positive organisms isolated from hospitalized patients and found to have minimal bacteriocidal activity against Stapylococcus aureus, coagulase-negative staphylococci (CoNS), Enterococcus faecalis and Enterococcus faecium [Biedenbach et al. 2010].
In contrast to current treatment, fidaxomicin has a narrow spectrum of activity and spares a majority of the gut flora [Louie et al. 2009a; Tannock et al. 2010]. From phase II clinical trials, fecal samples obtained from 23 patients who received fidaxomicin for treatment of CDAD were matched (demographically and symptomatically) to 8 patients with CDAD who received vancomycin for 10 days as standard treatment. In patients treated with vancomycin, there was a decrease in normal bacteria of the human colon and an overgrowth of enterobacteria [Tannock et al. 2010]. These findings suggest that vancomycin and fidaxomicin are both effective at inhibiting C. difficile. However, fidaxomicin does not alter the microbiota, which may be a protective mechanism against future recurrences.
Pharmacokinetics
Fidaxomicin is almost completely insoluble in water at pH value found in the GI tract with minimal systemic absorption as initially shown in animal models [Gerber and Ackermann, 2008; Swanson et al. 1991]. In hamsters, fidaxomicin was not detected in the serum when animals were treated with a single oral dose of 25 mg/kg. Low doses (0.2 mg/kg) protected clindamycin-treated hamsters from fatal colitis [Swanson et al. 1991]. These results were compared with vancomycin, which failed to prevent colitis [Swanson et al. 1991]. There have also been confirmatory studies in rats and monkeys showing minimal systemic absorption of fidaxomicin [Gerber and Ackermann, 2008]. In addition, there were no drug-related adverse effects appreciated in rats or monkeys following oral administration of fidaxomicin for 28 consecutive days at doses of up to 90 mg/kg/day [Shue et al. 2008].
In 2004, the initial phase I clinical trials were conducted as double-blind, randomized, placebo-controlled, dose-escalation studies in two parts, phase IA and phase IB, to evaluate the safety and pharmacokinetics of fidaxomicin [Shue et al. 2008]. In phase IA, 16 healthy volunteers received single escalating doses of fidaxomicin at 100, 200, 300 or 450 mg. Each patient received two escalating doses of the study medication in a crossover manner, with a washout period between treatments. The phase IB trial was designed to evaluate multiple oral doses of fidaxomicin. A total of 24 healthy volunteers received fidaxomicin at doses of 150, 300 and 450 mg, in three groups. At each dose level, six volunteers were randomized to receive the active drug and two volunteers received placebo.
In both phase IA and phase IB, blood, urine and fecal samples were collected. The plasma blood levels in all patients were mostly below 5 ng/ml, which was the lower limit of quantification. The highest plasma concentration reported (6.7 ng/ml at 4 h post dose) was in a healthy volunteer who received a 450 mg dose. There was no incremental accumulation of fidaxomicin in the plasma in the multidose arm. Fidaxomicin was eliminated with a half life of 0.93–2.77 h (calculated from the 450 mg dose) and was well tolerated with minimal adverse effects. There were no levels demonstrated in the urine in healthy volunteers. High fecal concentrations of fidaxomicin and its metabolite OP-1118 were confirmed in all volunteers, which were also shown to be dose related. The total fecal recovery of fidaxomicin and its metabolite from the 200 mg and 300 mg dose groups was 100%.
Clinical studies
In phase II clinical trials, patients over the age of 18 years with a positive C. difficile toxin result and clinical signs and symptoms of CDAD were included [Louie et al. 2009b]. However, only patients with a primary episode or first recurrence were eligible to participate. Patients with fulminant C. difficile infections (more than 12 bowel movements/day, severe abdominal symptoms, white blood cell count 30,000 cells/ml or toxic megacolon) were excluded from this study. The primary outcomes were clinical cure (resolution of diarrhea and abdominal comfort within the 10-day treatment period without additional therapy during the study period), time to resolution of diarrhea and total relief of symptoms of CDAD. A secondary outcome was recurrence within 6 weeks of therapy. A total of 45 patients were randomized to receive 50, 100 or 200 mg of fidaxomicin orally every 12 h (100, 200 or 400 mg/day) for 10 days. Clinical cure rates at the end of therapy were 71%, 80% and 94% for the 100, 200 and 400 mg/day treatment groups respectively. The time to resolution of diarrhea was 5.5 days, 3.5 days and 3.0 days for the increasing treatment groups. Only 2 of 41 clinical cure patients treated with fidaxomicin had recurrent C. difficile infections, 1 patient from the 100 mg/day group and 1 patient from the 400 mg/day group. Both of these patients responded to metronidazole with resolution of symptoms. There were minimal adverse events, which were determined to be unrelated to fidaxomicin. A majority of patients treated with fidaxomicin had low plasma concentrations under 20 ng/ml. In addition, fecal concentrations of fidaxomicin and its metabolite increased with administration of higher doses.
There have been two phase III clinical trials evaluating fidaxomicin versus vancomycin for CDAD treatment. The North American study was a prospective multicenter, double-blind, randomized trial [Louie et al. 2011]. Patients over the age of 18 were enrolled at 52 sites in the United States and 15 sites in Canada. CDAD was defined by the presence of diarrhea and C. difficile toxin A, B or both in a stool specimen within 48 h of randomization. However, patients could have received up to four doses of vancomycin or metronidazole in the 24 h period prior to randomization at the discretion of a treating physician not associated with the study. Patients with fulminant C. difficile infections (life-threatening infection, toxic megacolon), previous exposure to fidaxomicin, a history of ulcerative colitis or Crohn’s disease and more than one occurrence in the previous 3 months were excluded from the study [Louie et al. 2011].
Patients were randomized to receive fidaxomicin 200 mg every 12 h (with intervening placebo) or vancomycin 125 mg every 6 h. The primary outcome was clinical cure (resolution of diarrhea with less than three stools per day without a further need for medication 2 days after the study drug was finished). Clinical failure was the persistence of diarrhea and the need for additional therapy for treatment. Other outcomes were sustained response (resolution of diarrhea at the end of 10-day therapy plus survival without recurrence through 25 days beyond end of treatment) and clinical recurrence (development of three diarrheal stools per 24 h period within 4 weeks after the end of therapy as well as demonstration of C. difficile toxin A or B in the stool) [Louie et al. 2011; Optimer Pharmaceuticals, 2011].
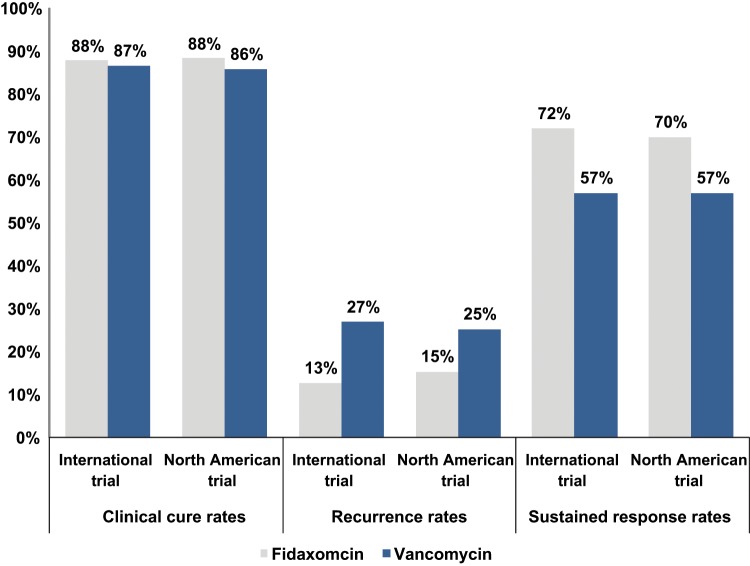
A total of 629 patients were enrolled and underwent randomization, 302 patients received fidaxomicin and 327 patients received vancomycin. Approximately 88.2% of patients in the fidaxomicin group and 85.8% of patients in the vancomycin group achieved clinical cure in the modified intention-to treat (miTT) population. The time to resolution of diarrhea was shorter in the fidaxomicin group than in the vancomycin group, 58 h versus 78 h. Treatment with fidaxomicin was associated with lower rates of recurrences than vancomycin, 15% versus 25.3% respectively in the miTT population. The overall sustained clinical response rates were higher in the fidaxomicin group versus the vancomycin group, 70% versus 57%, respectively [Optimer Pharmaceuticals, 2011]. The rates of recurrence in the fidaxomicin and vancomycin groups were comparable in patients with BI/NAP1/027 strain, 24.4% and 23.6% respectively. Sustained clinical response rates among the BI/NAP1/027 strains were 58% in the fidaxomicin group and 63% in the vancomycin group [Optimer Pharmaceuticals, 2011]. Among the non-BI strains, the sustained response rate was 83% versus 66% for fidaxomicin and vancomycin respectively [Optimer Pharmaceuticals, 2011]. The mean fecal concentration of fidaxomicin at the end of therapy was approximately 1225 μg/g, which was almost 5000 times as high as the MIC90 of 0.25 μg per milliliter from the study [Louie et al. 2011]. Furthermore, the plasma accumulation of fidaxomicin was low (22.8 ng/ml ± 26.5) and only detected after the first day of therapy.
A second phase III clinical trial was also conducted internationally. A total of 535 patients were enrolled from 100 sites in North America and Europe [Cornely et al. 2012]. The study protocol was identical to the North American trial with clinical cure as the primary outcome. Recurrence rates and sustained clinical response were also measured. In the miTT population, 87.7% of patients in the fidaxomicin group and 86.8% of patients in the vancomycin group met the criteria for clinical cure. Fidaxomicin was associated with lower rates of recurrences than vancomycin, 12.7% versus 26.9% respectively. In the mitt population, sustained clinical response was achieved in 76.6% of the fidaxomicin group versus 63.4% in the vancomycin group. The sustained clinical response in BI isolates was 52% in the vancomycin group and 65% in the fidaxomicin group [Optimer Pharmaceuticals, 2011]. The combined results of the clinical trials are shown in Figure 2.
Figure 2.
Results from clinical trials comparing fidaxomicin and vancomycin from the modified intention-to-treat population [Louie et al. 2009; Optimer Pharmaceutical, 2009; Cornely et al. 2012].
The results of phase III clinical trials were combined to evaluate differences in recurrence rates. In the subgroup analysis, recurrence rates were divided into two types, early and late recurrence. Early recurrences (within 2 weeks of completing therapy for C difficile infection) were more likely to be relapses of the primary infection while late recurrences (within 4 weeks after completing therapy) were more likely to be reinfection. Patients randomized to fidaxomicin had significantly less early recurrences compared with patients randomized to vancomycin [Golan et al. 2011]. Risk factors for late recurrences were more likely to be associated with long-term status such as hypoalbuminemia, cardiovascular diseases, renal impairment and older age [Golan et al. 2011].
The effects of concomitant systemic antibiotics and the efficacy of fidaxomicin and vancomycin were assessed in a subgroup analysis from the clinical trials [Mullane et al. 2011]. The effect of concurrent antibiotics administered during the treatment phase (days 1–10) as well as anytime during the study period (days 1–40) was analyzed. Vancomycin was inferior to fidaxomicin in achieving clinical cure in patients who received one or more concurrent antibiotics during the treatment phase (days 1–10), 79.4% versus 90% respectively. Concurrent antibiotic administration at any point in the study increased recurrence rates in both the vancomycin and fidaxomicin groups, 16.9% versus 29.2%. However, the recurrence rate in fidaxomicin remained lower than vancomycin, even with concurrent antibiotics. Sustained response rates (resolution of diarrhea without recurrence) in both groups decreased when patients received one or more concurrent antibiotic, 72.7% and 59.4%.
Resistance
C. difficile has minimal ability to develop spontaneous resistance to fidaxomicin in vitro and in clinical studies. In vitro, the resistance frequency for fidaxomicin against C. difficile was 2.8 × 10–8 at four and eight times the MIC, which was similar to both vancomycin and metronidazole [Swanson et al. 1991]. This feature was also confirmed in strains from phase III clinical trials. C. difficile strains were obtained from patients at baseline, prior to treatment. Over the course of treatment, no resistance to fidaxomicin developed [Goldstein et al. 2011]. However, in one single strain isolated from a cured patient, an elevated fidaxomicin MIC of 16 μg/ml was noted at recurrence. At baseline, prior to receiving therapy, the same strain had a fidaxomicin MIC of 0.06 μg/ml. A specific mutation in the RNA polymerase is the likely cause of fidaxomicin resistance [Tupin et al. 2010].
Current indications
Fidaxomicin was approved for treatment of CDAD in the United States in May 2011 and in Europe in December 2011 [Traynor, 2011]. It should be used only for infections that are known or strongly suspected to be caused by C. difficile and should not be used for treating systemic infections. Prescribing fidaxomicin without proven CDAD, or clinical suspicion of CDAD, is unlikely to benefit the patient and may increase the risk of drug-resistant bacteria [Optimer Pharmaceuticals, 2011]. The recommended dose of fidaxomicin is a 200 mg tablet twice daily for 10 days with or without food [Optimer Pharmaceuticals, 2011].
Safety
There are no contraindications to fidaxomicin listed in the prescribing information [Optimer Pharmaceuticals, 2011]. In phase III clinical trials, vomiting was the most commonly reported side effect but there were no clinical differences in adverse side effects between the fidaxomicin group and the vancomycin group, 62.3% versus 60.4 % respectively [Louie et al. 2011]. In the North American trial, there were more abnormal laboratory values in the fidaxomicin group compared with the vancomycin group, 4.7% versus 1.2 % respectively. The most common abnormal laboratory tests reported in the fidaxomicin group were hyperuricemia (four patients), increased aspartate transaminase and alanine transaminase (three patients) and leukopenia (two patients) [Louie et al. 2011]. The most common adverse reactions related to fidaxomicin are nausea, vomiting, abdominal pain, gastrointestinal hemorrhage, anemia and neutropenia [Optimer Pharmaceuticals, 2011].
Fidaxomicin does not have any reported cross resistance or antagonistic interaction with other antibiotics [Optimer Pharmaceuticals, 2011]. The safety and efficacy of fidaxomicin has not been studied in the pediatric population. There is no dose adjustment necessary in the older population or in patients with impaired renal function. There is a theoretical concern regarding the interaction between cyclosporine and fidaxomicin due to the P-glycoprotein (P-gp) efflux pump. Fidaxomicin, and its metabolite are substrates of the P-gp efflux, which is expressed in the GI tract. In contrast, cyclosporine is a P-gp inhibitor. When cyclosporine and fidaxomicin were coadministered, there were slight elevations in fidaxomicin in the plasma. However, there is no dose adjustment necessary when a P-gp inhibitor is administered with fidaxomicin [Optimer Pharmaceuticals, 2011].
Conclusion
CDAD is a major cause of healthcare-associated diarrhea and with few treatment therapies. Fidaxomicin was recently approved for treatment of CDAD and the first drug for CDAD to be approved in nearly 25 years. Its narrow spectrum of activity and ease of administration make it an attractive alternative to current therapies. Fidaxomicin has also been shown to be associated with less recurrent disease compared with vancomycin in clinical trials. It has a good safety profile in a wide population with minimal adverse side effects. Moreover, the ability of C. difficile to develop resistance against fidaxomicin is low. Future research goals should investigate the protective mechanism of fidaxomicin in preventing recurrent disease, fidaxomicin and its role in inflammatory bowel disease, and fidaxomicin as a prophylactic medication for CDAD.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors report no conflict of interest. Lauren Epstein is supported by a NIH T-32 training grant (grant number 5T32AI055412-07).
Contributor Information
Yoav Golan, Department of Medicine, Tufts Medical Center, Boston, MA, USA.
Lauren Epstein, Division of Geographic Medicine and Infectious Disease, Department of Medicine, Tufts Medical Center, 800 Washington St. #238, Boston, MA 02446, USA.
References
- Abrahamian F., Talan D., Moran G., Pinner R. (2006) Update on emerging infections from the Centers for Disease Control and Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk–four states, 2005. Ann Emerg Med 48: 55–59 [DOI] [PubMed] [Google Scholar]
- Ackermann G., Loffler B., Adler D., Rodloff A. (2004) In vitro activity of OPT-80 against Clostridium difficile. Antimicrob Agents Chemother 48: 2280–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nassir W., Sethi A., Li Y., Pultz M., Riggs M., Donskey C. (2008) Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother 52: 2403–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babakhani F., Gomez A., Robert N., Sears P. (2011) Postantibiotic effect of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. Antimicrob Agents Chemother 55: 4427–4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. (2002) Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 346: 334–339 [DOI] [PubMed] [Google Scholar]
- Bartlett J. (2008) The case for vancomycin as the preferred drug for treatment of Clostridium difficile infection. Clin Infect Dis 46: 1489–1492 [DOI] [PubMed] [Google Scholar]
- Biedenbach D., Ross J., Putnam S., Jones R. (2010) In vitro activity of fidaxomicin (OPT-80) tested against contemporary clinical isolates of Staphylococcus spp. and Enterococcus spp. Antimicrob Agents Chemother 54: 2273–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignardi G. (1998) Risk factors for Clostridium difficile infection. J Hosp Infect 40: 1–15 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2005) Severe Clostridium difficile-associated disease in populations previously at low risk–four states, 2005. MMWR Morbid Mortal Wkly Rep 54: 1201–1205 [PubMed] [Google Scholar]
- Citron D., Tyrrell K., Merriam C., Goldstein E. (2012) Comparative in vitro activities of LFF571 against Clostridium difficile and 630 other intestinal strains of aerobic and anaerobic bacteria. Antimicrob Agents Chemother 56: 2493–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Gerding D., Johnson S., Kelly C., Loo V., McDonald L., et al. Society for Healthcare Epidemiology of America & Infectious Diseases Society of America (2010) Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 31: 431–455 [DOI] [PubMed] [Google Scholar]
- Cornely O., Crook D., Esposito R., Poirier A., Somero M., Weiss K., et al. OPT-80-004 Clinical Study Group (2012) Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 12: 281–289 [DOI] [PubMed] [Google Scholar]
- Coronelli C., White R., Lancini G., Parenti F. (1975) Lipiarmycin, a new antibiotic from Actinoplanes. II. Isolation, chemical, biological and biochemical characterization. J Antibiot 28: 253–259 [DOI] [PubMed] [Google Scholar]
- Credito K., Appelbaum P. (2004) Activity of OPT-80, a novel macrocycle, compared with those of eight other agents against selected anaerobic species. Antimicrob Agents Chemother 48: 4430–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubberke E., Wertheimer A. (2009) Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol 30: 57–66 [DOI] [PubMed] [Google Scholar]
- Finegold S., Molitoris D., Vaisanen M., Song Y., Liu C., Bolanos M. (2004) In vitro activities of OPT-80 and comparator drugs against intestinal bacteria. Antimicrob Agents Chemother 48: 4898–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J., Baines S., Todhunter S., Huscroft G., Wilcox M. (2011) Nitazoxanide is active against Clostridium difficile strains with reduced susceptibility to metronidazole. J Antimicrob Chemother 66: 1407–1408 [DOI] [PubMed] [Google Scholar]
- Garey K., Ghantoji S., Shah D., Habib M., Arora V., Jiang Z., et al. (2011) A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. J Antimicrob Chemother 66: 2850–2855 [DOI] [PubMed] [Google Scholar]
- Gerber M., Ackermann G. (2008) OPT-80, a macrocyclic antimicrobial agent for the treatment of Clostridium difficile infections: a review. Exp Opin Invest Drugs 17: 547–553 [DOI] [PubMed] [Google Scholar]
- Ghantoji S., Sail K., Lairson D., DuPont H., Garey K. (2010) Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect 74: 309–318 [DOI] [PubMed] [Google Scholar]
- Golan Y., Louie T., Miller M., Mullane K., Weiss K., Lentnek A, et al. (2011) Risk of recurrence and time to recurrence following treatment of Clostridium difficile infection: patient characteristics and the differential effect of fidaxomicin vs. vancomycin. Presented at Digestive Disease Week, 7–10 May 2011, Chicago, IL, USA [Google Scholar]
- Goldstein E., Citron D., Sears P., Babakhani F., Sambol S., Gerding D. (2011) Comparative susceptibilities to fidaxomicin (OPT-80) of isolates collected at baseline, recurrence, and failure from patients in two phase III trials of fidaxomicin against Clostridium difficile infection. Antimicrob Agents Chemother 55: 5194–5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri M., Villain-Guillot P., Latouche J., Leonetti J., Bastide L. (2006) Mutation in the Bacillus subtilis RNA polymerase beta’ subunit confers resistance to lipiarmycin. Antimicrob Agents Chemother 50: 401–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht D., Galang M., Sambol S., Osmolski J., Johnson S., Gerding D. (2007) In vitro activities of 15 antimicrobial agents against 110 toxigenic clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother 51: 2716–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. (2009) Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect 58: 403–410 [DOI] [PubMed] [Google Scholar]
- Karlowsky J., Laing N., Zhanel G. (2008) In vitro activity of OPT-80 tested against clinical isolates of toxin-producing Clostridium difficile. Antimicrob Agents Chemother 52: 4163–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., LaMont J. (2008) Clostridium difficile – more difficult than ever. N Engl J Med 359: 1932–1940 [DOI] [PubMed] [Google Scholar]
- Louie T., Emery J., Krulicki W., Byrne B., Mah M. (2009a) OPT-80 eliminates Clostridium difficile and is sparing of bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother 53: 261–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie T., Miller M., Donskey C., Mullane K., Goldstein E. (2009b) Clinical outcomes, safety, and pharmacokinetics of OPT-80 in a phase 2 trial with patients with Clostridium difficile infection. Antimicrob Agents Chemother 53: 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie T., Miller M., Mullane K., Weiss K., Lentnek A., Golan Y., et al. OPT-80-003 Clinical Study Group (2011) Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 364: 422–431 [DOI] [PubMed] [Google Scholar]
- McDonald L., Killgore G., Thompson A., Owens R., Kazakova S., Jr, Sambol S., et al. (2005) An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 353: 2433–2441 [DOI] [PubMed] [Google Scholar]
- McDonald L., Owings M., Jernigan D. (2006) Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis 12: 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane K., Miller M., Weiss K., Lentnek A., Golan Y., Sears P., et al. (2011) Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis 53: 440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D., Aslam S., Logan N., Nallacheru S., Bhaila I., Borchert F., Hamill R. (2005) Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis 40: 1586–1590 [DOI] [PubMed] [Google Scholar]
- Optimer Pharmaceuticals (2011) Dificid (fidaxomicin) package insert. San Diego, CA [Google Scholar]
- Pepin J., Valiquette L., Alary M., Villemure P., Pelletier A., Forget K., et al. (2004) Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ 171: 466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan C., Stecher B., Tzivelekidis T., van Ham M., Rohde M., Hardt W., et al. (2009) Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog 5: no. e100062610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shue Y., Sears P., Shangle S., Walsh R., Lee C., Gorbach S., et al. (2008) Safety, tolerance, and pharmacokinetic studies of OPT-80 in healthy volunteers following single and multiple oral doses. Antimicrob Agents Chemother 52: 1391–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R., Hardy D., Shipkowitz N., Hanson C., Ramer N., Fernandes P., et al. (1991) In vitro and in vivo evaluation of tiacumicins B and C against Clostridium difficile. Antimicrob Agents Chemother 35: 1108–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G., Munro K., Taylor C., Lawley B., Young W., Byrne B., et al. (2010) A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology 156: 3354–3359 [DOI] [PubMed] [Google Scholar]
- Traynor K. (2011) Fidaxomicin approved for C. difficile infections. Am J Health System Pharm 68: 1276. [DOI] [PubMed] [Google Scholar]
- Tupin A., Gualtieri M., Leonetti J., Brodolin K. (2010) The transcription inhibitor lipiarmycin blocks DNA fitting into the RNA polymerase catalytic site. EMBO J 29: 2527–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuto C., Butler M., Ashley E., Brown J. (2010) Alternative therapies for Clostridium difficile infections. Pharmacotherapy 30: 1266–1278 [DOI] [PubMed] [Google Scholar]
- Zar F., Bakkanagari S., Moorthi K., Davis M. (2007) A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 45: 302–307 [DOI] [PubMed] [Google Scholar]