Abstract
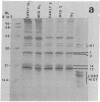
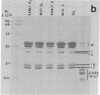
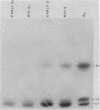
The phosphorylation of the high-mobility group (HMG) proteins at different stages of the cell cycle was studied in synchronized HeLa cells. HMG proteins were extracted and analyzed by NaDodSO4/polyacrylamide gel electrophoresis. Although the molecular weight distribution of HMGs remains unchanged, their total amounts increase by as much as 20-25% in the G1 and S phases when compared with amounts in G2. However, the most significant finding is that there is a 7-fold increase of 32P incorporation into HMG 14 in the G2 phase compared with that in G1, and a 2-fold increase of 32P incorporation into HMG 17 in early S phase relative to the incorporation in the G1 and G2 stages. In contrast, HMG 1 and HMG 2 are not phosphorylated. The clear demonstration of differential phosphorylation of HMG 14 and 17 at specific stages of the cell cycle warrants a serious consideration of their role in tissue-specific maintenance of the altered chromatin structure characteristic of potentially active or actively transcribed chromatin domains.
Full text
PDF
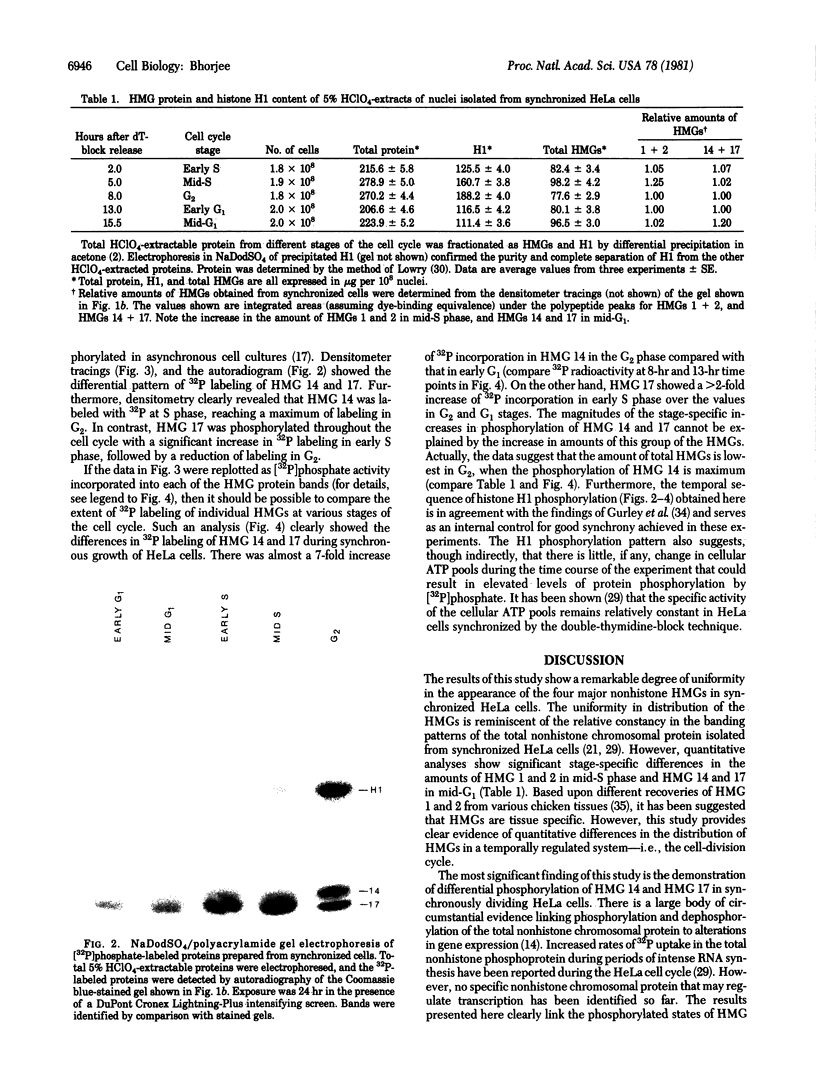
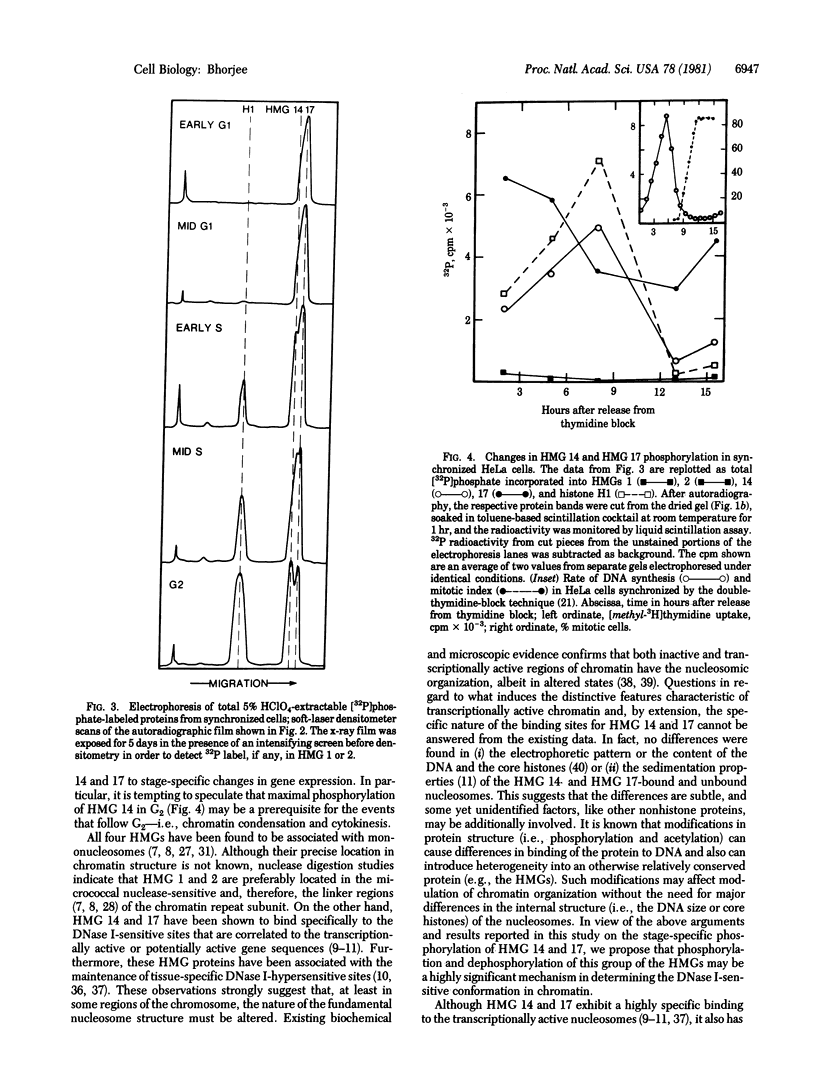

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhorjee J. S., Pederson T. Chromatin: its isolation from cultured mammalian cells with particular reference to contamination by nuclear ribnucleoprotein particles. Biochemistry. 1973 Jul 3;12(14):2766–2773. doi: 10.1021/bi00738a033. [DOI] [PubMed] [Google Scholar]
- Bhorjee J. S., Pederson T. Nonhistone chromosomal proteins in synchronized HeLa cells. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3345–3349. doi: 10.1073/pnas.69.11.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Hopkins R. B., Isenberg I. Immunological relatedness of high mobility group chromosomal proteins from calf thymus. J Biol Chem. 1978 Mar 10;253(5):1694–1699. [PubMed] [Google Scholar]
- Caplan A., Ord M. G., Stocken L. A. Chromatin structure through the cell cycle. Studies with regeneration rat liver. Biochem J. 1978 Aug 15;174(2):475–483. doi: 10.1042/bj1740475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. Summary: the molecular biology of the eukaryotic genome is coming of age. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1209–1234. doi: 10.1101/sqb.1978.042.01.122. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Gazit B., Panet A., Cedar H. Reconstitution of a deoxyribonuclease I-sensitive structure on active genes. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1787–1790. doi: 10.1073/pnas.77.4.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershey E. L., Kleinsmith L. J. Phosphorylation of nuclear proteins in avian erythrocytes. Biochim Biophys Acta. 1969 Dec 23;194(2):519–525. doi: 10.1016/0005-2795(69)90113-5. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Johns E. W. Are the high mobility group non-histone chromosomal proteins associated with 'active' chromatin? Biochim Biophys Acta. 1978 Jun 22;519(1):279–284. doi: 10.1016/0005-2787(78)90081-3. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Mathew C. G., Wright C. A., Venkov C. D., Johns E. W. Analysis of the high mobility group proteins associated with salt-soluble nucleosomes. Nucleic Acids Res. 1979 Dec 11;7(7):1815–1835. doi: 10.1093/nar/7.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Nicolas R. H., Johns E. W. An improved large scale fractionation of high mobility group non-histone chromatin proteins. Biochim Biophys Acta. 1975 Oct 20;405(2):280–291. doi: 10.1016/0005-2795(75)90094-x. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Sanders C., Johns E. W. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973 Sep 21;38(1):14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Walker J. M., Johns E. W. Studies on the degradation of high mobility group non-histone chromosomal proteins. Biochim Biophys Acta. 1978 Jun 22;519(1):233–242. doi: 10.1016/0005-2787(78)90076-x. [DOI] [PubMed] [Google Scholar]
- Gordon J. S., Rosenfeld B. I., Kaufman R., Williams D. L. Evidence for a quantitative tissue-specific distribution of high mobility group chromosomal proteins. Biochemistry. 1980 Sep 16;19(19):4395–4402. doi: 10.1021/bi00560a003. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Sequential phsophorylation of histone subfractions in the Chinese hamster cell cycle. J Biol Chem. 1975 May 25;250(10):3936–3944. [PubMed] [Google Scholar]
- Jackson J. B., Pollock J. M., Jr, Rill R. L. Chromatin fractionation procedure that yields nucleosomes containing near-stoichiometric amounts of high mobility group nonhistone chromosomal proteins. Biochemistry. 1979 Aug 21;18(17):3739–3748. doi: 10.1021/bi00584a015. [DOI] [PubMed] [Google Scholar]
- Karn J., Johnson E. M., Vidali G., Allfrey V. G. Differential phosphorylation and turnover of nuclear acidic proteins during the cell cycle of synchronized HeLa cells. J Biol Chem. 1974 Feb 10;249(3):667–677. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy W B., Wong N. C., Dixon G. H. Selective association of the trout-specific H6 protein with chromatin regions susceptible to DNase I and DNase II: possible location of HMG-T in the spacer region between core nucleosomes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2810–2814. doi: 10.1073/pnas.74.7.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew C. G., Goodwin G. H., Johns E. W. Studies on the association of the high mobility group non-histone chromatin proteins with isolated nucleosomes. Nucleic Acids Res. 1979 Jan;6(1):167–179. doi: 10.1093/nar/6.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison J. M. Enzyme synthesis in synchronous cultures. Science. 1969 Aug 15;165(3894):657–663. doi: 10.1126/science.165.3894.657. [DOI] [PubMed] [Google Scholar]
- Pederson T., Bhorjee J. S. Evidence for a role of RNA in eukaryotic chromosome structure. Metabolically stable, small nuclear RNA species are covalently linked to chromosomal DNA in HeLa cells. J Mol Biol. 1979 Mar 15;128(4):451–480. doi: 10.1016/0022-2836(79)90288-2. [DOI] [PubMed] [Google Scholar]
- Puck T. T. Phasing, Mitotic Delay, and Chromosomal Aberrations in Mammalian Cells. Science. 1964 May 1;144(3618):565–566. doi: 10.1126/science.144.3618.565-c. [DOI] [PubMed] [Google Scholar]
- Saffer J. D., Glazer R. I. The phosphorylation of high mobility group proteins 14 and 17 from Ehrlich ascites and L1210 in vitro. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1280–1285. doi: 10.1016/0006-291x(80)90628-2. [DOI] [PubMed] [Google Scholar]
- Sandeen G., Wood W. I., Felsenfeld G. The interaction of high mobility proteins HMG14 and 17 with nucleosomes. Nucleic Acids Res. 1980 Sep 11;8(17):3757–3778. doi: 10.1093/nar/8.17.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotirov N., Johns E. W. Quantitative differences in the content of the histone f2c between chicken erythrocytes and erythroblasts. Exp Cell Res. 1972 Jul;73(1):13–16. doi: 10.1016/0014-4827(72)90095-x. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Sterner R., Boffa L. C., Vidali G. Comparative structural analysis of high mobility group proteins from a variety of sources. Evidence for a high mobility group protein unique to avian erythrocyte nuclei. J Biol Chem. 1978 Jun 10;253(11):3830–3836. [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Allfrey V. G. Selective release of chromosomal proteins during limited DNAase 1 digestion of avian erythrocyte chromatin. Cell. 1977 Oct;12(2):409–415. doi: 10.1016/0092-8674(77)90117-9. [DOI] [PubMed] [Google Scholar]
- Watson D. C., Peters E. H., Dixon G. H. The purification, characterization and partial sequence determination of a trout testis non-histone protein, HMG-T. Eur J Biochem. 1977 Mar 15;74(1):53–60. doi: 10.1111/j.1432-1033.1977.tb11365.x. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of actively transcribed nucleosomes using immobilized HMG 14 and 17 and an analysis of alpha-globin chromatin. Cell. 1981 Feb;23(2):391–400. doi: 10.1016/0092-8674(81)90134-3. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]