Abstract
Background. Response rates and immunologic memory following measles vaccination are reduced in human immunodeficiency virus (HIV)–infected children in the absence of highly active antiretroviral therapy (HAART).
Methods. HIV-infected children 2 to <19 years old receiving HAART and with HIV loads <30 000 copies/mL, CD4% ≥15, and ≥1 prior measles-mumps-rubella vaccination (MMR) were given another MMR. Measles antibody concentrations before and 8, 32, and 80 weeks postvaccination were determined by plaque reduction neutralization (PRN). A subset was given another MMR 4–5 years later, and PRN antibody was measured before and 7 and 28 days later.
Results. At entry, 52% of 193 subjects were seroprotected (PRN ≥120 mIU/mL). Seroprotection increased to 89% 8 weeks postvaccination, and remained at 80% 80 weeks postvaccination. Of 65 subjects revaccinated 4–5 years later, 85% demonstrated memory based on seroprotection before or 7 days after vaccination. HIV load ≤400 copies/mL at initial study vaccination was associated with higher seroprotection rates, greater antibody concentrations, and memory. Grade 3 fever or fatigue occurred in 2% of subjects.
Conclusions. Measles revaccination induced high rates of seroprotection and memory in children receiving HAART. Both endpoints were associated with HIV viral load suppression.
Clinical Trials Registration: NCT00013871 (www.clinicaltrials.gov).
(See the editorial commentary by Maldonado, on pages 466–8.)
Early in the human immunodeficiency virus (HIV) epidemic, it was recognized that measles can cause severe disease in HIV-infected children [1–3]. Prior to highly active antiretroviral therapy (HAART), HIV-infected children had reduced response rates, lower antibody titers, and more rapid antibody decline following measles vaccination; lack of recall responses; and vaccine failures [3–17]. Endemic measles and measles outbreaks pose a risk to HIV-infected children, and the HIV epidemic may complicate efforts for global measles control [18, 19]. Therefore, it is important to assess vaccine-induced immunity against measles in HIV-infected children in the context of HAART [20]. P1024 was a multicenter study of the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) designed to evaluate immunogenicity of vaccines in HIV-infected children on HAART. P1061s was a substudy that evaluated immunologic memory following vaccination in P1024. This report focuses on immunogenicity, safety, and immunologic memory associated with measles vaccination.
METHODS
P1024 Population
HIV-infected children 2 to <19 years old at 39 US sites were eligible if they fit into the following strata based on pre-HAART nadir CD4% and screening CD4% (within 30 days before entry): stratum 1, <15% and <15%; stratum 2, <15% and ≥15%; stratum 3, 15 to <25% and ≥15%; stratum 4, ≥25% and ≥25%. Stratum 1 was excluded in the study of measles vaccination due to the requirement of a CD4% ≥15 for measles-mumps-rubella vaccine (MMR) administration. Other inclusion criteria included perinatal infection, treatment with the same HAART (≥3 antiretrovirals from ≥2 classes) regimen for ≥6 months, plasma HIV RNA viral load (VL) <30 000 copies/mL (Roche Amplicor Assay), and previous receipt of ≥1 dose of MMR, unless contraindicated by CD4 count [21–23].
P1024 Protocol
Informed consent was obtained and human experimentation guidelines of the US Department of Health and Human Services and participating institutions were followed. MMR (M-M-R II; Merck & Co, Whitehouse Station, NJ; 0.5 mL subcutaneously) was administered at the week 16 visit to subjects with a CD4% ≥15 and an absolute CD4 cell count ≥500/µL (age <6 years) or ≥200/µL (age ≥6 years) at the 2 preceding visits; pneumococcal polysaccharide vaccine was administered at the same visit and additional vaccines at other visits [21–23]. Measles antibody, plasma VL, and quantitative lymphocyte subsets were measured at entry and 8, 32, and 80 weeks postvaccination (study weeks 24, 48, and 96). Adverse reactions were assessed by diary and telephone 3, 7, 14, 21, and 28 days postvaccination, according to the Division of AIDS Standardized Toxicity Table for Grading Severity of Pediatric Adverse Experiences (http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf).
P1061s Protocol
Subjects enrolled in P1024 between June 2001 and March 2002 were eligible for P1061s, which enrolled between February 2006 and August 2006. Subjects who received MMR in P1024 with no grade ≥3 vaccine-related adverse event, did not receive MMR nor have proven measles infection since the conclusion of P1024, and had a CD4% ≥15 and an absolute CD4 cell count ≥200/µL on the 2 preceding measurements were eligible to have immunoglobin G memory response to measles vaccine evaluated in P1061s. MMR, hepatitis B, and pneumococcal (conjugate or polysaccharide) vaccines were administered at entry. Measles antibody was measured at entry and days 7 and 28, and VL and lymphocyte subsets were measured at entry. Adverse reactions were assessed by diary and telephone calls or study visits 3, 7, 21, and 28 days postvaccination.
Laboratory Assays and Immunologic Definitions
Measles neutralizing antibody concentrations were determined by plaque reduction neutralization (PRN) at the Centers for Disease Control and Prevention, Atlanta, GA, for P1024 serum samples and the Center for Biologics Evaluation and Research, Food and Drug Administration, Bethesda, MD, for P1061s serum samples [24, 25]. PRN titers, defined as the serum dilution that reduced the number of plaques by 50%, were calculated using the Kärber method. A 1:100 dilution of World Health Organization (WHO) II reference serum (dilution concentration = 50 mIU/mL) was included in each run and all PRN titers were multiplied by a correction factor equal to 50 divided by the WHO II titer measured concurrently [25]. Seropositivity was defined as PRN concentration ≥8 mIU/mL and seroprotection as PRN concentration ≥120 mIU/mL [26, 27].
Vaccine response in P1024 was defined by PRN concentration ≥120 mIU/mL (protective antibody response) 8 weeks postvaccination. Antibody increases that were ≥4-fold and geometric mean concentration (GMC) were also evaluated. Subjects with antibody concentrations <8 mIU/mL were assigned a concentration of 2 mIU/mL. Immunologic memory in P1061s was defined on the basis of seroprotection (PRN concentration ≥120 mIU/mL) at P1061s entry or day 7 (protective memory response) or ≥4-fold rise in antibody concentration between P1061s entry and day 7 in subjects seropositive at entry. Primary response was defined in P1024 as ≥4-fold antibody rise 8 weeks postvaccination among subjects seronegative at entry and in P1061s as ≥4-fold antibody rise by day 28 postvaccination in subjects seronegative at P1061s entry.
Statistical Analysis
Subjects with results at entry and 8 weeks postvaccination (±4 weeks) were included in P1024 analyses. P1061s MMR recipients with results at entry, day 7 (day 5–15), and day 28 (±8 days) were included in P1061s analyses. Fisher's exact test was used for comparison of proportions among groups, McNemar test for comparison of proportions between time points, t test for comparison of geometric mean concentrations (GMCs) among groups, and paired t test for comparison of GMCs between time points. Univariate regression analyses were performed to identify predictors of P1024 and P1061s measles antibody concentrations. Predictors with a P value <.1 were included in multivariate analyses. Stepwise regression was performed in the case of multiple collinear predictors.
RESULTS
P1024 Study Population
Of 263 subjects enrolled, 226 received MMR per protocol (Table 1; flow diagram online). Remaining subjects had inclusion/exclusion criteria violations (8), did not qualify for MMR based on CD4 criteria (19), did not receive MMR per protocol (9), or had other violations (1). One hundred ninety-three who received MMR per protocol had measles serology pre- and 8 weeks post-MMR vaccination and comprised the P1024 analysis group.
Table 1.
Characteristics of P1024 Measles Vaccine Recipients
| Characteristic | Total (N = 193) | Stratum 2 (N = 63) | Stratum 3 (N = 73) | Stratum 4 (N = 57) | P Valuea |
|---|---|---|---|---|---|
| Age at study MMR visit | |||||
| Median (y) | 9.8 | 10.9 | 10.0 | 6.8 | <.001 |
| ≥7 y (%) | 70 | 84 | 77 | 47 | <.001 |
| Sex (%) | |||||
| F | 55 | 52 | 56 | 56 | .90 |
| Race/ethnicity (%) | |||||
| White non-Hispanic | 13 | 14 | 11 | 14 | .99 |
| Black non-Hispanic | 57 | 57 | 56 | 58 | |
| Hispanic | 30 | 29 | 32 | 28 | |
| Asian/Pacific Islander | 1 | 0 | 1 | 0 | |
| CDC clinical classification (%) | |||||
| N: nonsymptomatic | 11 | 3 | 12 | 19 | .001 |
| A: mildly symptomatic | 34 | 35 | 26 | 44 | |
| B: moderately symptomatic | 35 | 30 | 45 | 28 | |
| C: severely symptomatic | 19 | 32 | 16 | 9 | |
| Pre-HAART nadir CD4% | |||||
| Median | 18 | 10 | 19 | 31 | NA |
| <15 (%) | 33 | 100 | 0 | 0 | |
| 15 to <25 (%) | 38 | 0 | 100 | 0 | |
| ≥25 (%) | 30 | 0 | 0 | 100 | |
| Screening CD4% | |||||
| Median | 34 | 30 | 33 | 40 | NA |
| <15 (%) | 0 | 0 | 0 | 0 | |
| 15 to <25 (%) | 11 | 22 | 10 | 0 | |
| ≥25 (%) | 89 | 78 | 90 | 100 | |
| CD4% at study MMR visit | |||||
| Median | 34 | 30 | 35 | 40 | <.001 |
| <15 (%) | 1 | 2 | 0 | 0 | .007 |
| 15 to <25 (%) | 13 | 22 | 12 | 4 | |
| ≥25 (%) | 87 | 76 | 88 | 96 | |
| Entry CD19% | |||||
| Median | 19 | 18 | 19 | 19 | .60 |
| CD19% at study MMR visit | |||||
| Median | 18 | 18 | 18 | 19 | .42 |
| HIV RNA level at study MMR visit | |||||
| Median, copies/mL | 278 | 386 | 260 | 245 | .008 |
| ≤400 copies/mL (%) | 63 | 51 | 62 | 77 | <.001 |
| 401–5000 copies/mL (%) | 22 | 19 | 32 | 14 | |
| >5000 copies/mL (%) | 15 | 30 | 7 | 9 | |
| Interval from last previous MMR to study MMR visit | |||||
| Median (y) | 4.8 | 6.4 | 5.0 | 3.4 | <.001 |
| Interval >2 y (%) | 84 | 89 | 86 | 77 | .20 |
| Number of MMR vaccines prior to entry (%) | |||||
| 1 | 20 | 16 | 16 | 30 | .27 |
| 2 | 70 | 71 | 75 | 61 | |
| 3 | 10 | 13 | 8 | 9 |
Data are percentages of subjects, unless otherwise indicated. Immunologic strata are as follows: stratum 2, pre-HAART nadir CD4 cell percentage <15% and screening CD4 cell percentage ≥15%; stratum 3, pre-HAART nadir CD4 cell percentage 15% to <25% and screening CD4 cell percentage ≥15%; stratum 4, pre-HAART nadir CD4 cell percentage ≥25% and screening CD4 cell percentage ≥25%. No subjects in study stratum 1 (pre-HAART nadir CD4 cell percentage <15% and screening CD4 cell percentage <15%) were included in the study of measles vaccination due to the requirement of a CD4% ≥15% for MMR administration.
Abbreviations: CDC, Centers for Disease Control and Prevention; HAART, highly active antiretroviral therapy; MMR, measles-mumps-rubella vaccine; NA, not applicable.
a Fisher's exact test for categorical variables and Kruskal–Wallis test for continuous variables.
P1024 Measles Antibody Concentrations—Immunologic Strata Combined
At entry, 83% were seropositive and 52% had protective PRN concentrations (Table 2). The percentage with protective concentrations increased to 89% 8 weeks postvaccination and 80% had protective concentrations 80 weeks postvaccination. Between entry and 8 weeks postvaccination, ≥4-fold antibody rises occurred in 44%, including 78% of those seronegative at entry (primary responses). The GMC increased from entry to 8 weeks postvaccination, then decreased 32 and 80 weeks postvaccination but remained greater than at entry.
Table 2.
Measles Serologic Status Before and After Measles Vaccination in P1024
| Endpoint and Study Time Pointa | All Strata Combined | Stratum 2 | Stratum 3 | Stratum 4 |
|---|---|---|---|---|
| Percent with PRN ≥8 mIU/mL at entry (no. of subjects evaluated) | 83 (193) | 76 (63) | 81 (73) | 95 (57)b |
| (95% CI) | (77–88) | (64–86) | (70–89) | (85–99) |
| Percent with PRN ≥120 mIU/mL (no. evaluated) | ||||
| Week 0 | 52 (193) | 37 (63) | 49 (73) | 74 (57)c |
| (95% CI) | (45–60) | (25–50) | (37–61) | (60–84) |
| 8 wk post-MMR (study week 24) | 89 (193)d | 86 (63)d | 89 (73)d | 93 (57)d |
| (95% CI) | (84–93) | (75–93) | (80–95) | (83–98) |
| 32 wk post-MMR (study week 48) | 82 (185)d,e | 82 (61)e | 81 (69)e | 82 (55) |
| (95% CI) | (75–87) | (70–91) | (70–90) | (69–91) |
| 80 wk post-MMR (study week 96) | 80 (179)e | 76 (59)e | 77 (66)e | 89 (54)e |
| (95% CI) | (74–86) | (63–86) | (65–87) | (77–96) |
| Percent with ≥4-fold PRN rise, week 0 to 8 wk post-MMR (no. evaluated) | ||||
| Subjects with PRN <8 mIU/mL at entry | 78 (32) | 60 (15) | 93 (14) | 100 (3) |
| (95% CI) | (60–91) | (32–84) | (66–100) | (29–100) |
| Subjects with PRN ≥8 mIU/mL at entry | 37 (161) | 46 (48) | 37 (59) | 30 (54) |
| (95% CI) | (30–45) | (31–61) | (25–51) | (18–44) |
| Geometric mean antibody concentration, mIU/mL | ||||
| Week 0 | 108 | 47 | 86 | 360f |
| (95% CI) | (77–152) | (27–83) | (48–153) | (207–627) |
| 8 wk post- MMR (study week 24) | 695g | 571g | 627g | 983g |
| (95% CI) | (556–868) | (360–903) | (453–868) | (668–1445) |
| 32 wk post-MMR (study week 48) | 401g,h | 317g,h | 397g,h | 526g |
| (95% CI) | (312–515) | (188–536) | (282–558) | (336–825) |
| 80 wk post-MMR (study week 96) | 361g,h | 247g,h | 361h | 544 |
| (95% CI) | (279–467) | (145–423) | (255–510) | (343–864) |
Study MMR vaccine administered at study week 16. Immunologic strata are as follows: stratum 2, pre-HAART nadir CD4 cell percentage <15% and screening CD4 cell percentage ≥15%; stratum 3, pre-HAART nadir CD4 cell percentage 15% to <25% and screening CD4 cell percentage ≥15%; stratum 4, pre-HAART nadir CD4 cell percentage ≥25% and screening CD4 cell percentage ≥25%.
Abbreviations: CI, confidence interval; HAART, highly active antiretroviral therapy; MMR, measles-mumps-rubella vaccine; PRN, plaque reduction neutralization antibody concentration.
a Week 0 serological results were included if obtained any time prior to study MMR vaccine administration. Serological results at 8, 32, and 80 weeks post-vaccination (study weeks 24, 48, and 96) were included if they were obtained within windows of ±4, ±8, and ±12 weeks, respectively.
b P = .01 (Fisher's exact test) for the difference of the proportions with measles antibody concentration ≥8 mIU/mL among immune strata.
c P < .001 (Fisher's exact test) for the difference of the proportions with measles antibody concentration ≥120 mIU/mL among immune strata. Pairwise comparisons between stratum 4 and strata 2 and 3 were statistically significant (P ≤ .007), but pairwise comparisons between strata 2 and 3 were not.
d P ≤ .004 (McNemar test) for the comparison of the proportion of subject with measles antibody concentration ≥120 mIU/mL vs the previous time point.
e P ≤ .04 (McNemar test) for the comparison of the proportions of subjects with measles antibody concentration ≥120 mIU/mL at 32 weeks postvaccination (study week 48) and 80 weeks postvaccination (study week 96) vs week 0.
f P < .001 (t test) for the difference among immune strata. Pairwise comparisons between stratum 4 and strata 2 and 3 were statistically significant (P < .001), but pairwise comparisons between strata 2 and 3 were not.
g P ≤ .02 (paired t test) for the comparison of geometric mean antibody concentration vs the previous timepoint.
h P < .001 (paired t test) for the comparison of geometric mean antibody concentration at 32 weeks postvaccination (study week 48) and 80 weeks postvaccination (study week 96) vs week 0.
P1024 Measles Antibody Concentrations According to Immunologic Strata
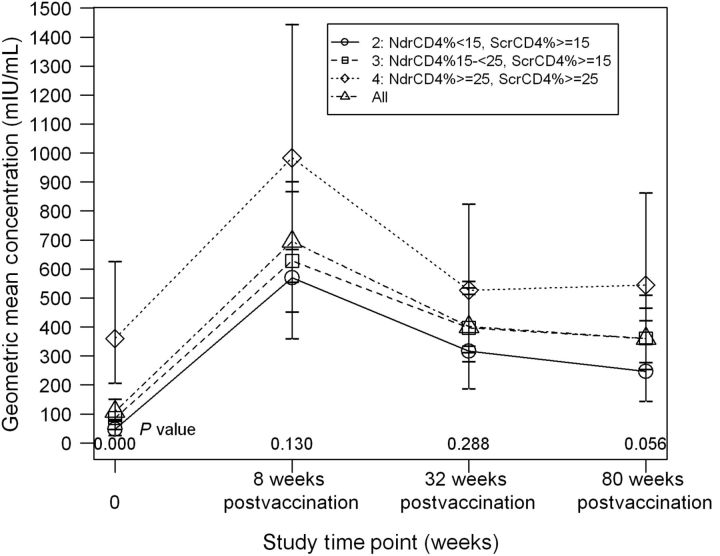
At entry, the percentages in stratum 4 who were seropositive (95%) and seroprotected (74%) were higher than for strata 2 and 3 (Table 2; Figure 1). The percentage with protective antibody concentrations increased in each stratum to similar levels 8 weeks postvaccination (86%–93%) and decreased modestly thereafter, with all strata maintaining higher proportions with protective levels 80 weeks postvaccination compared with entry. There were no significant differences among strata in the percentage with protective antibody values at any post-vaccination time point. The GMC at entry of stratum 4 was significantly higher than that of strata 2 and 3, with only stratum 4's GMC exceeding the seroprotective threshold. GMCs of all strata increased 8 weeks postvaccination and then decreased between 8 and 32 weeks postvaccination. By 80 weeks postvaccination, GMCs of strata 2 and 3, but not of stratum 4, remained significantly greater than baseline; all exceeded the seroprotective level. GMCs of stratum 4 were consistently higher than those of strata 2 and 3, but differences were not significant at 8 and 32 weeks postvaccination and marginally significant at 80 weeks postvaccination.
Figure 1.
P1024 geometric mean concentration (GMC) according to immunologic strata. GMCs of measles neutralizing antibody are shown at each P1024 study visit for all subjects combined and according to immunologic stratum. The GMC of stratum 4 was higher than that of strata 2 and 3 at entry (P < .001); differences among strata were not significant after study MMR vaccination, administered at study week 16. Time point 0 on the x-axis reflects the GMC of serum samples obtained at entry. Eight, 32, and 80 weeks postvaccination correspond to study weeks 24, 48, and 96, respectively. Abbreviations: Ndr, nadir; Scr, screening.
P1024 Measles Antibody Concentrations and VL
At entry, the proportion of subjects with PRN ≥120 mIU/mL was inversely related to VL group, but differences were not significant (Figure 2). Eight weeks postvaccination, this inverse relationship was significant and differences remained significant at 32 and 80 weeks postvaccination; pairwise comparisons revealed differences between VL ≤400 copies/mL versus VL 401–5000 and >5000 copies/mL, but not between the latter 2 groups. GMCs varied inversely with VL at entry and each time point following vaccination. Pairwise analyses showed that subjects with VL ≤400 copies/mL had a higher GMC versus those with >5000 copies/mL at entry and versus those with 401–5000 or >5000 copies/mL postvaccination; differences between the latter groups were not significant.
Figure 2.
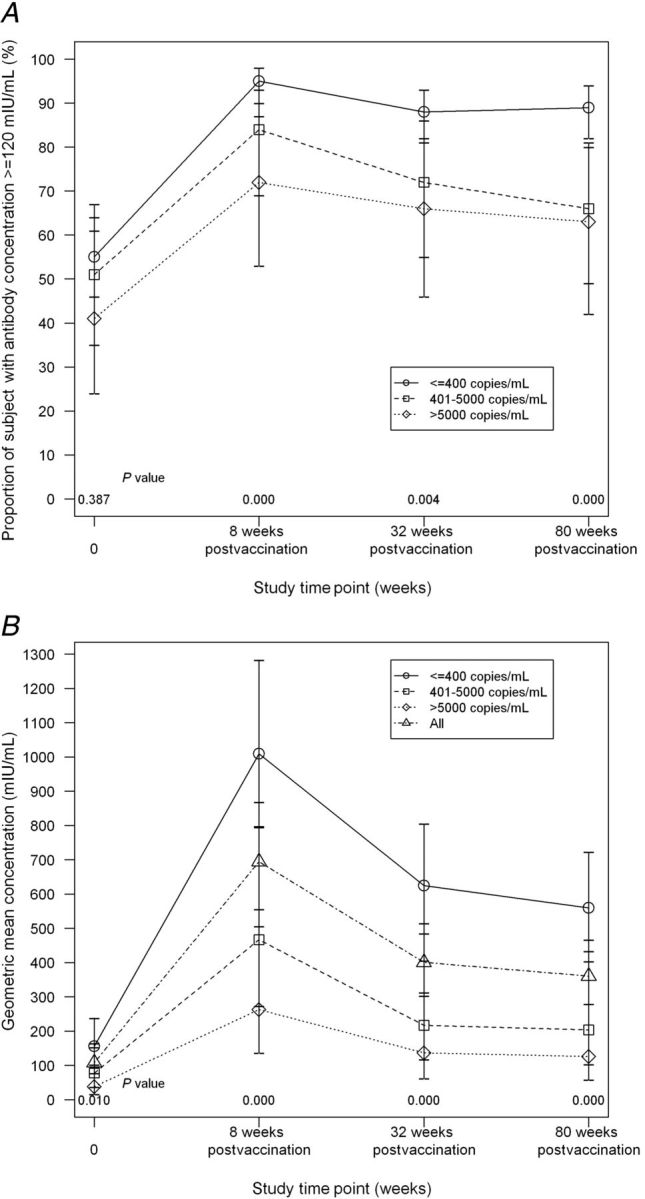
P1024 proportion of subjects with antibody concentration ≥120 mIU/mL according to P1024 HIV RNA group and P1024 geometric mean concentration (GMC) according to P1024 HIV RNA group. Proportion of subjects with protective measles neutralizing antibody concentrations (top panel, A) and GMCs of measles neutralizing antibody (bottom panel, B) are shown at each P1024 study visit according to P1024 HIV viral load group. The proportion with protective (≥120 mIU/mL) antibody concentrations was higher for subjects with ≤400 copies/mL than for each of the other viral load groups at each time point after study MMR vaccination, administered at study week 16. The GMC of subjects with a viral load ≤400 copies/mL was higher than that of subjects with a viral load >5000 copies/mL at entry (P = .004) and higher than that of each of the other 2 viral load groups at each time point after study MMR vaccination (P ≤ .004). Time point 0 on the x-axis reflects results of serum samples obtained at entry. Eight, 32, and 80 weeks postvaccination correspond to study weeks 24, 48, and 96, respectively. Bars represent 95% confidence intervals.
Predictors of P1024 Entry Measles Antibody Concentration
Univariate analyses identified the following predictors of higher entry antibody concentration: age <7 years; nadir CD4% prior to HAART ≥25; CD4% ≥25 and VL ≤400 copies/mL at the last MMR vaccination prior to entry; shorter interval from last MMR to entry; shorter duration of the entry HAART regimen; immune stratum 4; and CD4% ≥25, VL ≤400 copies/mL, or 401–5000 copies/mL, and higher total lymphocyte count at entry. Sex, race/ethnicity, and CD19% at entry were not associated with entry antibody concentration. In a multivariate analysis, age <7 years and entry VL ≤400 copies/mL or 401–5000 copies/mL remained associated with higher entry antibody concentration (P ≤ .02).
Predictors of P1024 Measles Antibody Concentration 8 Weeks Postvaccination
After adjusting for baseline antibody concentration, VL ≤400 copies/mL at entry (vs >5000 copies/mL) and VL ≤400 copies/mL at the MMR study visit (vs 401–5000 copies/mL and >5000 copies/mL) were associated in univariate analyses with higher antibody concentration 8 weeks after MMR vaccination. Longer duration of the entry HAART regimen was marginally associated with higher antibody concentration (P = .06). Age; sex; race/ethnicity; nadir CD4% prior to HAART; interval from last previous MMR to study MMR visit; immune stratum; CD4% at entry; and CD4%, CD19%, and total lymphocyte count at the MMR study visit were not associated with the antibody concentration 8 weeks after vaccination. Multivariate analysis found only VL ≤400 copies/mL at the MMR study visit (vs 401–5000 copies/mL and >5000 copies/mL) associated with higher measles antibody concentration 8 weeks postvaccination, after adjusting for baseline antibody concentration (P ≤ .03).
P1061s Study Population
Of 224 eligible P1024 subjects, 101 were enrolled in P1061s (flow diagram online). Of these, 80 met inclusion criteria, fulfilled CD4 criteria to receive MMR in P1024 and P1061s, lacked grade ≥3 adverse events following the P1024 dose of MMR, and received MMR per protocol in both studies; 65 of 80 had entry, day 7, and day 28 measles antibody data and were included in P1061s analyses. Their characteristics at P1024 entry and rates of measles seroprotection and GMCs at each P1024 time point were similar to those of the entire P1024 analysis group. Their median CD4% at P1061s entry was 35%; 12% had a CD4% 15%–<25% and 88% had a CD4% ≥25%, and for all 65, the CD4% at P1061s entry was consistent with their original P1024 immunologic stratum assignment (32%, 38%, and 29% in strata 2–4, respectively). Ninety-five percent were on HAART, 3% were on non-HAART antiretroviral therapy, and 2% were not receiving antiretroviral treatment. Sixty-eight percent had a VL ≤400 copies/mL, 18% were between 401–5000 copies/mL, and 13% had >5000 copies/mL. Median time from P1024 MMR vaccination to P1061s entry was 4.24 years (interquartile range, 4.13–4.38 years).
P1061s Measles Antibody Concentrations
Ninety-eight percent were seropositive and 75% had seroprotective antibody concentrations at entry, higher than the seroprotection rate at P1024 entry and only slightly lower than seroprotection rates following P1024 vaccination (Table 3). At day 7, 83% were seroprotected, and 85% had protective memory defined by PRN concentrations ≥120 mIU/mL at entry or day 7. By day 28, 95% achieved seroprotective antibody concentrations. Of the 64 subjects seropositive at entry, only 5% demonstrated memory defined by ≥4-fold antibody rise between entry and day 7, while 25% manifested ≥4-fold antibody rises between entry and day 28. The single subject seronegative at P1061s entry (and before and after P1024 revaccination) experienced a ≥4-fold seroprotective response by day 28.
Table 3.
Measles Serologic Status Before and After Measles Vaccination in P1061s
| Endpoint and Study Time Pointa | All Strata Combined | Stratum 2 | Stratum 3 | Stratum 4 |
|---|---|---|---|---|
| N | 65 | 21 | 25 | 19 |
| Percent with PRN ≥8 mIU/mL on day 0 (95% CI) | 98 (92–100) | 95 (76–100) | 100 (86–100) | 100 (82–100) |
| Percent with PRN ≥120 mIU/mL | ||||
| Day 0 (MMR administration) (95% CI) | 75 (63–85) | 71 (48–89) | 72 (51–88) | 84 (60–97) |
| Day 7 postvaccination (95% CI) | 83 (72–91) | 76 (53–92) | 88 (69–97) | 84 (60–97) |
| Day 28 postvaccination (95% CI) | 95 (87–99)b | 95 (76–100) | 92 (74–99) | 100 (82–100) |
| Percent with ≥4-fold rise in measles antibody concentration among subjects seropositive at entryc | ||||
| Day 0 to day 7 (95% CI) | 5 (1–13) | 5 (0–25) | 4 (0–20) | 5 (0–26) |
| Day 0 to day 28 (95% CI) | 25 (15–37) | 20 (6–44) | 32 (15–54) | 21 (6–46) |
| Geometric mean antibody concentration, mIU/mL (95% CI) | ||||
| Day 0 (MMR vaccination) | 295 | 238 | 316 | 342 |
| (213–409) | (122–465) | (182–547) | (196–599) | |
| Day 7 postvaccination | 407d | 338d | 379 | 550d |
| (296–561) | (172–662) | (228–631) | (314–965) | |
| Day 28 postvaccination | 834d,e | 839d,e | 812d,e | 857d,e |
| (636–1093) | (503–1401) | (486–1358) | (560–1311) |
Immunologic strata are as follows: stratum 2, pre-HAART nadir CD4 cell percentage <15% and screening CD4 cell percentage ≥15%; stratum 3, pre-HAART nadir CD4 cell percentage 15% to <25% and screening CD4 cell percentage ≥15%; stratum 4, pre-HAART nadir CD4 cell percentage ≥25% and screening CD4 cell percentage ≥25%. No statistically significant differences according to immune stratum were observed.
Abbreviations: CI, confidence interval; MMR, measles-mumps-rubella vaccine; PRN, plaque reduction neutralization antibody concentration.
a Day 7 serological results were included if they were obtained within days 5–15 after day 0 and day 28 serological results were included if they were obtained within a window of ±8 days.
b P = .02 (McNemar test) for the comparison of the proportion of subjects with measles antibody concentration ≥120 mIU/mL at day 28 vs day 7 and P ≤ .001 (McNemar test) for the comparison of the proportions of subjects with measles antibody concentration ≥120 mIU/mL at day 28 vs day 0.
c N = 64 subjects seropositive at entry, including 20 in stratum 2, 25 in stratum 3, and 19 in stratum 4.
d P ≤ .03 (paired t test) for the comparison of geometric mean antibody concentration vs the previous time point.
e P ≤ .004 (paired t test) for the comparison of geometric mean antibody concentration at day 28 vs day 0.
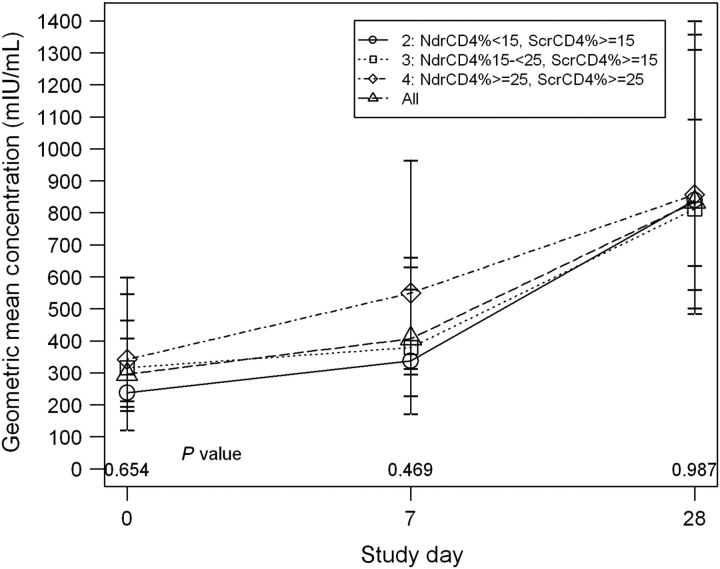
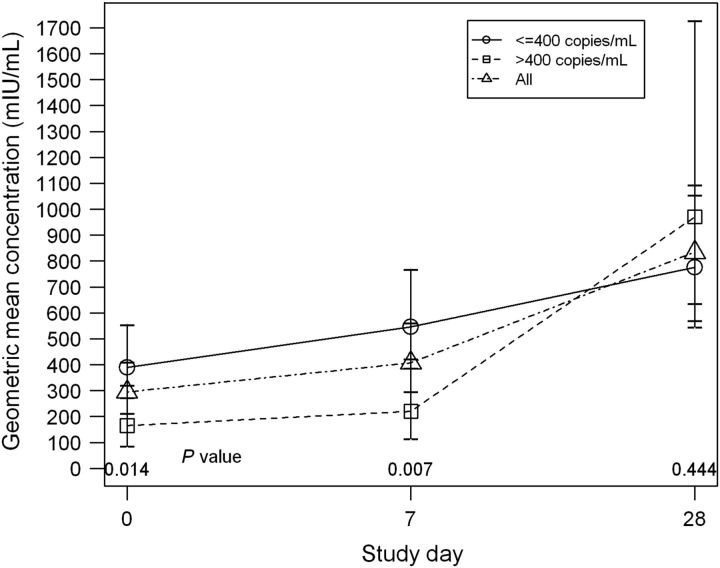
Differences according to immune strata in the percentages seroprotected at entry, day 7, or day 28 or in the proportions with memory (76%, 88%, 89% for seroprotection at entry or day 7 and 5%, 4%, 5% for ≥4-fold rise between entry and day 7, for strata 2–4, respectively) were not significant. The trend toward higher GMCs at entry and day 7 with increasing immune stratum was also not significant (Figure 3). There were no differences based on P1061s VL in the percentages seroprotected at entry, day 7, or day 28; proportions with memory; or in GMCs (data not shown). However, there were differences according to VL at the P1024 MMR vaccination visit in rates of seroprotection at day 7 (91% vs 67% for VL ≤400 copies/mL vs >400 copies/mL, respectively, P = .03; Fisher's exact test) and memory defined as seroprotection at day 0 or day 7 (91% vs 71%, P = .06) and in GMCs at P1061s entry and day 7 (Figure 4).
Figure 3.
P1061s geometric mean concentration (GMC) according to immunologic strata. GMCs of measles neutralizing antibody are shown at each P1061s study visit for all subjects combined and according to immunologic stratum. A trend toward higher GMCs with increasing stratum was present at P1061s entry and day 7, but differences were not significant. MMR vaccine was administered on P1061s day 0.
Abbreviations: Ndr, nadir; Scr, screening.
Figure 4.
P1061s geometric mean concentration (GMC) according to P1024 HIV RNA group. GMCs of measles neutralizing antibody are shown at each P1061s study visit for all subjects combined and according to HIV viral load ≤400 copies/mL vs >400 copies/mL at the P1024 MMR visit. The GMC of subjects with a viral load ≤400 copies/mL was higher than that of subjects with a viral load >400 copies/mL at P1061s entry and day 7. MMR vaccine was administered on P1061s day 0.
Univariate analysis identified the following predictors of higher day 7 measles antibody concentration in P1061s: antibody concentration 8 weeks post-P1024 MMR vaccination (P < .001), antibody concentration at P1061s entry (P < .001), and VL ≤400 copies/mL at P1024 MMR vaccination (P = .007). Age; race; sex; interval from previous MMR to P1024 vaccination; nadir CD4% prior to HAART; duration of the entry HAART regimen prior to P1024 vaccination; immune stratum; measles antibody concentration at P1024 entry; total lymphocyte count, CD4%, and CD19% at the P1024 MMR visit; being on HAART at P1061s entry; and total lymphocyte count, CD4%, CD19% and VL ≤400 copies/mL at P1061s entry were not significantly associated with day 7 antibody concentration.
Safety (P1024 and P1061s)
Among 193 subjects in the P1024 dataset, 4 (2%) experienced grade 3/severe systemic events (3 fever, 1 fatigue) judged possibly or probably related to MMR. One MMR recipient with insufficient serologic data experienced grade 3 fever and pharyngitis possibly or probably related to vaccination. No grade ≥3 adverse events were reported among 10 other subjects who received MMR vaccine but were excluded from analyses for protocol violations (4) or not fulfilling protocol CD4 criteria (6). No grade ≥3 adverse events related to MMR were reported in P1061s. No vaccine-related potentially life-threatening events or deaths were observed.
DISCUSSION
At P1024 entry, approximately half of subjects lacked protective antibody levels against measles, despite all having received MMR previously and 80% having received ≥2 doses. This reflects that most subjects were >2 (median 4.8) years removed from their last MMR, and many likely were not receiving HAART when previously vaccinated. The low seroprotection rate is consistent with low response rates to measles (re)vaccination (25%–75%) and of measles seroprevalence (5%–79%) in HIV-infected children not on HAART and reflects poor primary responses, impaired avidity, rapid waning of immunity, and defective memory [4–17]. Low levels and ongoing decline of measles antibody continue to be characteristic of children treated with HAART subsequent to vaccination [9, 28–32]. The low seroprotection rate prior to revaccination is also consistent with low antibody concentrations for Streptococcus pneumoniae, Bordetella pertussis, and hepatitis B virus at P1024 entry [21–23], and the assessment that HAART is unlikely to restore immunologic memory for vaccinations administered prior to HAART [32].
In contrast, measles seroprotection increased to 89% after revaccination in the context of HAART. Furthermore, we observed ≥4-fold antibody rises in 44% of subjects, including primary ≥4-fold responses in 78% of subjects seronegative at entry. Seroprotection rates of 93% in the highest immune stratum and 95% among subjects with VL <400 copies/mL rival ≥95% protection rates following 2 vaccine doses in HIV-uninfected children [33]. Although antibody concentrations fell beyond 8 weeks postvaccination, seroprotection fell only slightly, with 80% seroprotected 80 weeks postvaccination. These findings are consistent with smaller studies that showed response rates of 60%–90% in children revaccinated against measles after HAART [6, 28, 29, 31–32]. Some studies demonstrated persistence of antibody for 12–36 months, while others reported rapid declines and loss of detectable levels [31, 34].
In P1061s, 75% had measles antibody concentrations ≥120 mIU/mL at entry, only slightly lower than the percentage seroprotected at the conclusion of P1024. Memory was further evidenced by 85% having seroprotective antibody levels at entry or 7 days after the P1061s MMR dose. Parenthetically, the P1061s MMR dose likely stimulated a primary response in the single seronegative subject and produced antibody increases in several others who had antibody concentrations below the protective level at entry and day 7, indicating that an additional dose may induce and/or boost immunity in subjects who, despite HAART, had no or limited response or lacked memory following previous vaccination. Overall, we observed a surprisingly high seroprotection rate of 95% by day 28. Although studies are mixed as to persistence of memory following revaccination while on HAART, our finding of persisting memory 4–5 years after measles revaccination is consistent with studies which demonstrated protective antibody levels and measles-specific memory B cells several years after vaccination while on HAART [32, 34–36].
Responses to MMR in P1024 and detection of immunologic memory in P1061s were greatest in children with an undetectable VL at the time of P1024 vaccination and were not related to CD4% or CD19% measurements. For inactivated vaccines studied in P1024, both concurrent CD4% and VL were significant predictors of response [21–23]. Other studies found that response to measles and varicella-zoster vaccines in children on HAART was related more to suppression of VL than to CD4 values [29, 37, 38]. This suggests that responses to live vaccines may be particularly influenced by adverse effects of HIV replication on number and function of B (including memory cells) and T cells. HAART may mitigate these effects, reinforcing the importance of vaccinating when VL is maximally suppressed [14, 32, 35, 36, 39].
We observed varying immune responsiveness to different vaccines in the same population of HIV-infected children. P1024 responses were high for measles and pneumococcal conjugate vaccines, modest for pertussis vaccine, and weak for hepatitis B virus vaccine [21–23]. In P1061s, immunologic memory was demonstrated 4–5 years later for measles and pneumococcal vaccines, but in only a minority after hepatitis B vaccination [23, 40]. This demonstrates variability in response and memory induction to different immunogens in HIV-infected children on HAART.
Limitations of our study included that we did not know what antiretroviral therapy subjects may have received when given MMR prior to P1024, whether subjects who lacked measles antibody at entry had responded to previous MMR, and if subjects who lacked seroprotective antibody levels had ever attained seroprotective levels. Antibody measurements 8 weeks after P1024 vaccination may have missed peak responses. In P1061s, despite a high rate of memory based on seroprotection, far fewer subjects fulfilled the memory criterion based on ≥4-fold antibody rise by day 7 postvaccination, and only a minority manifested ≥4-fold rises by day 28. This suggests that neutralizing antibody present at entry in the majority was sufficient to inhibit replication of vaccine virus, consistent with memory but precluding rapid, large anamnestic responses, similar to HIV-uninfected children who most often do not attain ≥4-fold rises after revaccination. It is also consistent with our finding in P1024 that ≥4-fold rises were more frequent among initially seronegative subjects and that the magnitude of antibody rises tended to be lower in subjects with higher entry antibody concentrations (data not shown). Thus, the ≥4-fold rise criterion likely underestimated memory, and memory B cell assays would have been informative. It is also possible that, despite HAART, deficiencies in B cell memory and/or CD4 cell help in HIV-infected children limit the rate and/or magnitude of secondary antibody responses [32]. If memory kinetics are delayed, definitions of memory focused on seroprotection and ≥4-fold antibody rise by day 7 may have underestimated true proportions with memory, and antibody increases between days 7 and 28 in subjects seropositive at entry may have represented memory responses. Furthermore, it can be argued that all subjects seropositive at entry were immunologically primed and had some memory. Finally, the number of subjects in the datasets may have limited the power to discern predictors of vaccine response other than VL, multiple comparisons may have introduced chance associations in our exploratory analyses of predictors, and follow-up duration may have been inadequate to detect late adverse events (eg, pneumonitis or encephalitis).
Geographic overlap of the HIV epidemic and endemic measles transmission place HIV-exposed and HIV-infected children at risk [39, 41]. Infants born to HIV-infected mothers, regardless of whether they are HIV-infected, are at increased risk of measles infection, possibly related to lower levels of maternal measles antibody. HIV-infected children are at high risk of measles infection due to poor immunologic memory despite prior vaccination [39]. Measles morbidity and mortality are enhanced in HIV-infected populations, even among children receiving HAART [18, 19]. Although modeling suggests that the HIV epidemic has had limited impact on dynamics of measles transmission in developing countries because of the high mortality associated with HIV in the absence of antiretroviral therapy, the HIV epidemic may be expected to contribute more heavily to an increase in measles with escalating use of antiretroviral therapy and survival of HIV-infected children, if unprotected against measles [20]. Therefore, protecting HIV-infected children against measles with vaccination while on HAART is important not only for their own health, but also for global measles control. The present study reinforces the safety and potential value of measles (re)vaccination, when administered with an adequate CD4% in the context of HAART, to achieve high response rates with persisting immunologic memory [18, 20, 39, 41]. This strategy is of great importance to areas threatened simultaneously by both highly pathogenic viruses.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors appreciate the participation of patients and families in this study and the assistance of research personnel at the study sites.
Additional members of the P1024 and P1061s Protocol Teams. Shirley Jankelevich, MD, and Patrick Jean-Philippe, MD, Pediatric Medicine Branch, National Institute of Allergy and Infectious Diseases, Bethesda, MD; Jennifer Read, MD, Pediatric, Adolescent, and Maternal AIDS Branch, National Institute of Child Health and Human Development, Bethesda, MD; Victoria Hadhazy, MA, Alison Robbins, MA, and Beth Sheeran, MS, RD, PACTG/IMPAACT Operations Office, Rockville, MD; Jane C. Lindsey, ScD, and Lin-Ye Song, PhD, Statistical and Data Analysis Center, Harvard School of Public Health, Boston, MA; Janice Hodge, RN, BS, Mary Caporale, MSc, M.P.H., Nancy Webb, MS, and Courtney Ashton, Frontier Science and Technology Research Foundation, Amherst, NY; Dorothy Smith, MS, CPNP, University of Massachusetts Memorial Medical Center, Worcester, MA; Lourdes Angeli, RN, BSN, MPH, San Juan City Hospital, San Juan, PR; John Pella, CCRA, CCRC, MPH, Westat, Inc, Rockville, MD; Paul Tran, RPh, Pharmaceutical Affairs Branch, National Institute of Allergy and Infectious Diseases, Bethesda, MD; Grace Aldrovandi, MD, Children's Hospital of Los Angeles, Los Angeles, CA; Rachel Barrett, BS, University of Colorado School of Medicine, Denver, CO; William Kabat, BS, Chicago Children's Memorial Hospital, Chicago, IL; Alan Shaw, PhD, Merck and Co, West Point, PA; Daniel Isaacman, MD, and Velma Keeley, BS, Wyeth Pharmaceuticals, Collegeville, PA; Paul Willems, MD, GlaxoSmithKline Pharmaceuticals, Collegeville, PA; Carol Gore, Panorama Village, TX.
Participating sites and site personnel. Chicago Childrens’ Memorial Hospital (Ram Yogev, MD), UMDNJ-New Jersey Medical School (Barry Dashefsky, MD, Linda Bettica, RN, Paul Palumbo, MD), Harlem Hospital (Elaine Abrams, MD, Maxine Frere, RN, Lisa Gaye Robinson, MD), Metropolitan Hospital Center (Mahrukh Bamji, MD), Long Beach Memorial Hospital (Audra Deveikis, Susan Marks, Karen Elkins, Lisa Melton), San Juan City Hospital (Eleanor Jimenez, MD), Los Angeles County Medical Center (James Homans, MD, Ana Melendez, RN, Andrea Kovacs, MD), University of Florida–Jacksonville (Mobeen H. Rathore, MD, Ana Alvarez, MD, Ayesha Mirza, MD, Thomas Chiu, MD), University of California–San Diego (Stephen A. Spector, MD, Rolando Viani, MD, MTP, Mary Caffery, RN, MSN, Lisa Stangl, CPNP), State University of New York at Stony Brook (Denise Ferraro, RN, Silvia Muniz, Michell Davi, NP), University of Colorado School of Medicine and The Children's Hospital (Jody Maes, MD, Carol Salbenblatt, RN, Suzanne Paul, BSN, MSN, FNP, Emily Barr, CPNP, CNM, MSN; Grant No. M01 RR00069, General Clinical Research Centers Program, National Center for Research Resources, NIH), University of Chicago Children's Hospital, Schneider Children's Hospital of North Shore–Long Island Jewish Health System (Vincent R. Bonagura, MD, Susan J. Schuval, MD, Connie Colter, MS, CPNP), Children's Hospital of Oakland (Ann Petru, MD, Teresa Courville, RN, MN, Karen Gold, RN, FNP, Lauren Poole, RN, FNP), St. Christopher's Hospital for Children and Drexel University College of Medicine (Janet S. Chen, MD, Jill A. Foster, MD, Daniel H. Conway, MD, Gary Koutsoubis), Boston Medical Center and Boston University School of Medicine (Ann Marie Regan, PNP), State University of New York Downstate (H. Jack Moallem, MD, Edward Handelsman, MD, Denise Swindell, Jean Kaye, RN), Ramon Ruiz Arnau University (Wanda Figueroa, MD), Bronx Lebanon Hospital Center (Mavis Dummitt, RN, Anantha Kallury, BPharm, Murli Purswani, MD, Saroj Bakshi, MD), St. Lukes/Roosevelt Hospital Center (Emma Stuard, MD, Steven Arpadi, MD), University of Massachusetts Medical Center (Katherine Luzuriaga, MD, Dottie Smith), Children's Hospital of Boston (Sandra Burchett, MD, MS, Lynne Lewis, RN, MS, CPNP, Catherine Kneut, RN, MS, CPNP), North Broward Hospital District (Amy Inman, BS, Linh Tran, PharmD, Guillermo Talero, MD, Ann Puga, MD), University of Maryland (John Farley, MD, Mary MacFadden), University of California–San Francisco Moffitt Hospital (Diane Wara, MD), New York University School of Medicine (Siham Akleh, RN, Aditya Kaul, MD, Sulachni Chandwani, MD, Thomas Hastings, RN), University of Rochester Medical Center (Geoffrey A. Weinberg, MD, Susan Laverty, RN, Barbra Murante, MS, RN, PNP, Francis Gigliotti, MD), Yale University School of Medicine (Warren A. Andiman, MD, Leslie Hurst, MS, Sostena Romano, APRN), University of Puerto Rico and University Children's Hospital (Irma Rodriguez, MD), University of Connecticut Health Center/Connecticut Children's Medical Center (Juan C. Salazar, MD, MPH, Gail Karas, RN, Lorraine Wells, RN), Texas Children's Hospital and Baylor University (William Shearer, MD), Cook County Hospital (Jaime Martinez, MD), Cornell University–New York Presbyterian Hospital (Joseph Stavola, MD), Children's Hospital National Medical Center (Hans Spiegel, MD), University of Florida–Gainesville (Robert Lawrence, MD), Tulane University and Charity Hospital of New Orleans (Russell Van Dyke, MD, Cheryl Borne, RN, Margaret Cowie, BS), University of Alabama at Birmingham (Robert Pass, MD, Marilyn Crain, MD, Newana Beatty).
Financial support. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases (NIAID) cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) group. Support of the sites was provided by the NIAID and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C). This work was also supported in part by the General Clinical Research Center Units funded by the National Center for Research Resources, National Institutes of Health (NIH; including MO1-RR00069, General Clinical Research Centers Program).
Merck and Co. provided study vaccine used in P1061s. Overall support for the IMPAACT group was provided by the NIAID (U01 AI068632), the NICHD, and the National Institute of Mental Health (NIMH; AI068632). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Potential conflicts of interest. M. J. L. shares intellectual property, serves on an advisory board, and chairs an adjudication committee for Merck & Co. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients. JAMA. 1992;267:1237–41. [PubMed] [Google Scholar]
- 2.Krasinski K, Borkowsky W. Measles and measles immunity in children infected with human immunodeficiency virus. JAMA. 1989;261:2512–6. [PubMed] [Google Scholar]
- 3.Palumbo P, Hoyt L, Demasio K, Oleske J, Connor E. Population-based study of measles and measles immunization in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1992;11:1008–14. doi: 10.1097/00006454-199211120-00004. [DOI] [PubMed] [Google Scholar]
- 4.Al-Attar I, Reisman J, Muehlmann M, McIntosh K. Decline of measles antibody titers after immunization in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1995;14:149–51. [PubMed] [Google Scholar]
- 5.Arpadi SM, Markowitz LE, Baughman AL, et al. Measles antibody in vaccinated human immunodeficiency virus type 1-infected children. Pediatrics. 1996;97:653–7. [PubMed] [Google Scholar]
- 6.Aurpibul L, Puthanakit T, Sirisanthana T, Sirisanthana V. Response to measles, mumps, and rubella revaccination in HIV-infected children with immune recovery after highly active antiretroviral therapy. Clin Infect Dis. 2007;45:637–42. doi: 10.1086/520651. [DOI] [PubMed] [Google Scholar]
- 7.Brena AE, Cooper ER, Cabral HJ, Pelton SI. Antibody response to measles and rubella vaccine by children with HIV infection. J Acquir Immune Defic Syndr. 1993;6:1125–9. [PubMed] [Google Scholar]
- 8.Brunell PA, Vimal V, Sandu M, Courville TM, Daar E, Israele V. Abnormalities of measles antibody response in human immunodeficiency virus type 1 (HIV-1) infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:540–8. [PubMed] [Google Scholar]
- 9.Farquhar C, Wamalwa D, Selig S, et al. Immune responses to measles and tetanus vaccines among Kenyan human immunodeficiency virus type 1 (HIV-1)–infected children pre- and post-highly active antiretroviral therapy and revaccination. Pediatr Infect Dis J. 2009;28:295–9. doi: 10.1097/INF.0b013e3181903ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowlkes A, Witte D, Beeler J, et al. Persistence of vaccine-induced measles antibody beyond 12 months: a comparison of response to one and two doses of Edmonston-Zagreb measles vaccine among HIV-infected and uninfected children in Malawi. J Infect Dis. 2011;204:S149–57. doi: 10.1093/infdis/jir135. [DOI] [PubMed] [Google Scholar]
- 11.Helfand RF, Witte D, Fowlkes A, et al. Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi. J Infect Dis. 2008;198:1457–65. doi: 10.1086/592756. [DOI] [PubMed] [Google Scholar]
- 12.Hilgartner MW, Maeder MA, Mahoney EM, et al. Response to measles, mumps, and rubella revaccination among HIV-positive and HIV-negative children and adolescents with hemophilia. Hemophilia growth and development study. Am J Hematol. 2001;66:92–8. doi: 10.1002/1096-8652(200102)66:2<92::AID-AJH1023>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Moss WJ, Scott S, Mugala N, et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis. 2007;196:347–55. doi: 10.1086/519169. [DOI] [PubMed] [Google Scholar]
- 14.Nair N, Moss WJ, Scott S, et al. HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus-specific immunoglobulin G after vaccination and infection. J Infect Dis. 2009;200:1031–8. doi: 10.1086/605648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudy BJ, Rutstein RM, Pinto-Martin J. Responses to measles immunization in children infected with human immunodeficiency virus. J Pediatr. 1994;125:72–4. doi: 10.1016/s0022-3476(94)70125-3. [DOI] [PubMed] [Google Scholar]
- 16.Thaithumyanon P, Punnahitananda S, Thisyakorn U, Praisuwanna P, Ruxrungtham K. Immune responses to measles immunization and the impacts on HIV-infected children. Southeast Asian J Trop Med Public Health. 2000;31:658–62. [PubMed] [Google Scholar]
- 17.Walter EB, Katz SL, Bellini WJ. Measles immunity in HIV-infected children. Pediatr AIDS HIV Infect. 1994;5:300–4. [PubMed] [Google Scholar]
- 18.Aurpibul L, Puthanakit T, Kanjanavanit S, Sirisanthana T, Sirisanthana V. Measles outbreak in an orphanage: HIV-infected children on antiretroviral therapy are still at risk. Pediatr Infect Dis J. 2010;29:167–9. doi: 10.1097/INF.0b013e3181b99e15. [DOI] [PubMed] [Google Scholar]
- 19.Moss WJ, Fisher C, Scott S, et al. HIV type 1 infection is a risk factor for mortality in hospitalized Zambian children with measles. Clin Infect Dis. 2008;46:523–7. doi: 10.1086/526525. [DOI] [PubMed] [Google Scholar]
- 20.Scott S, Mossong J, Moss WJ, Cutts FT, Cousens S. Predicted impact of the HIV-1 epidemic on measles in developing countries: results from a dynamic age-structured model. Int J Epidemiol. 2008;37:356–67. doi: 10.1093/ije/dyn007. [DOI] [PubMed] [Google Scholar]
- 21.Abzug MJ, Pelton SI, Song LY, et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2006;25:920–9. doi: 10.1097/01.inf.0000237830.33228.c3. [DOI] [PubMed] [Google Scholar]
- 22.Abzug MJ, Song LY, Fenton T, et al. Pertussis booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. Pediatrics. 2007;120:e1190–202. doi: 10.1542/peds.2007-0729. [DOI] [PubMed] [Google Scholar]
- 23.Abzug MJ, Warshaw M, Rosenblatt HM, et al. Immunogenicity and immunologic memory after hepatitis B virus booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis. 2009;200:935–46. doi: 10.1086/605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albrecht P, Herrmann K, Burns GR. Role of virus strain in conventional and enhanced measles plaque neutralization test. J Virol Methods. 1981;3:251–60. doi: 10.1016/0166-0934(81)90062-8. [DOI] [PubMed] [Google Scholar]
- 25.Cohen BJ, Audet S, Andrews N, Beeler J. Plaque reduction neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine. 2007;26:59–66. doi: 10.1016/j.vaccine.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–42. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 27.Dine MS, Hutchins SS, Thomas A, Williams I, Bellini WJ, Redd SC. Persistence of vaccine-induced antibody to measles 26–33 years after vaccination. J Infect Dis. 2004;189:S123–30. doi: 10.1086/380308. [DOI] [PubMed] [Google Scholar]
- 28.Bekker V, Scherpbier H, Pajkrt D, Jurriaans S, Zaaijer H, Kuijpers TW. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics. 2006;118:e315–22. doi: 10.1542/peds.2005-2616. [DOI] [PubMed] [Google Scholar]
- 29.Berkelhamer S, Borock E, Elsen C, Englund J, Johnson D. Effect of highly active antiretroviral therapy on the serological response to additional measles vaccinations in human immunodeficiency virus-infected children. Clin Infect Dis. 2001;32:1090–4. doi: 10.1086/319591. [DOI] [PubMed] [Google Scholar]
- 30.Tejiokem MC, Gouandjika I, Beneiguel L, et al. HIV-infected children living in Central Africa have low persistence of antibodies to vaccines used in the Expanded Program on Immunization. PLoS One. 2007;2:e1260. doi: 10.1371/journal.pone.0001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melvin AJ, Mohan KM. Response to immunization with measles, tetanus, and Haemophilus influenza type b vaccines in children who have human immunodeficiency virus type 1 infection and are treated with highly active antiretroviral therapy. Pediatrics. 2003;111:e641–4. doi: 10.1542/peds.111.6.e641. [DOI] [PubMed] [Google Scholar]
- 32.Sutcliffe CG, Moss WJ. Do children infected with HIV receiving HAART need to be revaccinated? Lancet Infect Dis. 2010;10:630–42. doi: 10.1016/S1473-3099(10)70116-X. [DOI] [PubMed] [Google Scholar]
- 33.American Academy of Pediatrics. Measles. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red book: 2009 report of the committee of infectious diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2009. pp. 448–9. [Google Scholar]
- 34.Aurpibul L, Puthanakit T, Sirisanthana T, Sirisanthana V. Persistence of measles, mumps, and rubella protective antibodies 3 years after revaccination in HIV-infected children receiving antiretroviral therapy. Clin Infect Dis. 2010;50:1415–8. doi: 10.1086/652150. [DOI] [PubMed] [Google Scholar]
- 35.Belaunzaran-Zamudio PF, Garcia-Leon ML, Wong-Chew RM, et al. Early loss of measles antibodies after MMR vaccine among HIV-infected adults receiving HAART. Vaccine. 2009;27:7059–64. doi: 10.1016/j.vaccine.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 36.Pensieroso S, Cagigi A, Palma P, et al. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci USA. 2009;106:7939–44. doi: 10.1073/pnas.0901702106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin MJ, Gershon AA, Weinberg A, et al. Immunization of HIV-infected children with varicella vaccine. J Pediatr. 2001;139:305–10. doi: 10.1067/mpd.2001.115972. [DOI] [PubMed] [Google Scholar]
- 38.Levin MJ, Gershon AA, Weinberg A, et al. Administration of live varicella vaccine to HIV-infected children with current or past significant depression of CD4+ T cells. J Infect Dis. 2006;194:247–55. doi: 10.1086/505149. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson A, Chiodi F. Measles outbreak in Africa – is there a link to the HIV epidemic? PLoS Pathog. 2011;7:1–3. doi: 10.1371/journal.ppat.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abzug MJ, Pelton SI. Prevention of invasive pneumococcal disease in HIV-infected children: expanding the toolbox. J Infect Dis. 2009;199:1109–11. doi: 10.1086/597389. [DOI] [PubMed] [Google Scholar]
- 41.Rainwater-Lovett K, Moss WJ. The urgent need for recommendations on revaccination of HIV-infected children after successful antiretroviral therapy. Clin Infect Dis. 2010;51:634–5. doi: 10.1086/655769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.