Abstract
Background. Recent mathematical models suggested that frequent human immunodeficiency virus (HIV) testing with immediate initiation of antiretroviral therapy (ART) to individuals with a positive test result could profoundly curb transmission. The debate about ART as prevention has focused largely on parameter values. We aimed to evaluate structural assumptions regarding linkage to care and population mobility, which have received less attention.
Methods. We modified the linkage structure of published models of ART as prevention, such that individuals who decline initial testing or treatment do not link to care until late-stage HIV infection. We then added population mobility to the models. We populated the models with demographic, clinical, immigration, emigration, and linkage data from a South African township.
Results. In the refined linkage model, elimination of HIV transmission (defined as an incidence of <0.1%) did not occur by 30 years, even with optimistic assumptions about the linkage rate. Across a wide range of estimates, models were more sensitive to structural assumptions about linkage than to parameter values. Incorporating population mobility further attenuated the reduction in incidence conferred by ART as prevention.
Conclusions. Linkage to care and population mobility are critical features of ART-as-prevention models. Clinical trials should incorporate relevant data on linkage to care and migration to evaluate the impact of this strategy.
There has been growing enthusiasm about the potential for prevention of human immunodeficiency virus (HIV) transmission through the use of antiretroviral therapy (ART) early in the course of HIV disease [1, 2]. While earlier models examined the prevention benefits of treatment in the United States [3, 4], a high-profile article by Granich et al explicitly addressed the question of whether yearly testing and immediate access to ART for individuals with diagnosed HIV infection could eliminate HIV transmission in a high-burden setting like South Africa [5]. This model of “ART as prevention,” alternatively referred to as “test and treat,” generated considerable debate concerning the validity of key model parameters, biologic and programmatic [6–10]. The structural assumptions of this model have received less scrutiny.
Field trials are now planned to assess whether frequent testing combined with early access to ART can reduce community HIV incidence under real-world conditions [11]. Among the programmatic parameters that have been debated is the estimate of linkage to care—the proportion of individuals who agreed to testing and, if positive for HIV, ART initiation. Many studies have shown high rates of loss to follow-up after HIV testing [12–14], such that one-half or fewer of the individuals meeting clinical or CD4 T-cell criteria for ART initiation actually start treatment. Less attention has been placed on the model assumption that individuals failing to link to care remained in the same model compartment to be linked the following year; in effect, the probability of accepting care did not change upon prior failed linkage, such that nearly all individuals would engage with care within a few years. This key assumption merits further examination: when individuals who decline treatment or are lost to follow-up ultimately engage (or re-engage) in care may be a key factor influencing outcomes of strategies of early testing and treatment.
In South Africa, high rates of domestic and international migration have been observed in recent years, fueled by economic and social changes, as well as by political instability in neighboring Zimbabwe [15]. The impact of migration on the effects of ART-as-prevention studies or programs used in discrete communities is not known.
In real-world settings, the movement of individuals in and out of communities and treatment programs may substantially attenuate the gains of ART as prevention. We adapted and modified previously published models of ART as prevention and incorporated data from a well-studied township in Cape Town, South Africa, to assess the impact of linkage to care and migration on model projections.
METHODS
Overview
To simulate HIV infection and disease, we first provide the structure of a simple natural history and treatment model adapted from previous models of HIV [5] and offer details of how we modified it to include a phase of acute infection wherein transmission probability is greater. We then describe further structural adjustments to the model that were made to examine the impact of linkage to care and migration on the resulting dynamics. We report comparisons of the results of these models in terms of long-term HIV incidence, prevalence, and ART coverage in the community. We explain the model's equations in detail in the Supplementary Materials. Here, we present an overview of the model structure and assumptions.
Model 1 Structure
In the base model (model 1; Figure 1A), individuals enter the model at the age of 15 years and are susceptible to HIV infection. Susceptible individuals (S) are infected through contact with infected individuals. Following infection, individuals enter an acute stage (A), wherein they are more infectious. In principle, they could have their disease diagnosed at this stage and initiate ART. In practice, diagnosis of acute HIV infection in resource-poor settings is likely rare.
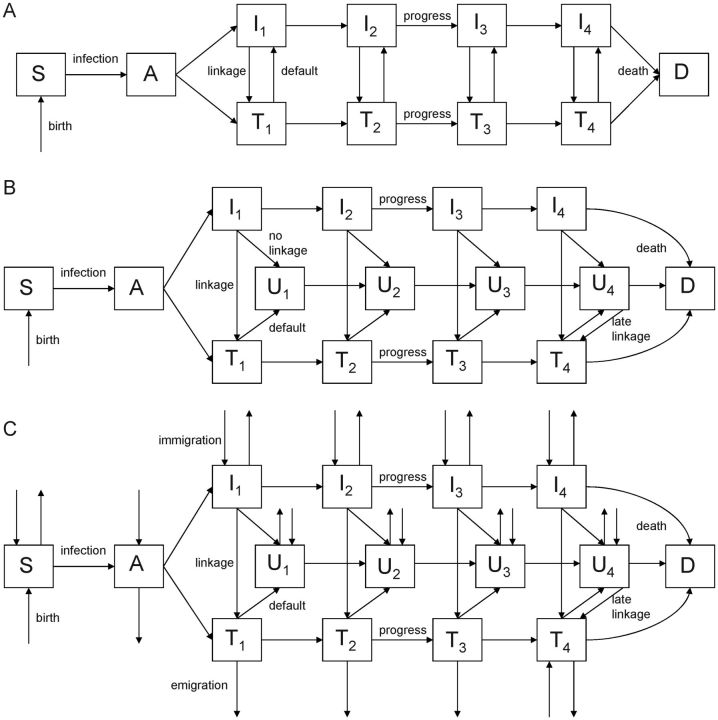
Figure 1.
In model 1, individuals who do not link in 1 year have an unchanged probability of linking in subsequent years (linkage is conditionally independent, as in Granich et al [5]). Model 2 incorporates a revised formulation of linkage to care, in which individuals who do not link initially join an unlinked compartment and link in late-stage disease. In model 3, we built upon the revised linkage structure and added migration (immigration and emigration). Mortality unrelated to human immunodeficiency virus (HIV) infection may occur from any compartment (background mortality; not shown). A, acute infection; D, dead; I, infected, not receiving antiretroviral therapy; S, susceptible; T, receiving antiretroviral therapy; U, unlinked to care (individuals who decline treatment or are lost to follow-up). Subscripts to the I, U, and T compartments denote stages of HIV infection progression.
Following recovery from acute infection, individuals enter a series of stages of chronic infection (I), progressing through them toward HIV-related death. We modeled 4 stages of chronic HIV infection, and the duration spent in each stage is one-quarter of the life expectancy for individuals with untreated HIV infection. Consistent with the Granich model and in contrast to World Health Organization stages or CD4 T-cell counts, the chronic disease stages are defined to model a specified mean life expectancy for individuals with untreated HIV disease [5]. As a result, progression through stages is only forward. From any stage of untreated HIV infection, individuals can be tested at annual rate τ and initiate ART (T). During ART, individuals progress more slowly toward HIV-related death, and their infectiousness is lower. Default from treatment returns individuals to an untreated state (I). We have incorporated background mortality data, such that individuals can die from HIV-unrelated causes in any compartment.
Revised Models: Models 2 and 3
In model 1, individuals who are not linked to treatment (ie, those who decline HIV testing or treatment or are lost to follow-up before ART initiation) remain in the untreated population (I) but effectively have an equal probability of agreeing to testing and/or treatment in the following year (or testing interval). Because the probability of linkage to care (ie, accepting both testing and treatment) is likely to be conditionally dependent with respect to prior response—that is, people who have not linked before are not as likely to link in subsequent attempts—we created a modified model (model 2; Figure 1B). In model 2, individuals who decline testing or are lost to follow-up before starting ART move to an “unlinked” compartment (U). These individuals progress through HIV disease at the same rate as untested individuals, but they do not link back to care until the final stage of HIV infection, when they present with symptomatic disease. Similarly, individuals who default from treatment move to the unlinked compartments and relink to care in the final stage of HIV infection. In the final stage of HIV infection, linkage among those who defaulted from treatment or who did not link after initial testing was assumed to be 100% (we relaxed this assumption in sensitivity analysis; Supplementary Materials).
In model 3 (Figure 1C), we added immigration into and emigration from the population, building on the linkage structure from model 2. Because we assumed that individuals were not immigrating from other areas where ART-as-prevention trials were taking place, immigration into ART compartments at the early stage of HIV infection was not permitted. Individuals could immigrate into all other compartments and could emigrate from any compartment in the model.
Study Population and Input Parameters
Masiphumelele is a well-defined peri-urban township, 40 km south of Cape Town. The adult population in 2010 was 12 809 (Supplementary Materials). Since 2002, complete censuses have been performed every other year in this township, yielding detailed data on demographic characteristics, population growth, and rates of immigration and emigration. A recent cross-sectional survey in this community provided HIV prevalence, CD4 T-cell count among HIV-infected individuals, and ART coverage data. Reports from the township's primary ART clinic provided data on loss to follow-up. Studies of HIV prevalence were used to calculate HIV incidence and the transmission parameter (Supplementary Materials). A recent study in this community yielded an estimate of 53% for the key parameter of linkage to care [16].
For biological parameters, including survival following HIV infection, relative transmissibility of HIV during acute HIV infection, and relative transmissibility of HIV during ART, we used estimates from the literature. Model parameters and their sources are shown in Table 1. We used the same set of parameters, when applicable, for all 3 models.
Table 1.
Parameter Definitions and Ranges for Models
| Parameter | Symbol | Base Value | Range | Reference |
|---|---|---|---|---|
| Adult population | … | 12 809 | … | Census (Supplementary Materials) |
| HIV prevalence in community | … | 0.23 | 0.20–0.25 | [26] |
| Proportion of HIV-infected patients receiving ART | … | 0.35 | 0.30–0.42 | [26] |
| HIV prevalence among immigrants | Θ | 0.18 | 0.14–0.23 | Authors’ unpublished data |
| Proportion of immigrants with CD4 T-cell count of <200 cells/μL during ART | ω | 0.59 | 0.33–0.82 | Authors’ unpublished data |
| Linkage to care (ART) proportion | (1 − γ) | 0.53 | … | [16] |
| Immigration rate | δ | 0.10/y | 0.08–0.12 | Authors’ unpublished data |
| Emigration rate | ψ | 0.04/y | 0.02–0.06 | Census (Supplementary Materials) |
| HIV-unrelated background mortality | μ | 0.017/y | … | [21] |
| Default rate of patients receiving ART | 0.07/y | 0.06–0.09 | [27] | |
| Incidence of HIV infection | … | 0.025/y | 0.021–0.028 | Supplementary Materials |
| Median survival from time of HIV infection | 4/(ρ + μ) + 0.2 | 11.7y | 11.4–12.1 | [28] |
| Median survival during immediate ART | 4/(σ + μ) | 21.0y | … | [5] |
| Birth rate | β | 0.021/y | … | Census (Supplementary Materials) |
| Rate of recovery from acute infection | α | (2m)−1 | … | [29–31] |
| Relative infectiousness during acute phase of infection | ξ | 10 | 8.5–26 | [29–31] |
| Testing rate | τ | 1/y | … | Assumption |
| Relative infectiousness during ART | ε | 0.04 | 0.02–0.08 | [32–34] |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
Analytic Approach
We first compared the 2 models of linkage to care (model 1 and model 2), simulating HIV incidence and prevalence under an ART-as-prevention program with once yearly HIV testing of the entire population with undiagnosed infection. We varied the linkage proportion within each model, using the value from the Granich model (92%) [5] and a recent estimate from Masiphumelele (53%) [16].
Next, we used model 3 to assess the impact of immigration and emigration on the incidence, prevalence, and proportion of the total population receiving ART, comparing findings with those of model 1, which differed by linkage structure and absence of migration, and with those of model 2, which differed only by absence of migration.
Primary model outcomes, examined at 10-, 20-, and 30-year horizons, were incidence of HIV infection, prevalence of HIV infection, and proportion of the population covered by ART. We emphasize the time to achieving an HIV incidence of <0.1% per annum because this has been described as “elimination of HIV infection” in previous models [5, 17].
Parameter Uncertainty and Sensitivity Analysis
We used Latin hypercube sampling for uncertainty and sensitivity analysis [18], using model 3. We varied model parameters over 95% binomial confidence intervals (for parameters derived from primary data) or literature estimates (using triangular distributions) when primary data were not available. Parameter ranges are shown in Table 1. We drew 1000 times from these parameter distributions, taking the median and 95% prediction intervals. We compared model projections of prevalence, incidence, and ART coverage at 30 years.
We compared the relationship between model parameters and outputs by calculating partial rank correlation coefficients (PRCCs). The PRCC is a nonparametric test that quantifies the relationship between 2 variables, in this case, the model parameter value and the outcome of interest (incidence or prevalence of HIV infection) [19]. We plotted PRCCs as a function of time in the model, following the methods of Blower et al [20].
In 2-way sensitivity analysis, we assessed the HIV incidence in the population at 30 years with varying immigration rates and linkage rates, using model 3.
RESULTS
Linkage
Model results were first compared to assess the impact of linkage rates and model structure without factoring in migration. With model 1, HIV incidence in 30 years was reduced from 2.5% to 0.03%, with 92% linkage to care, and to 0.05%, with 53% linkage to care (Table 2 and Figure 2A). At 92% linkage, incidence fell to <0.1% at 15.2 years, and at 53% linkage, it crossed this threshold at 21.8 years. At 30 years, prevalence was 2.0% and 2.6% at 92% and 53% linkage, respectively (Figure 2B).
Table 2.
Projections of Incidence and Prevalence of Human Immunodeficiency Virus (HIV) Infection and Percentage of Entire Population Receiving Antiretroviral Therapy (ART) Over Time Under ART-as-Prevention Programs, According to Model and Linkage Proportion
| Variable | Incidence (%) |
Prevalence (%) |
Population Receiving ART (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2030 | 2040 | 2020 | 2030 | 2040 | 2020 | 2030 | 2040 | |
| Model 1 | |||||||||
| Linkage 92% | 0.15 | 0.07 | 0.03 | 10.8 | 4.8 | 2.0 | 10.0 | 4.5 | 1.9 |
| Linkage 53% | 0.24 | 0.11 | 0.05 | 11.6 | 5.6 | 2.6 | 10.0 | 4.8 | 2.2 |
| Model 2 | |||||||||
| Linkage 92% | 0.43 | 0.26 | 0.15 | 12.3 | 6.8 | 3.9 | 8.9 | 4.8 | 2.7 |
| Linkage 53% | 0.76 | 0.49 | 0.33 | 14.4 | 9.4 | 6.3 | 8.0 | 5.3 | 3.5 |
| Model 3 | |||||||||
| Linkage 92% | 0.52 | 0.49 | 0.47 | 14.3 | 13.1 | 12.9 | 9.6 | 8.6 | 8.5 |
| Linkage 53% | 0.91 | 0.86 | 0.85 | 15.9 | 14.8 | 14.6 | 7.2 | 6.6 | 6.5 |
Figure 2.
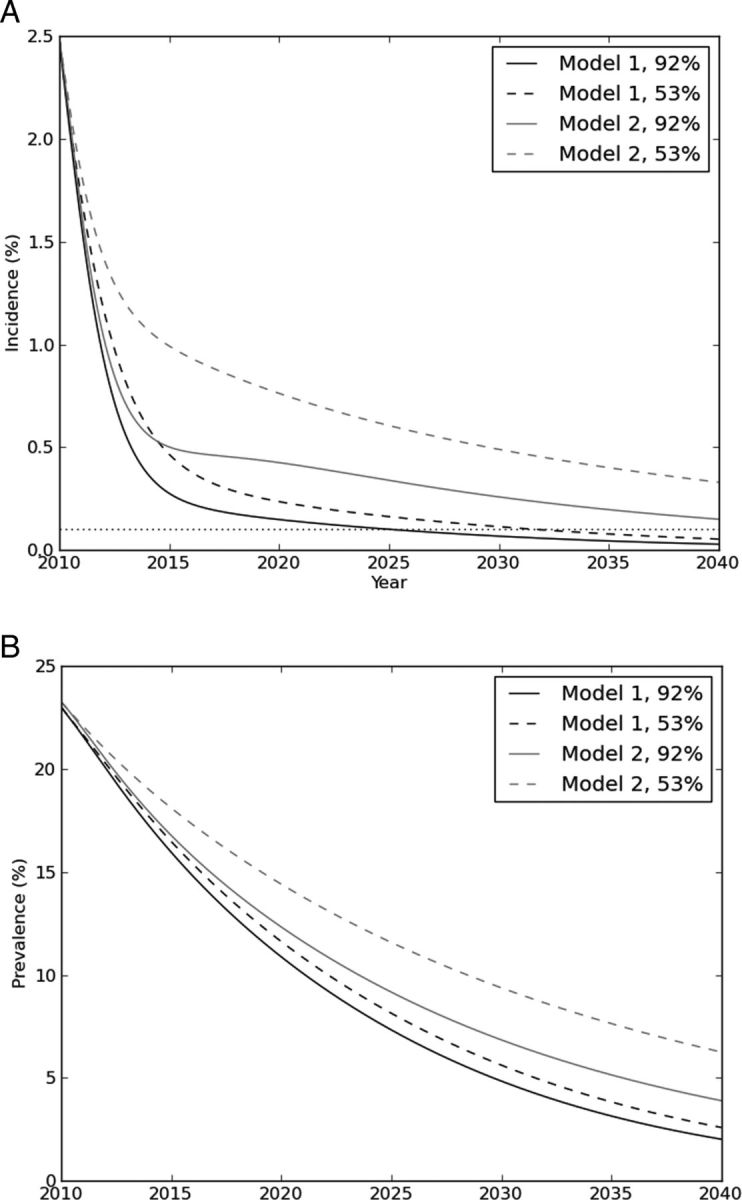
Incidence (A) and prevalence (B) (vertical axes) of human immunodeficiency virus (HIV) infection during antiretroviral therapy as prevention in linkage model 1 (individuals who do not link to care have an unchanged probability of linking in subsequent years) versus linkage model 2 (individuals who do not to link to care do not link until late-stage HIV infection), stratified by linkage proportion. The horizontal axis in both graphs is time in years following initiation of ART-as-prevention programs. The higher linkage proportion estimate (92%; used by Granich et al [5]) is depicted by solid lines, and an estimate from the study community (53%) is depicted by dashed lines. The horizontal dotted line in panel A indicates incidence of 0.1% (“elimination”). HIV incidence and prevalence decline substantially under both models, but incidence did not decrease to <0.1% at either linkage proportion in model 2.
In model 2, HIV elimination did not occur at either linkage rate. Incidence was reduced to 0.15% and 0.33% with 92% and 53% linkage, respectively (Table 2 and Figure 2A). At 30 years, prevalence was 3.9% and 6.3% at 92% and 53% linkage, respectively (Figure 2B).
At each level of linkage and each time horizon, incidence was higher in model 2 than in model 1. The 30-year incidence and prevalence projected in model 1 with a linkage of 53% (0.05% and 2.6%, respectively) was lower than the projections from model 2 with a linkage of 92% (0.15% and 3.9%, respectively).
Migration
Model 3 was used to assess the impact of immigration into and emigration from the community. In this model, HIV incidence did not fall to <0.1% at 30 years at either linkage rate (Table 2). Prevalence was initially lower in the model with migration (Figure 3B) because of the lower prevalence of HIV infection among immigrants (and the higher prevalence among emigrants). At 53% linkage, the prevalence in the migration model rose above the prevalence in the model without migration by 5.3 years, and it remained higher. At 30 years, prevalence in the migration model was nearly 15%. While prevalence and incidence were declining in the model without migration, in the migration model they reached equilibrium (14.6% and 0.85%, respectively; Figure 3A and 3B).
Figure 3.
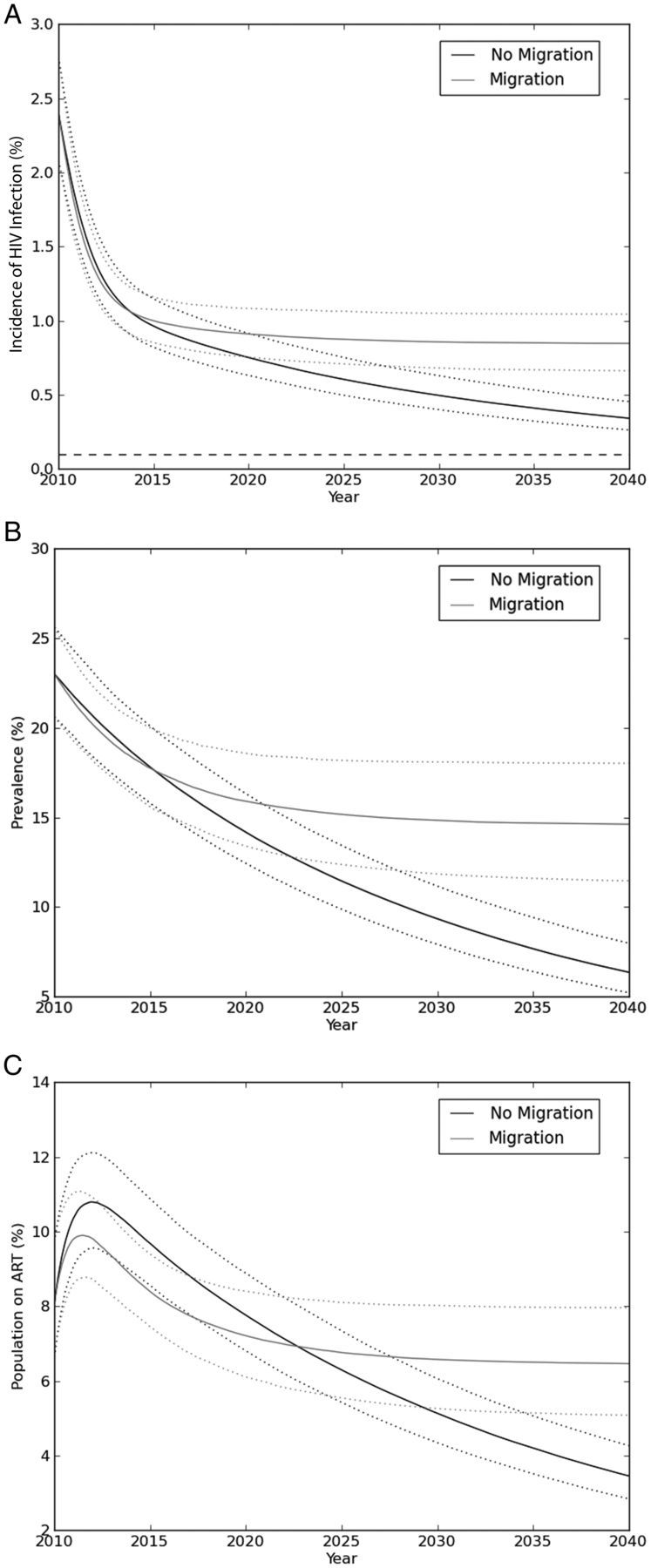
Human immunodeficiency virus (HIV) incidence (A), prevalence (B), and proportion of the population receiving antiretroviral therapy (ART; C) (vertical axes) in models with (gray lines) and without (black lines) migration, using a linkage proportion of 53%. Dotted lines indicate 95% confidence intervals. The horizontal axis in all graphs is time in years following initiation of ART-as-prevention programs. HIV incidence and prevalence initially decline substantially in both models, but they plateau at a higher incidences and prevalences in the model with migration. The proportion of individuals in the community who are receiving ART is initially lower in model 3, which accounts for migration, due to the lower prevalence of HIV infection among migrants and to the emigration of individuals receiving ART; after 12.7 years, the proportion of individuals receiving ART in the model accounting for migration is higher.
The proportion of the total population receiving ART was initially lower in the model accounting for migration because of the lower prevalence of HIV infection among migrants, emigration of individuals receiving ART, and immigration of individuals not receiving ART (Figure 3C). By 12.7 years, the proportion of the population receiving ART in the migration model was higher because the higher HIV prevalence outweighed the effects of emigration by individuals receiving ART or the dilution effect of immigration by individuals not receiving ART.
Parameter Sensitivity Analyses
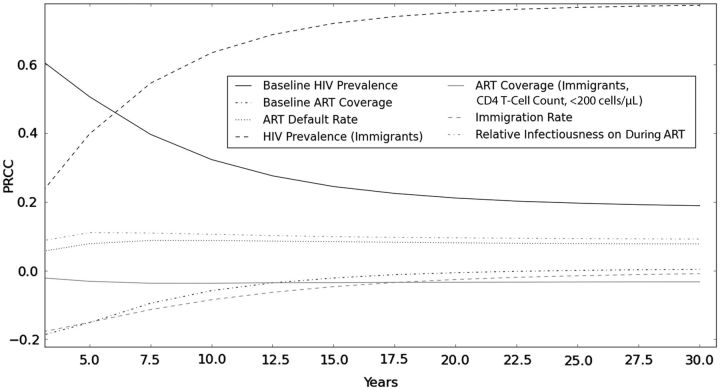
In sensitivity analysis of parameters using model 3, the results were most sensitive to HIV prevalence among migrants, an effect that increased over time (PRCC at 30 years, 0.8; Figure 4). The results were also modestly sensitive to the proportion of immigrants coming into the community of ART recipients. Early in the simulations, the results were sensitive to the relative baseline prevalence of HIV infection (PRCC, 0.6) and community ART coverage (PRCC, −0.2), but the effects were reduced over time (PRCC, 0.2 and 0.0, respectively).
Figure 4.
Time-dependent partial rank correlation coefficients of model parameters in the full model (model 3, accounting for revised linkage and migration) with human immunodeficiency virus (HIV) incidence. The horizontal axis indicates time from initiation of an antiretroviral therapy (ART)-as-prevention program. Values above or below 0 on the y-axis correspond to the positive or negative effects of parameters on projected HIV incidence.
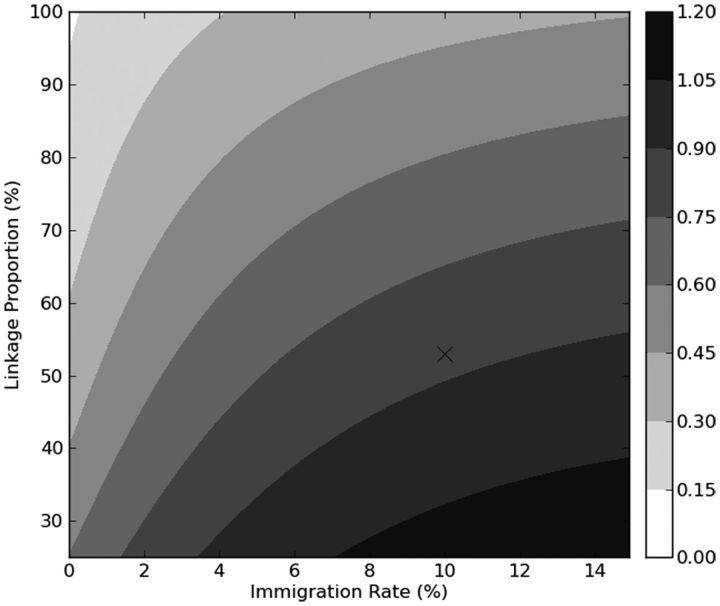
In 2-way sensitivity analysis assessing the impact of immigration rate and linkage rate (Figure 5), a high linkage rate and low immigration rate were needed to approach elimination of transmission, although incidence did not decrease to <0.1% by 30 years at any combination of linkage and migration due to default from ART.
Figure 5.
Projected annual incidence of human immunodeficiency virus (HIV) infection at 30 years, according to linkage rate and immigration rate using model 3 (incorporating revised linkage and migration). Darker shades indicate higher HIV incidence at 30 years, with incidence values indicated in the shaded bar (right). HIV incidence is higher at 30 years for higher immigration rates and lower linkage rates. X, base case.
If the prevalence of HIV infection among immigrants declined by 5% per year, the impact of migration on HIV incidence and prevalence would be negligible (absolute difference, 0.01% and 0.85%, respectively); for any lesser decline, a substantial effect was observed (Supplementary Figure A1).
DISCUSSION
We examined whether the projected benefits of annual testing and provision of ART for prevention of HIV transmission would be robust to real-world demographic and programmatic challenges. We made simple adjustments to a high-profile mathematical model of ART as prevention [5] and found that incorporating alternative assumptions about linkage to care and population mobility substantially attenuated the anticipated reduction in HIV incidence and prevalence. By using parameters from a well-studied community in South Africa, we projected that an ART-as-prevention program in this setting would be unlikely to achieve anticipated goals of “elimination” of HIV transmission in a 30-year horizon, even under optimistic assumptions about HIV testing and acceptance of treatment.
While assumptions about key model parameters were the focus of much discussion about the model used by Granich et al, we found that certain structural assumptions pertaining to linkage to care and population migration may be equally or more important. Under the Granich model, individuals who declined testing or treatment or were otherwise lost to follow-up were equally likely to engage in care in subsequent years. Therefore, even if the acceptance (linkage) rate was only 53%, by 3 years, 90% of individuals would be in care. At the 92% linkage used in the Granich study, 99.9% of individuals would be in care within 3 years. Our findings suggest that this assumption is important: if individuals who declined testing or treatment did not engage in care until late-stage disease, the anticipated gains of ART as prevention were substantially attenuated.
There is scant literature on when individuals who decline testing or treatment re-engage with care. Clinical experience as providers suggests that model 1, in which the probability of re-engaging with care after declining testing or treatment is conditionally independent with respect to prior response, is less close to reality than model 2, in which individuals re-present for care in late-stage disease. The importance of this function highlights the need to better characterize the health-seeking behavior of individuals who decline HIV testing or treatment or are lost to follow-up after initial testing.
Sub-Saharan Africa has been undergoing rapid urbanization [21]. Migration to South African urban and peri-urban townships has been driven by both domestic migration and immigration from neighboring countries, given the relatively greater economic opportunity and political stability offered by the country. In particular, unrest in neighboring Zimbabwe has led to massive emigration from the country, with most immigrating to South African townships. The community described in this study has seen high levels of migration; however, other community studies in South Africa have reported even higher rates [22]. Model projections were highly sensitive to the immigration rates, even though the prevalence of HIV infection among migrants was lower than the baseline prevalence of HIV infection in the community.
Several clinical trials to test the impact of ART as prevention at a community level are being planned or are underway [11]. In settings with substantial migration or in which individuals who decline testing or treatment are unlikely to agree in subsequent years, the anticipated reduction in HIV prevalence and incidence may be considerably lower. Studies must be adequately powered to detect smaller or slower declines in HIV incidence or prevalence. However, the effects of linkage to care and migration on HIV incidence and prevalence, while dramatic in the long term, may not appear within the 5-year horizon of a clinical trial (Figure 3). This highlights the difficulty of extrapolating findings from short-term clinical trials to longer-term HIV-associated dynamics within a population.
The refined linkage structure (models 2 and 3) modified the base model at 2 points: pre-ART loss to follow-up (ie, failed linkage to care) and post-ART loss to follow-up (ie, default from treatment). We separately assessed a model in which individuals who defaulted from treatment returned to the initial infectious compartments (eg, I1 and I2) and could return to care earlier. The results remained pessimistic, though slightly less so (Supplementary Figure A2). There are few data to inform the rate of returning to care upon treatment default.
As with all mathematical models, these results must be interpreted within the context of model assumptions and the quality of available data. While the qualitative results were robust in broad parameter sensitivity analyses, we caution against overly interpreting the quantitative projections in light of parameter uncertainty and the structural limitations of these parsimonious models. Consistent with the Granich model, we assumed a single sexual risk group with regard to HIV transmission and did not model concurrency or complex mixing. A recent model relaxing these assumptions found that the impact of ART as prevention was qualitatively similar, though attenuated, when assortative sexual mixing was considered [17]. We assumed that perinatal HIV transmission accounts for a minority of new infections and did not incorporate it in these models. We did not allow HIV-infected individuals to receive a diagnosis during the brief period of acute infection, as antibody tests are usually negative at that time and HIV RNA levels are not commonly used to diagnose new infections in South Africa. We used a treatment default rate of 7% per year that was based on data in this community, which is higher than that used in the original Granich model (1.5%); even higher rates have been observed in some programs [23]. We did not incorporate heterogeneity in viral load response and its relationship to transmission. We assumed that all individuals were offered testing yearly for HIV infection, which we acknowledge is optimistic; lower HIV testing coverage would render our results conservative with respect to the attenuated benefits.
We assumed that 53% of individuals with HIV infection were linked to care, which was based on results from a recent community-based study. This linkage proportion is far lower than that used by Granich et al in their analysis [5]. However, 53% is comparable to—and higher than—the reported proportion of ART-eligible individuals successfully linked to care following testing in other studies in South Africa [12, 13].
For our base-case estimates, we assumed that HIV prevalence was not declining in the larger population from which individuals were emigrating, though we relaxed this assumption in sensitivity analysis, finding that high rates of decline (eg, 5% per annum) in HIV prevalence among immigrants offsets their impact. Given the absence of effective, large-scale prevention interventions, we believe this assumption is reasonable, at least for shorter horizons.
Finally, this analysis did not attempt to identify and explicate all of the potential deficiencies of the published models of ART as prevention, but rather focused on 2 key structural aspects of these models that have received less published scrutiny. Our aim was to inform future modeling analyses for ART as prevention, as well as relevant data that programs implementing this strategy should collect to inform their results. While the results with revised linkage and incorporation of migration were substantially more pessimistic, the reduction in incidence was nevertheless considerable.
Early initiation of ART can markedly reduce the risk of transmission of HIV among serodiscordant partners [24]; this finding, together with the improved toxicity profiles of newer antiretroviral agents, has generated enthusiasm for expanded testing and early initiation of ART to curb the HIV epidemic in high-transmission settings. Community-based trials are now underway to assess whether the findings of reduced transmission among monogamous partners in a clinical trial setting will translate into an effective HIV control measure at a population level [25]. However, the anticipated benefits of such strategies may be overestimated if realistic demographic and behavior components are not considered. Our findings highlight the important role of migrant populations and of individuals who decline care or are lost to follow-up in sustaining HIV epidemics where ART-as-prevention strategies are pursued.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Corina Rusu for assistance in preparation of this manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, which played no role in the study design, methods, interpretation of results, the content of this manuscript, or the decision to submit it for publication.
Financial support. This work was supported by the National Institute of General Medical Sciences (U54 GM088558), the National Institute of Allergy and Infectious Diseases (R01 AI058736), the National Institute of Mental Health (R01 MH087328-02), the AIDS Clinical Trials Group (U01 AI068636), and the Doris Duke Charitable Foundation (Clinical Scientist Development Award).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Montaner JSG, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368:531–6. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 2.El-Sadr WM, Affrunti M, Gamble T, Zerbe A. Antiretroviral therapy: a promising HIV prevention strategy? J Acquir Immune Defic Syndr. 2010;55(Suppl 2):S116–21. doi: 10.1097/QAI.0b013e3181fbca6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287:650–4. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 4.Velasco M, Castilla V, Sanz J, et al. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr. 2009;50:148–52. doi: 10.1097/QAI.0b013e31819367e7. [DOI] [PubMed] [Google Scholar]
- 5.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Mastro TD, Cates W., Jr Universal voluntary HIV testing and immediate antiretroviral therapy. Lancet. 2009;373:1077. doi: 10.1016/S0140-6736(09)60640-1. author reply 1080–1. [DOI] [PubMed] [Google Scholar]
- 7.Epstein H. Universal voluntary HIV testing and immediate antiretroviral therapy. Lancet. 2009;373:1078–9. doi: 10.1016/S0140-6736(09)60643-7. author reply 1080–1. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh Y-H, de Arazoza H. Universal voluntary HIV testing and immediate antiretroviral therapy. The Lancet. 2009;373:1079–80. doi: 10.1016/S0140-6736(09)60645-0. [DOI] [PubMed] [Google Scholar]
- 9.Ruark A, Shelton JD, Halperin DT, Wawer MJ, Gray RH. Universal voluntary HIV testing and immediate antiretroviral therapy. Lancet. 2009;373:1078. doi: 10.1016/S0140-6736(09)60642-5. author reply 1080–1. [DOI] [PubMed] [Google Scholar]
- 10.Wilson DP. Universal voluntary HIV testing and immediate antiretroviral therapy. Lancet. 2009;373:1077–8. doi: 10.1016/S0140-6736(09)60641-3. [DOI] [PubMed] [Google Scholar]
- 11.Vermund SH, Hodder SL, Justman JE, et al. Addressing research priorities for prevention of HIV infection in the United States. Clin Infect Dis. 2010;50(Suppl 3):S149–55. doi: 10.1086/651485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kranzer K, Zeinecker J, Ginsberg P, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLoS One. 2010;5:e13801. doi: 10.1371/journal.pone.0013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassett IV, Regan S, Chetty S, et al. Who starts antiretroviral therapy in Durban, South Africa?… not everyone who should. AIDS. 2010;24(Suppl 1):S37–44. doi: 10.1097/01.aids.0000366081.91192.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Losina E, Bassett IV, Giddy J, et al. The “ART” of linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5:e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polzer T. Population movements in and to South Africa. 2010 http://www.migration.org.za/report/polzer-t-2010-population-movements-and-south-africa-acms-fact-sheet-june-2010. Accessed 17 January 2012. [Google Scholar]
- 16.Govindasamy D, van Schaik N, Kranzer K, Wood R, Mathews C, Bekker L-G. Linkage to HIV care from a mobile testing unit in South Africa by different CD4 count strata. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e31822e0c4c. 58:344–52. http://www.ncbi.nlm.nih.gov/pubmed/21836524. Accessed 29 August 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodd PJ, Garnett GP, Hallett TB. Examining the promise of HIV elimination by “Test and Treat” in hyper-endemic settings. AIDS. 2010;24:729–35. doi: 10.1097/QAD.0b013e32833433fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein M. Large sample properties of simulations using Latin hypercube sampling. Technometrics. 1987;29:143–51. [Google Scholar]
- 19.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int Stat Rev. 1994;62:229–43. [Google Scholar]
- 20.Blower SM, Porco TC, Darby G. Predicting and preventing the emergence of antiviral drug resistance in HSV-2. Nat Med. 1998;4:673–8. doi: 10.1038/nm0698-673. [DOI] [PubMed] [Google Scholar]
- 21.United Nations Population Fund. State of the world population 2007: unleashing the potential of urban growth. New York: United Nations Population Fund: 2007. [Google Scholar]
- 22.Camlin CS, Hosegood V, Newell M-L, McGrath N, Bärnighausen T, Snow RC. Gender, migration and HIV in rural KwaZulu-Natal, South Africa. PLoS One 5. 2010;5(7):e11539. doi: 10.1371/journal.pone.0011539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.HIV Prevention Trials Network. Initiation of antiretroviral treatment protects uninfected sexual partners from HIV infection (HPTN Study 052) 2011 http://www.hptn.org/web%20documents/PressReleases/HPTN052PressReleaseFINAL5_12_118am.pdf. Accessed 5 July 2011. [Google Scholar]
- 25.Granich R, Gupta S, Suthar AB, et al. Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Curr HIV Res. 2011 doi: 10.2174/157016211798038597. 9(6):446–69. http://www.ncbi.nlm.nih.gov/pubmed/21999779. Accessed 12 December 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kranzer K, van Schaik N, Karmue U, et al. High prevalence of self-reported previously undiagnosed HIV despite high coverage of HIV testing: a cross-sectional population based sero-survey in a South African community. PLoS One. 2011;6(9):e25244. doi: 10.1371/journal.pone.0025244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranzer K, Lewis JJ, Ford N, et al. Treatment interruption in a primary care antiretroviral therapy program in South Africa: cohort analysis of trends and risk factors. J Acquir Immune Defic Syndr. 2010;55:e17–23. doi: 10.1097/QAI.0b013e3181f275fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Lancet. 2000;355:1131–7. [PubMed] [Google Scholar]
- 29.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 30.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 31.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 32.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 33.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.