Abstract
Background. Vulnerability of younger women to human papillomavirus 16 (HPV16) infection has been attributed to the predominance of ectocervical columnar epithelia in this age group. However, squamous metaplastic tissue may be more influential. We examined the extent of ectopy and metaplastic activity as risks for HPV16 acquisition in a prospective cohort.
Methods. Participants were HPV16 negative at the first two visits. Follow-up occurred every 4 months. Ectopy was quantitatively measured on colpophotographs. We calculated metaplastic rate as the difference in ectopy between visits. Cox proportional hazards models were constructed, adjusting for several covariates.
Results. Analyses included 198 women (mean baseline age 17 years) for 1734 visits. Mean follow-up was 4.4 years. Incident HPV16 was detected in 36 (18%) women. Metaplastic rate between the two visits before HPV16 detection was significantly associated with incident infection (hazard ratio [HR], 1.17; confidence interval [CI], 1.02–1.33; P = .02). However, ectopy was not significant, whether measured before or concurrent to HPV16 detection (HR range, 0.99–1.00; CI range, .97–1.02; P range, .47–.65).
Conclusions. Dynamic metaplasia rather than the sheer extent of ectopy appears to increase risk for incident HPV16 in healthy young women. This in vivo observation is consistent with the HPV life cycle, during which host cell replication and differentiation supports viral replication.
Human papillomavirus (HPV) infection, the cause of cervical cancer, is estimated to be the most common sexually transmitted infection (STI) in the United States [1]. Among more than 100 genotypes, HPV type 16 is the most common type globally and has been implicated in approximately 56% of all cervical cancers [2–5]. As with most STIs, HPV rates are highest in younger females [6, 7]. A recent meta-analysis of HPV detection (by polymerase chain reaction or Hybrid Capture 2 technique) in women with normal cervical cytology included data from 644 793 women in North America. Women younger than 25 years of age were estimated to have the highest prevalence rate of 23.2%, compared with 8.7% in women 25–34 years old and less than 5% in women 35 years and older. Of the HPV burden in North America, HPV16 was estimated to produce 24% of all prevalent infections [5]. In addition to age, risks for HPV acquisition include behavioral factors such as higher number of new sexual partners and earlier age of first sex [8, 9]. However, in a study in Columbia, young age remained at a significant risk after adjusting for these behavioral factors [10], underscoring the potential biologic vulnerability of younger women to HPV. Because 5%–10% of infections acquired during adolescence remain persistent, constituting the ultimate necessary factor for the development of cervical cancer, understanding the biologic vulnerability of young women remains critical.
Interest in cervical vulnerability to HPV has focused on the cervical transformation zone, that is, the epithelial area where squamous cell cancers arise [11]. Prepubertally, an abrupt squamo-columnar junction is typically found on the ectocervix, where the proximal single-cell-layer columnar epithelium is adjacent to the distal nonkeratinized stratified squamous epithelium [12]. In the setting of pubertal hormones, the physiologic process of squamous metaplasia is activated, such that uncommitted generative cells of the columnar epithelium are transformed into squamous epithelium through a remodeling process that includes active cell replication and differentiation. This area of cellular activity is termed the “transformation zone.” “Ectopy” refers to the predominance of columnar and metaplastic epithelia visible on the ectocervix. As cervical maturation proceeds over time and areas of ectopy diminish, the transformation zone typically becomes located more proximally toward the endocervix. Thus ectopy is often in a dynamic state during adolescence. In theory, the setting of active metaplastic change is ideal for HPV infection because HPV requires host cell differentiation and replication for its own viral replication and survival [13, 14].
However, the specific roles of cervical ectopy and active metaplasia in the natural history of HPV infection are not well understood largely because of the inherent difficulty in measuring the cervical epithelium in epidemiological studies. In a few cross-sectional studies of older women, significant associations between ectopy and HPV prevalence, including HPV16, have been found [15–17]. To our knowledge, prior studies have not examined these associations prospectively or addressed the dynamic process of squamous metaplastic activity in young women. Our study objectives were to: (1) examine the risk of HPV16 acquisition among women with differing extents of ectopy and (2) examine squamous metaplastic activity as a risk factor for HPV16 acquisition in a prospective cohort of sexually active, healthy young women. We were specifically interested in HPV16 because of its clinical relevance as the most common cause of cervical cancer.
METHODS
Participants
Participants were selected from a larger ongoing cohort study of the natural history of HPV, described elsewhere [18, 19]. The original cohort enrolled women who were 13–21 years of age, sexually active for a maximum of 5 years, not pregnant, had no history of surgical cervical procedures or immunosuppression, and were willing to attend study visits at 4-month intervals. During follow-up, women who had cervical intraepithelial neoplasia grade 2 or 3 confirmed by histology were excluded. From October 2000 to September 2002, 258 women were enrolled from a family planning clinic.
For our current analyses, we selected women who had negative HPV16 results on their cervical HPV DNA tests for at least the first 2 consecutive visits starting at baseline and attended at least 1 follow-up visit thereafter. Our final dataset consisted of 198 women. All data were collected before HPV vaccines were available to this population. The Committee on Human Subject Research, University of California, San Francisco approved the study. Voluntary written informed consent was obtained from each participant.
Data Collection
Women were seen at 4-month intervals for an interview to assess sociodemographic characteristics, social and sexual behaviors, and medical history; a pelvic examination to obtain cervical and vaginal samples; and colpophotography to document the cervical epithelium [20]. HPV DNA of 37 types was detected by Roche reverse line blot assay applied to cervical lavage samples [21], which were collected using a plastic pipette to direct 10 cc of sterile normal saline over the cervix, recollect the saline, repeat for a total of 3 passes over the cervix, and recollect for testing. Bacterial vaginosis, Trichomonas vaginalis, and yeast were diagnosed clinically by routine saline and potassium hydroxide wet prep microscopy applied to vaginal samples [22]. Under colposcopy, after application of 3% acetic acid, colpophotographs (35-mm film) were taken at 10× and 16× magnifications.
At baseline visits, annual visits, and when symptomatic, Chlamydia trachomatis and Neisseria gonorrhoeae were tested by using commercial nucleic acid amplification assays. At baseline and annual visits, blood was drawn for HPV16 serology, detected by an HPV16 L1 antibody binding assay using glutathione S-transferase fusion proteins on a Luminex platform [23–25].
Quantitative Measurement of Cervical Epithelium
Colpophotographs were digitized (1024 × 1536 pixels), and epithelial areas of interest were measured using computerized planimetry techniques, as described previously [20]. In brief, colpophotographs were viewed in Adobe Photoshop CS2 (San Jose, CA) by the investigators (L. Y. H., A. B. M.) who were blinded to all other patient data. Ectopy was defined as columnar and early-mid squamous metaplasia. As identified by standard clinical colposcopic features [26, 27], columnar epithelium is characterized by redness that becomes faintly acetowhite and translucent upon application of acetic acid, grape-like villous structures, long stromal papillae, deep clefts, and an irregular surface. As villi begin to fuse in early-mid metaplasia, areas appear flatter and more densely acetowhite; islands of columnar epithelia may be seen. These areas contrast with mature squamous epithelium that appears pink and smooth. Ectopy areas in the colpophotographs were manually outlined, represented by pixel counts, summed, and expressed as a percentage of the total cervical face, yielding the measurement “percent-ectopy of the cervix.” This proportional measure allowed comparisons among visits because photographic conditions such as angle of view and level of magnification necessary for the optimal view vary. The rate of squamous metaplasia was calculated as the difference in percent-ectopy between adjacent visits, divided by time (months) elapsed between visits. A negative change from one visit to the next (going from a larger percent-ectopy to a smaller percent-ectopy) was assumed to represent squamous metaplastic activity.
Data Analyses
Almost all variables were time dependent and measured at each visit. The outcome of interest was HPV16 incidence, defined as the first positive result during follow-up after the initial 2 negatives. We focused on HPV16 because of its clinical relevance as the most common cause of cervical cancer and its high prevalence and incidence rates, yielding a sufficient sample for analyses. The 2 main predictors of interest were percent-ectopy and rate of squamous metaplasia. We examined the following potential confounders: age; years since first sex; new sexual partners (within prior 8 months); current hormonal contraceptive use; condom use since last visit; current pregnancy; current smoking; history of laboratory-documented infections with C. trachomatis, N. gonorrhoeae, bacterial vaginosis, or yeast; and concurrent infection with HPV types besides HPV16. These covariates were chosen based on prior evidence of their possible influences on HPV acquisition [8–10, 28–33], ectopy [12, 34, 35], and/or squamous metaplastic activity [20]. Additionally, HPV16 serology was measured using the earliest sample drawn prior to any positive detection of incident HPV16 during the study and was the only fixed variable in the analyses. We considered HPV16 serology to represent history of a previous exposure to HPV16 and included it as a covariate because exposure prior to study participation may influence the subsequent risk for HPV16 acquisition during the study. Because larger areas of ectopy may increase the risk for incident HPV16, it is plausible that women with ectopy had a previous infection with HPV16 before study participation.
Because the influence of percent-ectopy and metaplastic rate on HPV acquisition is plausibly time dependent, we examined these factors as time-varying predictors in Cox proportional hazards models for incident HPV16 infection. The measures of percent-ectopy were defined as current values (ie, observed at the same visit as the measurement of incident infection) and also as lagged values (ie, observed at the previous 2 visits). Thus for an incident infection observed at time t, 3 versions of percent-ectopy measures were evaluated: at time t, (t − 1), and (t − 2). Each version was evaluated in its own Cox proportional hazards models for HPV acquisition. Similarly, 2 versions of metaplastic rate were calculated—for the interval between (t − 2) and (t − 1) and the interval between (t − 1) and (t)—and examined in separate models. Percent-ectopy and metaplastic rate were considered to be continuous variables. We kept the single units of measurement (ie, 1%) to retain the detail of the quantitative colpophotographic measures and to appropriately reflect the distribution of the metaplastic rate in our dataset, although we acknowledge that a 1% unit would not be clinically appreciable. The plausibility of the linearity assumption for regression models was evaluated using graphical methods. Because the lengths of between-visit intervals varied due to late or missed visits, all models included interval length as a time-varying covariate. Because infection by multiple HPV types may influence risk for HPV16 acquisition, all models were adjusted for the detection of any other HPV type concurrent to the HPV16. Due to the limited sample size, we avoided entering more than 5 variables into a single model. The potential confounders were measured at the same timepoint as the main predictor under analysis, except for the fixed variable, HPV16 serology. Statistical analyses were performed using SAS version 9.2 (Cary, NC).
RESULTS
Participant Characteristics and Acquisition of HPV16 Infections
The 198 women attended a total of 1734 study visits. The mean number of visits per woman was 9 (SD, 3); the mean time in the study was 4.4 years (SD, 1.1). The median interval between visits was 4.4 (interquartile range [IR], 3.9–8.1) months. Self-reported race/ethnicity was 36 (18.3%) Asian, 31 (15.7%) African-American, 41 (20.8%) Caucasian, 82 (41.6%) Latina/Hispanic, 7 (3.6%) Mixed/Other. The mean age at baseline was 17 years (SD, 1.3); mean age of menarche was 12.2 (SD, 1.4); and mean age at first vaginal sex was 15 (SD, 1.5). During follow-up, incident HPV16 was detected in 36 (18%) women (Table 1), comprising 22 (61%) women who had no other HPV types detected concurrently and 14 (39%) women for whom multiple HPV types were detected concurrently. Of the entire sample, HPV16 serology was positive for 37 (19%) women, including 9 women who had an incident HPV16 infection.
Table 1.
Characteristics of the Study Participants (N = 198 Women, 1734 Visits)
| Women With no HPV16 Detected During Study (n = 162 women, 1406 visits) | Women With Incident HPV16 During Study (n = 36 women, 328 visits) | P Valuea | |
|---|---|---|---|
| Characteristics at Study Entryb | Mean (SD) | Mean (SD) | |
| Age, years | 17.1 (1.3) | 17 (1.4) | .89 |
| Age at menarche, years | 12.2 (1.4) | 12.4 (1.2) | .46 |
| Age at first vaginal sex, years | 15 (1.6) | 14.8 (1.4) | .49 |
| Number of lifetime partners | 4 (4) | 7 (11) | .09 |
| Race/ethnicity | n (%) | n (%) | |
| African-American | 24 (15) | 7 (19) | |
| Asian | 28 (17) | 8 (22) | |
| Hispanic/Latina | 70 (44) | 12 (33) | |
| Mixed/Other | 4 (2) | 3 (8) | |
| White | 35 (22) | 6 (17) | |
| History of pregnancy | 46 (29) | 8 (22) | .44 |
| History of Chlamydia trachomatis infection | 19 (12) | 3 (8) | .54 |
| History of Neisseria gonorrhoeae infection | 6 (4) | 1 (3) | .99 |
| History of Trichomonas vaginalis infection | 4 (3) | 0 (0) | .99 |
| Serology positive for HPV16 | 28 (18) | 9 (25) | .31 |
| Characteristics at Study Visitsc | n (%) | n (%) | |
| Had new partners during last 8 mo | 553 (40) | 185 (57) | <.01 |
| Current hormonal contraception use | 499 (36) | 102 (32) | .14 |
| Always used condoms since last visit | 38 (3) | 14 (4) | .13 |
| Current pregnancy | 67 (5) | 18 (6) | .58 |
| Current smoking | 526 (38) | 104 (32) | .06 |
| Chlamydia trachomatis infection at any prior visit | 48 (4) | 16 (5) | .21 |
| Neisseria gonorrhoeae infection at any prior visit | 5 (0.4) | 3 (1) | .18 |
| Trichomonas vaginalis infection at any prior visit | 11 (1) | 5 (2) | .20 |
| Infection with HPV types other than type 16 | 409 (29) | 135 (41) | <.01 |
| Actual months between adjacent visits | 4.5 (median) | 4.4 (median) | .72 |
Abbreviation: HPV, human papillomavirus.
a Chi-square test, Fisher exact test, or t test as appropriate.
b Frequencies for the variables that were measured at study entry are reported using the number of women as the denominator.
c Frequencies of the variables that were measured repeatedly at each study visit are reported using the total number of visits as the denominator.
Incident HPV16 Infection and Cervical Ectopy
The mean percent-ectopy at baseline was 19%; mean percent-ectopy at study end was 3%; and mean percent-ectopy at all 1734 visits was 9% (range 0–100). No significant associations were found between incident HPV16 and percent-ectopy (Table 2), whether percent-ectopy was measured at the incident visit (t) (hazard ratio [HR] = 0.99, adjusted for interval length and concurrent other HPV; 95% confidence interval [CI], .97–1.02; P = .47); at visit (t − 1) (HR, 0.99; CI, .97–1.01; P = .47); or at visit (t − 2) (HR, 1.0; CI, .99–1.02; P = .65). The findings were unchanged in the multivariate models adjusting for each of the possible confounders (data not shown).
Table 2.
Measures of the Cervical Epithelium as Predictors for Incident HPV 16 Infection Detected at a Given Visit (t) (N = 198 Women, Including 36 Incident Infections, 1734 Visits)
| Predictors Examined in Separate Cox Proportional Hazards Regression Models | Unadjusted HR | 95% CI | P Value | Adjusted HRa | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Percent ectopy at visit (t) | 0.99 | .96–1.01 | .29 | 0.99 | .97–1.02 | .47 |
| Percent ectopy at visit (t − 1)b | 0.99 | .97–1.01 | .33 | 0.99 | .97–1.01 | .47 |
| Percent ectopy at visit (t − 2)c | 1.00 | .99–1.02 | .63 | 1.00 | .99–1.02 | .65 |
| Rate of metaplasia between visit (t − 1) and visit (t) | 0.93 | .75–1.15 | .50 | 0.93 | .73–1.18 | .56 |
| Rate of metaplasia between visit (t − 2) and visit (t − 1) | 1.18 | 1.04–1.34 | .01 | 1.17 | 1.02–1.33 | .02 |
Abbreviation: CI, confidence interval; HPV, human papillomavirus; HR, hazard ratio.
a Adjusted for concurrent infection with other HPV type and for actual interval time between visits.
b Visit (t − 1) is the nearest visit prior to visit (t).
c Visit (t − 2) is the nearest visit prior to visit (t − 1).
Incident HPV16 Infection and Rate of Squamous Metaplasia
The overall mean rate of squamous metaplasia was 4.2% maturation per year. The metaplastic rate was significantly associated with HPV16 acquisition when measured between visit (t − 2) and (t − 1) (HR, 1.17, adjusted for interval length and concurrent other HPV; CI, 1.02–1.33; P = .02; Table 2). Thus each 1% metaplastic change per month was associated with a 17% increased risk for subsequent HPV16 incidence. In contrast, for the interval between visit (t − 1) and (t), no significant association was found (HR, 0.93; CI, .73–1.18; P = .56). Figure 1 shows an example of the sequence of colpophotographs for 1 woman who experienced an incident HPV16 infection.
Figure 1.
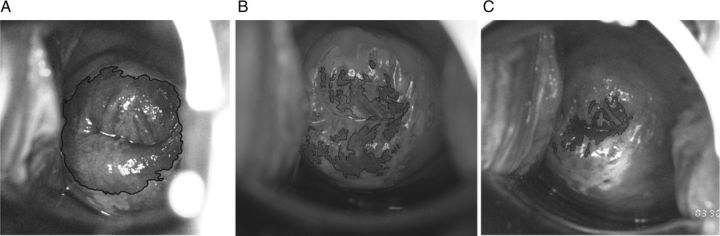
Colpophotographs taken at consecutive visits for 1 woman. Ectopy, defined as areas of columnar and early-mid-metaplastic epithelium, is shown here by a manual outline (solid black line) and measured as a percentage of the total cervical face. A, Ectopy is 62% at visit 1. B, Ectopy is 25% at visit 2. C, Ectopy is 9% at visit 3. For this woman, HPV 16 DNA detection by cervical sampling was negative at visits 1 and 2 and positive at visit 3.
In all multivariate models that adjusted for possible confounders, the rate of squamous metaplasia measured between visits (t − 2) and (t − 1) remained significant, with the adjusted HRs ranging from 1.16 to 1.18, CIs ranging from .02 to 1.35, and P values ranging from .01 to .03. Covariates that were independently associated with HPV16 incidence in the multivariate models were the following: age (HR, 1.27; CI, 1.0–1.61; P = .05); years since first sex (HR, 1.18; CI, .99–1.41; P = .06); new sexual partners in the past 4–12 months before HPV16 detection (HR, 2.16; CI, 1.02–4.56; P = .04); other genital infections (HR, 2.53; CI, .97–6.61; P = .06); and concurrent other HPV types (in the various models, HR range, 2.97–3.97; CI range, 1.45–7.89; P range < .01). The remaining covariates were not found to be significant (data not shown).
DISCUSSION
To our knowledge, this is the first prospective study of associations between cervical ectopy, squamous metaplastic activity, and incident HPV16 infection. The observed overall decrease in ectopy from baseline to study end was not surprising because cervical maturation over time would be expected in young women. The mean metaplastic rate (4.2% per year) was also consistent with our previously published results [20]. Among healthy young women, evidence of squamous metaplastic change significantly increased the risk for subsequent incident detection of HPV16. More specifically, each 1% change in metaplastic tissue (rate of metaplasia) contributed a 17% increased risk of HPV16 detection in the subsequent 4–8 months. In contrast, the sheer extent of ectopy was not found to be a risk factor, whether measured concurrently or up to several months prior to the incident HPV16. These findings were unchanged when adjusted for other factors thought to be associated with ectopy, squamous metaplastic activity, and/or HPV acquisition, including age, years since first sex, recent new sexual partners, hormonal contraception use, condom use, pregnancy, smoking, other genital infections, serology, and concurrent other HPV types.
The prospective study design and the quantitative documentation of the cervical epithelia were major strengths of this study. The prospective design allowed us to measure the predictors and covariates at a wide spectrum of timepoints in relation to the incident HPV16. Study visits were relatively frequent, conducted every 4 months typically, allowing us to better estimate the timepoint of incident infection. However, because biological acquisition of HPV is not immediately detectable due to the inherent limits of clinical HPV detection, consideration of the predictors at several timepoints was advantageous from this standpoint. The use of colpophotography and the quantitative approach to the ectopy measurement were additional strengths that minimized misclassification of the cervical epithelium. The proportional measure of “percent-ectopy” allowed comparison of colpophotographs taken at different visits and allowed a rare look at change in the ectocervix over time. Previous studies of cervical ectopy often relied on only qualitative nonmagnified visual inspection without acetic acid application, which is prone to errors related to subjectivity and obscuring factors such as friability.
An interesting finding was that the salient time interval of metaplasia that was associated with HPV16 acquisition was the 4–8 months prior to HPV16 detection, whereas metaplastic activity at the 0–4 month interval leading up to HPV16 detection was not significant. Given our understanding of the natural history of HPV infection, the significance of the 4–8 month interval is biologically plausible because an incubation or latency period of several weeks to months is estimated to follow the initial infection at the basal epithelial cells [36], and HPV may not be detected by our current technologies until several months after the initial basal cell infection. The HPV life cycle and viral replication are dependent on host cell replication and differentiation [13, 14], supporting the notion that metaplastic activity would be a risk factor for infection. The lack of significance of metaplastic activity from the 0–4 month interval appears surprising and may be spurious, requiring replication in other studies. However, our observations of time intervals for risk associated with metaplasia appear consistent with a prior study of sociodemographic and behavioral risk factors among female college students [8]. Winer et al found that new sex partners during the 5–8 month and 8–12 month intervals prior to incident HPV16 detection was a strong risk factor for incident HPV16, whereas new partners at 0–4 months before detection was not significant. The strongest association was found for partners at the 5–8 month interval, paralleling our findings regarding metaplastic activity. Also, as in our study, new partners was a significant factor when measured at approximately 4–12 months prior to the incident HPV16 detection rather than closer to the incident detection. Consistent results regarding the salient time interval for exposure to new partners were seen in another analysis of repeated HPV infections with new HPV types among our larger cohort as well [37]. Furthermore, our findings parallel our prior case-control study of low-grade squamous intraepithelial lesions (LSIL), a benign manifestation of HPV infection. Incident LSIL was significantly associated with a more rapid rate of metaplastic change (also measured quantitatively) that preceded the LSIL event but was not associated with ectopy measured at study baseline [38]. Taken together, these studies suggest that active squamous metaplasia may be an important contributor to both the establishment of HPV16 infection and the development of a clinical cervical lesion.
In contrast with our study, in a cross-sectional population-based study, Castle et al examined tissue affinity of HPV types and reported that a greater extent of ectopy was associated with trends of increasing positivity of several oncogenic types (grouped as α-9 types 16, 31, 33, 35, 52, 58, 67) and decreasing positivity of nononcogenic types (grouped as α-3/15 types 61, 71, 72, 81, 83, 84, 89) [15]. However, comparison with our study is limited for several reasons. Given the cross-sectional design of the Castle et al study, it was impossible to distinguish between persistent and incident HPV. We also focused on HPV16, whereas Castle et al evaluated high-risk and low-risk HPV groups and did not report on HPV16 alone. Furthermore, Castle and colleagues sampled an older adult population. Older women are less likely to have “active” rates of squamous metaplasia and more likely to have persistent infections. Another difference was our evaluation of ectopy as a proportional measure of the total cervical face, in contrast with measurement of an absolute pixel count to represent ectopy by Castle et al. Comparison of our findings with the two other available cross-sectional studies of ectopy and HPV infection would be inappropriate because these studies focused on ectopy in older women who presented with genital symptoms (discharge or bleeding) rather than healthy young women [16, 17].
Limitations of our study include the specific focus on HPV16 and the inherent limitations of available methods for HPV sampling and colpophotography. Future studies with larger sample sizes should be conducted to investigate the role of squamous metaplasia and ectopy in other HPV types since findings regarding biological risks for HPV16 cannot be assumed to apply to all other genital HPV types. We were unable to conduct an extensive investigation of the other types due to their lower incidence rates and the limited follow-up time of our cohort. The HPV sampling by directed cervical lavage was another possible limitation. Currently available methods for clinical cervical HPV detection include cervical lavage and cervical swabs. A prior pilot study by our group demonstrated excellent correlation (κ coefficient = 0.87; CI, .76–.98) between lavage and swab results [39]. However, the most definitive and direct approach to diagnose HPV infection of the ectocervical tissue would be an analysis of tissue samples obtained by cervical biopsy. An important limitation of our clinical HPV DNA testing is the inability to identify the initial HPV infection at the cellular level. Although our clinical measurement of metaplastic activity preceded the clinical HPV detection, it is possible that undetected HPV acquisition at the cellular level could have actually preceded the clinically apparent metaplastic activity. In that case, the apparent metaplasia could have represented cellular proliferation mediated by HPV16. These issues would be difficult if not impossible to evaluate in a cohort study because timing and exact location of infection would be impossible to pinpoint and repeated biopsy in women with normal cytology would be likely unethical. Finally, although colpophotography is more accurate than unaided visual exams in detecting ectopy, it has several limitations. First, colpophotography itself is inherently limited in that the optimal angle of view and quality of image capture from visit to visit varies clinically for any given woman. We attempted to address this issue by using the proportional measure of percent-ectopy. Second, for those with metaplasia restricted to the endocervix, we were unable to measure the metaplastic rate because this area is not visible on colpophotography. Third, the use of colpophotography and computerized planimetry to measure the columnar and metaplastic epithelium remains limited by the subjectivity of colposcopic impression itself. However, we believe it is currently the best available methodology for quantitative measurement.
The relationships between cervical ectopy, squamous metaplastic activity, and HPV infection in young women are likely complex. However, our study demonstrated that active squamous metaplasia rather than the sheer extent of ectopy on the ectocervix appears to increase the risk for subsequent detection of HPV16. This in vivo observation is consistent with the life cycle of HPV, which is dependent on host cell replication and differentiation for its own replication and survival.
Notes
Acknowledgments. We thank Michael Pawlita, MD; Denise A. Galloway, PhD; and Joseph Carter, PhD, for their diligent support and expertise in performing the HPV serology testing. We thank Cheryl Godwin De Medina, BA, our study coordinator, for her careful work with patients at the clinical site and assistance in data collection.
Financial support. This work was supported by the following grants from multiple institutes of the National Institutes of Health (NIH): the National Cancer Institute (grant number R37CA51323) for A. B. M., Y. M., S. F., J. J.; the National Institute of Allergy and Infectious Disease (grant number K23AI076670) for L. H., Y. M.; the National Institute of Child Health and Development (grant number T32HD044331) for L. H.; and the National Center for Research Resources, University of California San Francisco-Clinical Translational Sciences Institute (grant number UL1 RR024131). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2009. Atlanta, Georgia: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 2.Muñoz N, Bosch FX, De Sanjose S, et al. Epidemiological classification of human papillomavirus types associated with cervical cancer. NEJM. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the international agency for research on cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–8. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 4.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 6.Franceschi S, Herrero R, Clifford GM, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119:2677–84. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 7.Trottier H, Burchell AN. Epidemiology of mucosal human papillomavirus infection and associated diseases. Public Health Genomics. 2009;12:291–307. doi: 10.1159/000214920. [DOI] [PubMed] [Google Scholar]
- 8.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–26. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 9.Kahn JA, Rosenthal SL, Succop PA, Ho GY, Burk RD. Mediators of the association between age of first sexual intercourse and subsequent human papillomavirus infection. Pediatrics. 2002;109:E5. doi: 10.1542/peds.109.1.e5. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz N, Mendez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–87. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 11.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 12.Moscicki AB, Singer A. The cervical epithelium during puberty and adolescence. In: Jordan JA, Singer A, Jones H, Shafi M, editors. The cervix. 2nd ed. Massachusetts: Blackwell Publishing Professional; 2006. pp. 81–101. [Google Scholar]
- 13.von Knebel Doeberitz M. New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur J Cancer. 2002;38:2229–42. doi: 10.1016/s0959-8049(02)00462-8. [DOI] [PubMed] [Google Scholar]
- 14.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110:525–41. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 15.Castle PE, Jeronimo J, Schiffman M, et al. Age-related changes of the cervix influence human papillomavirus type distribution. Cancer Res. 2006;66:1218–24. doi: 10.1158/0008-5472.CAN-05-3066. [DOI] [PubMed] [Google Scholar]
- 16.Monroy OL, Aguilar C, Lizano M, Cruz-Talonia F, Cruz RM, Rocha-Zavaleta L. Prevalence of human papillomavirus genotypes, and mucosal IgA anti-viral responses in women with cervical ectopy. J Clin Virol. 2010;47:43–8. doi: 10.1016/j.jcv.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Rocha-Zavaleta L, Yescas G, Cruz RM, Cruz-Talonia F. Human papillomavirus infection and cervical ectopy. Int J Gynaecol Obstet. 2004;85:259–66. doi: 10.1016/j.ijgo.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki AB. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26:222–32. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 19.Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678–83. doi: 10.1016/S0140-6736(04)17354-6. [DOI] [PubMed] [Google Scholar]
- 20.Hwang LY, Ma Y, Benningfield SM, et al. Factors that influence the rate of epithelial maturation in the cervix in healthy young women. J Adolesc Health. 2009;44:103–10. doi: 10.1016/j.jadohealth.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 23.Rowhani-Rahbar A, Carter JJ, Hawes SE, et al. Antibody responses in oral fluid after administration of prophylactic human papillomavirus vaccines. J Infect Dis. 2009;200:1452–5. doi: 10.1086/606026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clifford GM, Shin HR, Oh JK, et al. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev. 2007;16:1874–9. doi: 10.1158/1055-9965.EPI-07-0349. [DOI] [PubMed] [Google Scholar]
- 25.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51:1845–53. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 26.Ferris DG, Cox JT, O'Connor DM, Wright VC, Foerster J. Modern colposcopy, textbook and atlas. 2nd ed. Dubuque, Iowa: Kendall/Hunt Publishing; 2004. Normal and abnormal colposcopic features; pp. 175–249. [Google Scholar]
- 27.Jordan JA, Singer A. Colposcopy. In: Jordan J, Singer A, Jones H, Shafi M, editors. The cervix. 2nd ed. Massachusetts: Blackwell Publishing Professional; 2006. pp. 445–61. [Google Scholar]
- 28.Nielsen A, Iftner T, Munk C, Kjaer SK. Acquisition of high-risk human papillomavirus infection in a population-based cohort of Danish women. Sex Transm Dis. 2009;36:609–15. doi: 10.1097/OLQ.0b013e3181a96d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Human papillomavirus infections among couples in new sexual relationships. Epidemiology. 2010;21:31–7. doi: 10.1097/EDE.0b013e3181c1e70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhoeven V, Baay M, Weyler J, et al. Concomitant Chlamydia trachomatis and human papilloma virus infection cannot be attributed solely to sexual behaviour. Eur J Clin Microbiol Infect Dis. 2004;23:735–7. doi: 10.1007/s10096-004-1194-5. [DOI] [PubMed] [Google Scholar]
- 31.Watts DH, Fazzari M, Minkoff H, et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis. 2005;191:1129–39. doi: 10.1086/427777. [DOI] [PubMed] [Google Scholar]
- 32.Mendez F, Muñoz N, Posso H, et al. Cervical coinfection with human papillomavirus (HPV) types and possible implications for the prevention of cervical cancer by HPV vaccines. J Infect Dis. 2005;192:1158–65. doi: 10.1086/444391. [DOI] [PubMed] [Google Scholar]
- 33.Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 34.Moscicki AB, Ma Y, Holland C, Vermund SH. Cervical ectopy in adolescent girls with and without human immunodeficiency virus infection. J Infect Dis. 2001;183:865–70. doi: 10.1086/319261. [DOI] [PubMed] [Google Scholar]
- 35.Singer A. The cervical epithelium and subepithelium during pregnancy and the puerperium. In: Jordan J, Singer A, Jones H, Shafi M, editors. The cervix. 2nd ed. Massachusetts: Blackwell Publishing Professional; 2006. pp. 102–21. [Google Scholar]
- 36.Stanley M. HPV—immune response to infection and vaccination. Infect Agent Cancer. 2010;5:19. doi: 10.1186/1750-9378-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moscicki AB, Ma Y, Jonte J, et al. The role of sexual behavior and human papillomavirus persistence in predicting repeated infections with new human papillomavirus types. Cancer Epidemiol Biomarkers Prev. 2010;19:2055–65. doi: 10.1158/1055-9965.EPI-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moscicki AB, Burt VG, Kanowitz S, Darragh T, Shiboski S. The significance of squamous metaplasia in the development of low grade squamous intraepithelial lesions in young women. Cancer. 1999;85:1139–44. doi: 10.1002/(sici)1097-0142(19990301)85:5<1139::aid-cncr18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Moscicki AB, Widdice L, Ma Y, et al. Comparison of natural histories of human papillomavirus detected by clinician- and self-sampling. Int J Cancer. 2010;127:1882–92. doi: 10.1002/ijc.25199. [DOI] [PMC free article] [PubMed] [Google Scholar]


