Abstract
Background.A total of 738 volunteer blood donors who were positive for anti–hepatitis C virus (HCV) were assessed for risk factors and outcomes for up to 15 years within the study and up to 54 years from the estimated onset of infection.
Methods.A third-generation recombinant immunoblot assay (RIBA) was performed to distinguish true from false anti-HCV reactivity. Findings of HCV polymerase chain reaction classified subjects as having chronic HCV infection or as having recovered. Liver biopsy specimens were staged by Ishak fibrosis score and graded by histologic activity index.
Results.Of 738 anti-HCV–positive subjects, 469 (64%) had positive RIBA results, 217 (29%) had negative results, and 52 (7%) had indeterminate results. Primary independent risk factors were injection drug use (odds ratio [OR], 35.0; P < .0001), blood transfusion (OR, 9.9; P < .0001), and intranasal cocaine use, including 79 “snorters” who repeatedly denied injection drug use or blood transfusion (OR, 8.5; P < .0001). Classification and regression tree and random forest analyses confirmed these risk factors. A total of 384 RIBA-positive donors (82%) were HCV RNA positive; of these, liver biopsy specimens from 185 (48%) showed no fibrosis in 33%, mild fibrosis in 52%, bridging fibrosis in 12%, and cirrhosis in 2% a mean duration of 25 years after infection. Analysis of 63 repeat biopsy specimens showed that 8% progressed ≥2 Ishak stages over 5 years (mean progression, 0.06 Ishak stages/year).
Conclusions.Injection drug use and blood transfusion before 1990 are dominant risk factors for HCV acquisition; intranasal cocaine use may be a surreptitious route of parenteral spread. After a mean of 25 years of HCV infection, histologic outcomes were relatively mild: 85% had no or mild fibrosis, and only 2% had cirrhosis. Nearly one-fifth spontaneously recovered.
While the frequency of new hepatitis C virus (HCV) infections in the United States has declined considerably in the past decade, the disease burden from cumulative infections over the past 50 years is substantial and is rising, as the long duration of individual infections allows evolution into cirrhosis and hepatocellular carcinoma (HCC) [1, 2]. The full spectrum of HCV-related outcomes is difficult to discern because milder disease often goes unrecognized, and slowly evolving chronic sequelae generally are not amenable to prospective ascertainment. The introduction of routine donor screening for antibody to HCV in 1990 afforded the opportunity to place otherwise unselected subjects into long-term follow-up to assess (1) the proportion of HCV-infected individuals who become chronic carriers, (2) the risk factors for HCV acquisition, (3) the long-term outcomes based on findings of liver histologic evaluations, and (4) the relationship of liver fibrosis to a variety of demographic, virologic, serologic, and biochemical indices. This cohort of anti-HCV–positive blood donors was previously analyzed in 1995 [3]. Reported herein is a further decade of data accrual, allowing for up to 15 years of follow-up within the study and a mean interval of 25 years from the time of a parenteral exposure that presumably initiated the infection to the time of liver biopsy.
METHODS
Screening and Confirmation of HCV Infection
At study initiation, a first-generation enzyme immunoassay (EIA) was used by the American Red Cross (ARC; EIA1.0; Ortho Diagnostics, Raritan, NJ) and the National Institutes of Health (NIH) (EIA1.0; Abbott Laboratories, Abbott Park, IL) to screen donated blood for HCV antibodies. A more sensitive second-generation EIA (EIA2.0; Ortho Diagnostics and Abbott Laboratories) was introduced in 1992. The specificity of anti-HCV–positive reactions was tested by second and/or third-generation recombinant immunoblot assays (RIBA HCV 2.0 SIA; Chiron, Emeryville, CA) [4]. Donors who were RIBA positive were considered to have confirmed antibody to HCV and, thus, to have been infected with HCV. Donors who were RIBA negative were considered to have false-positive antibody reactivity by EIA and, thus, not to have been infected with HCV. The HCV antibody status of donors with an indeterminate result of RIBA could not be determined, and donors with this result underwent testing for HCV RNA but were otherwise excluded from analysis.
At least 1 sample from each participant was tested in duplicate for HCV RNA, using reverse-transcription polymerase chain reaction (PCR; COBAS Amplicor HCV Test, version 2.0; Roche, Branchburg, NJ; limit of detection, 100 IU/mL) [3]. Quantitative serum HCV RNA levels were measured by the COBAS Amplicor HCV Monitor Test, version 2.0 (Roche), and genotyping was performed by the INNO-LiPA 2.0 assay (Innogenetics, Ghent, Belgium). Samples that were obtained prior to licensure of these PCR assays were retrieved from frozen storage for later testing.
Enrollment of Participants
Volunteer blood donors from the Greater Chesapeake and Potomac Region of the ARC and from the NIH Department of Transfusion Medicine who tested anti-HCV positive on replicate testing and provided informed consent were enrolled beginning in August 1990. The study protocol was approved by the ARC and NIH institutional review boards and has been reviewed annually.
Initial Evaluation and Follow-up
On the initial visit, the donor was interviewed by a physician, who completed an extensive questionnaire that recorded demographic characteristics, blood donation history, sexual history, and past medical history, including assessments of alcohol use, illicit drug use, and other potential risk factors for HCV infection. Participants completed a second questionnaire in private about illicit drug use. A physical examination was performed, and samples for biochemical and hematologic blood tests were obtained at baseline. A physician performed an interim history and physical examination annually, and laboratory testing was repeated biannually for the duration of study. RIBA-positive subjects continue to be followed up in this ongoing study; donors who were repeatedly RIBA negative and HCV RNA negative were discharged from the study after 6–12 months of follow-up because they were considered to have been unexposed to HCV and to have had false-positive results of EIA.
Histologic Evaluation of Liver Biopsy Specimens
Biopsy specimens were obtained from 185 HCV RNA-positive, treatment-naive participants. All specimens were read by the same hepatic pathologist (D.E.K.) without knowledge of the patient's clinical history. A biopsy specimen was considered adequate if it contained >10 portal tracts. The extent of liver fibrosis was scored from 0 to 6, using the Ishak fibrosis scale (0 = no fibrosis, 1–2 = portal fibrotic expansion, 3–4 = bridging fibrosis, and 5–6 = cirrhosis) [5]. Necrosis and inflammation were graded using a modification of the histologic activity index (HAI) on a scale of 0–18 [6, 7].
Mortality Follow-up
Vital status, date of death, and cause-specific mortality from 1990 through 2005 were obtained by searching the National Death Index (National Center for Health Statistics, Hyattsville, MD).
Statistical Analysis
Analyses were performed with standard statistical packages (SPSS v15.0 for Windows, SPSS, Chicago, IL; SAS v9.2, SAS Institute, Cary, NC). Only data collected prior to HCV treatment were included. Associations between categorical variables were assessed with the unadjusted χ2 statistic or a 2-tailed Fisher exact test.
Univariate analysis of variance was used to assess associations between continuous variables and group status. When normality could not be assumed, the Kruskal-Wallis test was used. The Bonferroni method was used to adjust for multiple comparisons. When an a priori order in the group status was assumed, the Jonckheere nonparametric test for trend was used, with a 2-sided P value. For comparisons of proportions to population proportions, the exact binomial test was used.
A multivariate logistic regression model was used for analysis of risk factors. A forward selection method with a significance level of 0.05 for entry was used, and odds ratios (ORs) with 95% confidence intervals were calculated based on the profile likelihood. Subjects who were EIA positive but RIBA negative and HCV RNA negative were used as controls in the risk factors analysis. Results of the forward logistic regression analysis were confirmed using backward and stepwise selection methods. Classification and regression tree (CART) and random forest analyses were used to confirm the findings of logistic regression and were performed with R statistical computing language.
RESULTS
Enrollment, Demographic Characteristics, and RIBA Status
Seven hundred and thirty-eight anti-HCV–positive blood donors were enrolled: 692 (94%) were enrolled from the ARC, 36 (5%) were enrolled from the NIH, and 10 (1%) were enrolled from other blood centers. A total of 454 anti-HCV–positive ARC donors were enrolled from 1990 through 1994 (11% of all anti-HCV–positive ARC donors in the region); 238 ARC donors were enrolled from 1995 through 2005 (43% of all anti-HCV–positive donors). Correspondingly, 28 anti-HCV–positive NIH donors (84% of the total detected) were enrolled from 1990 through 1994, and 8 (17% of the total) were enrolled from 1995 through 2005.
Demographic data on 1 040 713 blood donors who donated at the ARC between 1990 and 2005 were compared with the 692 ARC donors enrolled in the study. The populations had a similar sex distribution, but study participants were older (41.4 vs 38.0 years; P < .01), more likely to be African American (14% vs 8.9%; P < .01), and less likely to be first-time donors (23.8% vs 76.5%; P < .01).
Demographic data on 14 400 NIH volunteer blood donors who donated during the study period were compared to data for the 36 who were enrolled. Enrolled donors were similar with respect to sex and African American race (13.9% vs 6.5%; P = .16), were younger (41.9 vs 49.0 years; P < .01), and were less likely to be first-time donors (13.9% vs 80.1%; P < .01). Study participants from the ARC and the NIH were compared, and there were no differences in sex, age, African American race, or first-time donor status.
Among anti-HCV–positive blood donors, 469 (64%) were positive by the third-generation RIBA, 217 (29%) were negative, and 52 (7%) had indeterminate results. Characteristics of RIBA-positive and RIBA-negative individuals are compared in Table 1.
Table 1.
Characteristics of Blood Donors, by Hepatitis C Virus (HCV) Antibody Status
| Characteristics | Results of Third-Generation RIBAa |
P | |
|---|---|---|---|
| Positive (n = 469) | Negative (n = 217) | ||
| Age, mean ± SD (years) | 40±10 | 44 ± 12 | <.001 |
| Female sex | 215 (46) | 96 (44) | .742 |
| Race | |||
| White | 372 (79) | 190 (88) | .010 |
| African American | 85 (18) | 15 (7) | <.001 |
| No college education | 212 (45) | 49 (23) | <.001 |
| First-time donor | 145 (31) | 18 (8) | <.001 |
| History of STD | 127 (27) | 22 (10) | <.001 |
| MSM | 19 (4) | 1 (0.5) | .007 |
| ALT level, mean (IU/L)b | 54 | 22 | <.001 |
Data are no. (%) of donors, unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; MSM, men who have sex with men; RIBA, recombinant immunoblot assay; STD, sexually transmitted disease.
a All subjects had positive anti-HCV findings by enzyme immunoassay (EIA). Donors with positive results of RIBA are considered to have been infected with HCV. Donors with negative results of RIBA are considered to have false-positive anti-HCV findings by EIA.
b At initial evaluation, ALT level was elevated in 48% of RIBA-positive donors, compared with 6% of RIBA-negative donors (P < .001).
Risk Factors Analysis
Independent risk factors for HCV infection in the multivariate logistic regression analysis (Table 2) included, in order of entry into forward and stepwise logistic regression models, intranasal cocaine use (OR, 6.4; P < .0001), blood transfusion prior to 1991 (OR, 9.9; P < .0001), history of injection drug use (IDU; OR, 35.0; P < .0001), sexual promiscuity (>5 partners/year, history of sexually transmitted disease, exchanging sex for drugs or money, or a combination of these factors; OR, 2.3; P < .001), ear piercing (OR, 1.8; P < .01), and occupational exposure to human blood (OR, 3.8; P = .018). Ear piercing was a significant risk factor in 70 (28%) of 253 RIBA-positive men (P < .0001) but not in women.
Table 2.
Multivariate Logistic Regression of Risk Factors for Hepatitis C Virus Infection
| Risk Factora | Results of Third-Generation RIBA |
Multivariate Logistic Regression Analysisb |
||
|---|---|---|---|---|
| Positive, no. (%) (n = 469) | Negative, no. (%) (n = 217) | Odds Ratio (95% CI) | P | |
| IDU | 195 (42) | 2 (1) | 35.0 (10.4–218.0) | <.0001 |
| Blood transfusion | 126 (27) | 17 (8) | 9.9 (5.6–18.3) | <.0001 |
| Intranasal cocaine use | 292 (62) | 23 (11) | 6.4 (3.8–11.2) | <.0001 |
| Intranasal cocaine use without IDU or blood transfusion | 79 (49)c | 20 (10)c | 8.5 (4.9–15.1) | <.0001 |
| Occupational exposure | 27 (6) | 5 (2) | 3.8 (1.3–12.4) | .0176 |
| Sexual promiscuity | 243 (52) | 48 (22) | 2.3 (1.4–3.7) | .0006 |
| Malesd | 148 (58) | 36 (30) | 3.3 (2.1–5.3) | <.0001 |
| Femalese | 95 (44) | 12 (13) | 5.5 (3.0–11.2) | <.0001 |
| Ear piercing | 273 (58) | 86 (40) | 1.8 (1.2–2.8) | .0088 |
| Malesd | 70 (28) | 1 (<1) | 45.9 (9.9–815) | .0002 |
| Femalese | 203 (95) | 85 (89) | … | NS |
| Male sex | 254 (54) | 121 (56) | … | NS |
| Tattooing | 102 (22) | 9 (4) | … | NS |
| Acupuncture | 29 (6) | 5 (2) | … | NS |
Data are ordered by odds ratio.
Abbreviations: CI, confidence interval; IDU, injection drug use; NS, nonsignificant; RIBA, recombinant immunoblot assay.
aOrder of entry into the model (forward selection method): (1) intranasal cocaine use, (2) blood transfusion, (3) IDU, (4) sexual promiscuity, (5) ear piercing, and (6) occupational exposure.
b Ellipses indicate that the risk factor did not meet criteria for entry into the model.
c Results from analysis of a subset of 361 subjects who denied IDU and blood transfusion (163 RIBA positive, 198 RIBA negative).
d Results from analysis of a subset of 375 males (254 RIBA positive, 121 RIBA negative).
e Results from analysis of a subset of 311 females (215 RIBA positive, 96 RIBA negative).
Among 292 RIBA-positive subjects who snorted cocaine, 213 (73%) also reported a history of IDU or blood transfusion prior to 1991; 79 (27%) who snorted cocaine repeatedly denied IDU or other parenteral risk factors both in personal interviews and on a questionnaire. Among 70 men who had ear piercing as a risk factor, 67 (96%) had also snorted cocaine, had received a blood transfusion prior to 1991, had a history of IDU, or had a documented needlestick exposure to human blood.
The CART analysis identified the same risk factors for HCV infection and in the same order of importance as did the forward, backward, and stepwise logistic regression methods, except that CART did not identify occupational exposure as an important variable. Random forest analysis confirmed the importance of the risk factors identified by logistic regression; occupational exposure and sex were ranked as least important. CART and random forest analyses confirmed findings of the subset analyses of 361 subjects, presented in Table 2.
Survey of Intranasal Cocaine Use
Six hundred and ninety-two donors completed a detailed survey on cocaine use. Of 273 RIBA-positive subjects who used intranasal cocaine, 236 (86%) had shared straws or other snorting devices, 87 (32%) had experienced epistaxis during or after intranasal use, and 67 (25%) observed epistaxis in others with whom they were sharing materials. Longer duration of intranasal cocaine use was associated with positive RIBA results (P = .01) but not with detection of HCV RNA. Intranasal cocaine use was a significant independent risk factor for HCV infection, whether analyzed in the entire population (P < .0001) or in the subset of 79 who snorted cocaine but denied IDU and blood transfusion (P < .0001; Table 2).
Detection of HCV RNA by PCR and Follow Up of HCV RNA–Positive Subjects
Among 469 RIBA-positive blood donors, 384 (82%) were HCV RNA positive, and 85 (18%) were repeatedly HCV RNA negative. Of the 85 RIBA-positive, HCV RNA–negative donors, RIBA was performed a mean of 7 times over a mean period of 2.75 years, during which results remained persistently positive. The longest interval of RIBA-positive, HCV RNA–negative status documented in this study was 9.7 years. Patients who were RIBA positive, HCV RNA negative on at least 2 occasions were presumed to have been exposed to HCV and spontaneously recovered. All 217 RIBA-negative donors and 52 persistently RIBA-indeterminate donors tested negative for HCV RNA. Among RIBA-positive subjects, age, sex, and race were not significantly different between those who were HCV RNA positive and those who were HCV RNA negative: mean age, 40.2 versus 38.6 years (P = .18), male sex, 54% versus 53% (P = .81), and white race, 78% versus 85% (P = .19).
At the time the database was frozen for analysis, 257 of 384 HCV RNA–positive subjects (67%) were still being actively followed; 95 (37%) were treated for HCV infection. Since this was a natural history study, outcomes in these patients were only analyzed up to the time that treatment was initiated.
Among 258 HCV RNA–positive repeat blood donors, 65% were donating potentially HCV-infected blood for >10 years, and 42% had donated ≥10 times.
ALT Levels and Clinical Liver Disease in HCV RNA–Positive Subjects
Elevated alanine aminotransferase (ALT) levels were found at initial evaluation in 214 HCV RNA–positive subjects (56%). Over an average follow-up of 5.7 years, the mean ALT level was 62 U/L (range, 13–344 U/L), compared with 22 U/L in 354 among HCV RNA–negative subjects (P < .001). Fifty-seven HCV RNA–positive subjects (15%) had persistently normal ALT levels; 7 (12%) underwent biopsy, with all having an Ishak fibrosis score of ≤ 1. The pattern of mean ALT level elevations is shown in Table 3.
Table 3.
Pattern of Mean Alanine Aminotransferase (ALT) Level Elevations Among Hepatitis C Virus RNA–Positive Blood Donors
| ALT Level, Mean | No. (%) | No. (%) Biopsied | Mean HAI |
No. (%) Severe Fibrosisa |
||
|---|---|---|---|---|---|---|
| Normalb | 127 (33) | 42 (33) | 6.19 | 3 (7) | ||
| Elevated | 255 (67) | 142 (55) | 7.50 | 24 (17) | ||
| 1–2 × ULN | 185 (48) | 94 (51) | 7.08 | P < .001c | 13 (14) | P < .001d |
| 2–5 × ULN | 64 (17) | 45 (70) | 8.20 | 10 (22) | ||
| >5 × ULN | 6 (2) | 3 (50) | 10.33 | 1 (33) | ||
Abbreviations: HAI, histologic activity index; ULN, upper limit of normal.
a Defined as Ishak stage 3–6 (bridging fibrosis or cirrhosis).
b Persistently normal in 57 of 127 donors (45%). Of these 57, 7 (12%) underwent biopsy, with none having severe fibrosis.
c There is a significant trend of increasing HAI with increasing ALT group.
d There is a significant trend of severe fibrosis with increasing ALT group.
Fifty-one of 384 HCV RNA–positive patients (13%) had physical signs of chronic liver disease: icteric sclerae was detected in 6, spider angiomata in 40, collateral venous circulation in 1, palmar erythema in 9, splenomegaly in 2, and encephalopathy in 1; none had ascites.
Extent of Liver Disease at Biopsy
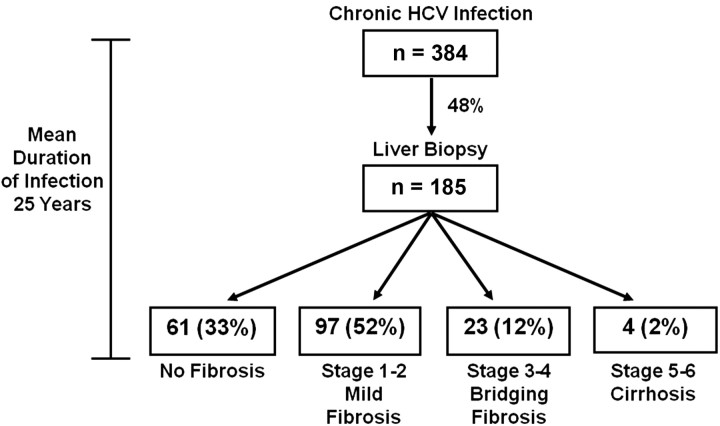
On initial liver biopsy of 185 chronically infected subjects, 61 (33%) had no fibrosis, 97 (52%) had mild fibrosis, 23 (12%) had bridging fibrosis, and 4 (2.2%) had cirrhosis (Figure 1). One patient developed HCC. Associations with liver fibrosis are shown in Table 4. Both age at infection and duration of HCV infection were extrapolated from the reported date of probable exposure, specifically, blood transfusion prior to 1991, the first year of IDU, or the date of a well-defined needlestick exposure; 125 (69%) of 185 biopsied patients had these defined risk factors. On the basis of these risk exposures, the mean age at the onset of HCV infection was 21 years (range, birth to 59 years), and the mean duration between infection and the last liver biopsy specimen obtained in the study was 25 years (range, 9–43 years). Within this time frame, the duration of infection was not associated with increasing severity of fibrosis.
Figure 1.
Stage of liver fibrosis among 185 hepatitis C virus (HCV)–positive patients undergoing initial liver biopsy. After a mean of 25 years based on the interval since a known parenteral exposure, 85% had no or minimal fibrosis, 2% had cirrhosis, and 12% had bridging fibrosis that might progress to cirrhosis.
Table 4.
Characteristics of 185 Hepatitis C Virus–Positive Patients Who Underwent Liver Biopsy, Staged by Ishak Fibrosis Score
| Characteristic | Score |
P | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3–6 | ||
| Patients (no. [%]) | 61 (33) | 64 (35) | 33 (18) | 27 (15) | |
| Age at infection, mean (years)a | 19.3 | 21.7 | 21.4 | 24.5 | .037 |
| Age at initial biopsy, mean (years) | 40.4 | 43.3 | 43.3 | 45.4 | .009 |
| Duration of infection, mean (years)a | 23.9 | 25.9 | 24.5 | 24.0 | .555 |
| Sex | |||||
| Male | 26 (28) | 33 (35) | 21 (22) | 14 (15) | .284 |
| Female | 35 (39) | 31 (34) | 12 (13) | 13 (14) | |
| Race | |||||
| White | 55 (34) | 53 (33) | 31 (19) | 23 (14) | .221 |
| African American | 5 (25) | 10 (50) | 1 (5) | 4 (20) | |
| Alcohol consumption (no. drinks/week) | |||||
| Current use | 5 | 7.1 | 6 | 6.5 | .993 |
| Peak use | 16 | 25.1 | 39.2 | 29.3 | .044 |
| Total peak use (no. drinks/year × total years) | 7910 | 11 476 | 11 388 | 18 663 | .174 |
| Body mass index, mean (kg/m2) | 25.5 | 26.1 | 27.9 | 31 | .004 |
| HAI inflammation, mean | 6.1 | 7.5 | 7.3 | 9.3 | <.001 |
| ALT level, mean (IU/L) | 57 | 70 | 77 | 87 | <.001 |
| Peak ALT level, mean (IU/L) | 113 | 124 | 138 | 142 | .006 |
| AST level, mean (IU/L) | 39 | 46 | 48 | 66 | <.001 |
| Peak AST level, mean (IU/L) | 70 | 80 | 81 | 102 | <.001 |
| GGTP level, mean (IU/L) | 57 | 57 | 70 | 97 | .025 |
| Total bilirubin level, mean (mg/dL) | 0.6 | 0.7 | 0.7 | 0.7 | .064 |
| Albumin level, mean (g/dL) | 4.3 | 4.3 | 4.4 | 4.3 | .888 |
| Platelet count, mean (platelets/mm3) | 248 | 229 | 240 | 188 | <.001 |
| Prothrombin time, mean (s) | 12.2 | 12.4 | 12.3 | 12.7 | .062 |
| Partial thromboplastin time, mean (s) | 27.9 | 28.7 | 27.4 | 28.7 | .928 |
| Alpha-1 fetoprotein level, mean (ng/mL) | 8 | 5 | 5 | 15 | .014b |
| Alkaline phosphatase level, mean (IU/L) | 77 | 72 | 79 | 87 | .289 |
| Lactate dehydrogenase level, mean (IU/L) | 161 | 164 | 172 | 180 | .008 |
| Creatine kinase level, mean (IU/L) | 124 | 134 | 132 | 104 | .909 |
| Viral measurements | |||||
| Quantitative RNA load, mean (× 106 copies/mL) | 3.43 | 3.17 | 4.55 | 1.96 | .189 |
| Genotype | |||||
| 1 | 46 (34) | 49 (36) | 22 (16) | 19 (14) | .414 |
| 2 | 10 (32) | 9 (29) | 9 (29) | 3 (10) | |
| 3 | 1 (14) | 2 (29) | 1 (14) | 3 (43) | |
| 4 | 0 | 1 | 0 | 1 | |
| 6 | 0 | 1 | 0 | 0 | |
| Mixed | 0 | 2 | 1 | 0 | |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGTP, γ-glutamyl peptidase; HAI, histologic activity index.
a For 125 subjects, inferred from the date of blood transfusion, the first year of injection drug use, or the date of a well-defined needlestick exposure.
b P = .024 after excluding data for 1 study subject, who had hepatocellular carcinoma.
Sex, race, education level, alcohol use at the time of HCV diagnosis, and total peak alcohol use were not significantly associated with the stage of fibrosis; body mass index was positively correlated with worsening fibrosis (P = .005; Table 4). Patients with bridging fibrosis or cirrhosis had higher HAI scores than those with mild or no fibrosis (P < .001). Elevated levels of serum markers of liver inflammation were highly associated with increasing stage of fibrosis (ALT and aspartate aminotransferase levels, P < .002; lactate dehydrogenase level, P = .010). The alpha-1 fetoprotein level was only significant when one patient who developed HCC was included (P = .006).
Among biopsied patients, 182 (98%) were genotyped, and 136 (76%) were genotype 1; 111 (60%) of biopsied patients had quantitative HCV RNA load measured, with a mean level of 3.32 × 106 copies/mL (range, 1.14 × 103 to 4.81 × 107 copies/mL; median, 1.20 × 106 copies/mL). Neither genotype nor HCV load were associated with a more severe stage of liver fibrosis.
Extent of Liver Disease at Repeat Liver Biopsy
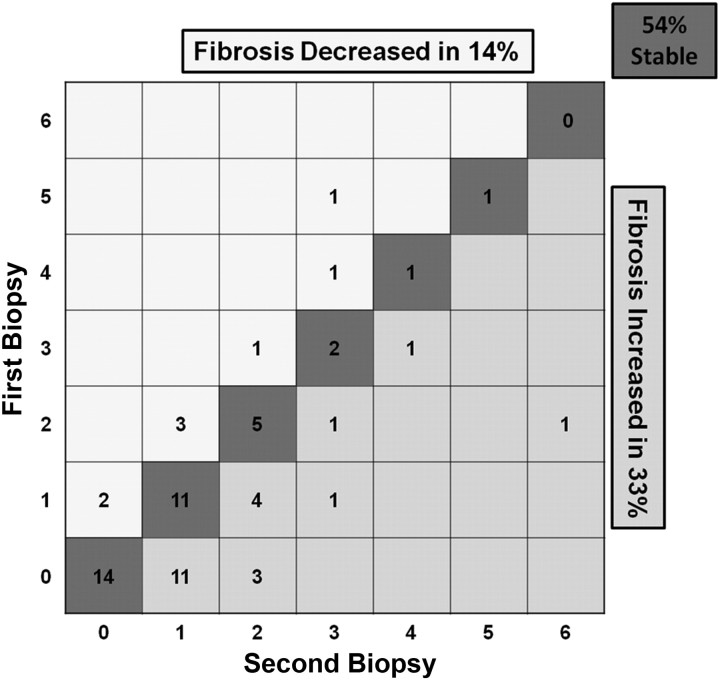
Sixty-three patients (34%) underwent a second biopsy after a mean interval of 4.6 years between biopsies. Over that interval, 21 (33%) had fibrosis that increased by at least 1 Ishak stage (5 increased by at least 2 stages), 34 (54%) had no change, and 8 (14%) showed a decrease of ≥1 Ishak stage (Figure 2). The mean progression rate between biopsies was 0.06 Ishak stages per year (0.28 for patients whose fibrosis increased).
Figure 2.
Fibrosis progression among 63 hepatitis C virus (HCV)–positive patients undergoing repeat liver biopsy. After a mean interval between biopsies of 4.6 years, 54% showed no change in interval biopsies, 14% showed lessened fibrosis, and 33% showed increased fibrosis by at least 1 stage; 5 (8%) increased by ≥2 stages.
Treated Versus Untreated HCV RNA–Positive Subjects
Among 384 HCV RNA–positive subjects, 95 (25%) were treated with interferon with or without ribavirin, or with pegylated interferon plus ribavirin. Compared with those who were not treated, subjects who received treatment were of similar age (40.2 vs 40.1 years; P = .97) and similar sex (48% vs 56% were male; P = .18). However, subjects who received treatment were more likely to be white (89% vs 74%; P < .01) and had a higher mean ALT level (78.5 vs 56.4; P < .01). When liver histologic findings were compared between biopsied subjects who were and those who were not treated, treated subjects had a higher mean Ishak fibrosis stage (1.65 vs 0.87; P < .01) and HAI (7.90 vs 6.64; P < .01).
Mortality
From 1990 to 2005, there were 28 deaths (4%) in the total study population, and 22 (79%) of those who died were HCV RNA positive (OR, 3.5; P < .01). The mean age at death was 51 years. Among those chronically infected with HCV, only 2 (9%) died from liver-related causes, one from HCC and the other from complications of cirrhosis.
DISCUSSION
Informed decisions for the treatment of chronic HCV infection require knowledge of the natural history of the disease because the key issue is not which drug or dosage to use, but whether treatment is indicated. Given that current treatments are arduous, expensive, and fraught with adverse events, and given that fibrosis progression is neither linear nor inevitable, one has to balance the probability of disease progression against the near certainty of deleterious drug-induced side effects. Early retrospective studies overestimated the severity of chronic HCV infection by focusing on those with established chronic liver disease while excluding the much larger number of silent infections [8–10]. This study prospectively followed asymptomatic individuals found to be anti-HCV positive at the time of blood donation. Although the study is biased by limiting enrollment to volunteer blood donors, we believe it provides a valid model for assessing transmission patterns in low-risk populations, the rate of spontaneous recovery in immunocompetent individuals, and the long-term outcomes of HCV infection.
Epidemiologic comparisons between RIBA-positive and RIBA-negative (EIA false-positive) controls demonstrated striking differences. Although all donors denied IDU at the time of donation, in subsequent private interviews with a physician, 41% of RIBA–positive donors admitted to IDU at some point in their life, compared with only 1% of RIBA-negative controls; none were current drug addicts. Thus, even in a presumed low-risk population, IDU was the greatest risk factor for HCV acquisition, with an OR of 35.0 (P < .0001). Unexpectedly, intranasal cocaine use was an additional strong independent risk factor in a multivariate logistic regression analysis and in CART and random forest analyses. Although intranasal cocaine use often overlapped IDU, there were 79 RIBA-positive subjects who snorted cocaine and repeatedly denied IDU or blood transfusion, and cocaine snorting remained a strong independent risk in this subset (OR, 8.5; P < .0001). Although one can never be certain of the veracity of IDU denial, there is plausibility to the concept that cocaine snorting might transmit HCV, in that (1) 86% of those who snorted admitted to the shared use of snorting devices, a previously implicated risk factor [11, 12]; (2) cocaine is known to denude mucous membranes, allowing direct access to blood vessels; (3) HCV RNA has been detected in nasal secretions [13]; (4) approximately 30% of subjects who snorted either experienced or observed nosebleeds during shared intranasal cocaine use; and (5) anti-HCV positivity was significantly associated with the duration of cocaine use. Thus, intranasal cocaine use may be a covert parenteral route of viral transmission, a route that might be applicable to human immunodeficiency virus and hepatitis B virus infection, as well as to HCV infection.
Over a mean interval of 25 years from onset of infection to liver biopsy, only 14% had severe histologic outcomes, and only 2% had cirrhosis; 85% had no or minimal fibrosis. Other studies have shown a similarly low proportion of severe histologic outcomes during the first 2–3 decades of HCV infection [14–17]. Further, this low incidence of severe outcomes is a worst-case scenario because biopsied patients had higher average ALT levels than nonbiopsied subjects, as there was reluctance to biopsy the approximate 30% who had normal or low-level ALT elevations. Thus, although this study has a selection bias based on the propensity to biopsy and treat those with the most severe clinical or biochemical profiles, this bias would be in the direction of observing more severe histologic outcomes rather than the relatively mild outcomes actually observed. If subjects with spontaneous recovery (15%) are factored into the outcome analysis, the number of acutely infected patients who progress to severe outcomes would be proportionately less. However, there is a further caveat to this outcome analysis. Despite a mean duration of follow-up of 25 years between the time of probable exposure and the time of the last available biopsy, patients biopsied in this study were still relatively young and had not reached the 30–40-year disease duration that seems critical to fibrosis progression in HCV infection [18]. Indeed, the histologic progression observed between 5-year-interval biopsies in one-third of our patients portends worse outcomes for some in the ensuing decades. Nonetheless, it is probable that those who have shown no or little fibrosis progression over 25 years will have a nonprogressive or slowly progressive course that will provide time for more effective and safer therapies to emerge and induce sustained virologic responses that appear tantamount to cure [19]. Clearly, a subset of patients will have progressive histologic deterioration either because treatment was not accessed or because antiviral therapy failed to achieve a sustained virologic response. Identification of silent HCV carriers and access to treatment remain major public health hurdles, but among treated subjects the number who will not achieve a sustained virologic response has been reduced dramatically with the recent licensure of protease inhibitors [20, 21]. Since the majority of HCV-infected individuals will not be treated in the near term, continued long-term follow-up is critically needed to provide better estimates of clinical and histologic outcomes after ≥3 decades of HCV infection, although one small study has shown relatively benign outcomes even after a mean observation period of 45 years [22]. Although the prognosis for the individual patient with HCV infection can be increasingly optimistic, the global burden of this disease is staggering on the basis of the sheer magnitude (estimated 100 million) of those who are already chronically infected.
Notes
Financial support. This work was supported by the Intramural Research Program of the Clinical Center, National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Alter MJ. Hepatitis C virus infection in the United States. J Hepatol. 1999;31:88–91. doi: 10.1016/s0168-8278(99)80381-x. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Conry-Cantilena C, VanRaden M, Gibble J, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med. 1996;334:1691–6. doi: 10.1056/NEJM199606273342602. [DOI] [PubMed] [Google Scholar]
- 4.Tobler LH, Lee SR, Stramer SL, et al. Performance of second and third-generation RIBAs for confirmation of third-generation HCV EIA-reactive blood donations. Retrovirus Epidemiology Donor Study. Transfusion. 2000;40:917–23. doi: 10.1046/j.1537-2995.2000.40080917.x. [DOI] [PubMed] [Google Scholar]
- 5.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 6.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–20. [PubMed] [Google Scholar]
- 7.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 8.Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–6. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 9.Kiyosawa K, Sodeyama T, Tanaka E, et al. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology. 1990;12:671–5. doi: 10.1002/hep.1840120409. [DOI] [PubMed] [Google Scholar]
- 10.Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687–95. doi: 10.1002/hep.510280632. [DOI] [PubMed] [Google Scholar]
- 11.Karmochkine M, Carrat F, Dos Santos O, Cacoub P, Raguin G. A case-control study of risk factors for hepatitis C infection in patients with unexplained routes of infection. J Viral Hepat. 2006;13:775–82. doi: 10.1111/j.1365-2893.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- 12.Tortu S, McMahon JM, Pouget ER, Hamid R. Sharing of noninjection drug-use implements as a risk factor for hepatitis C. Subst Use Misuse. 2004;39:211–24. doi: 10.1081/ja-120028488. [DOI] [PubMed] [Google Scholar]
- 13.McMahon JM, Simm M, Milano D, Clatts M. Detection of hepatitis C virus in the nasal secretions of an intranasal drug-user. Ann Clin Microbiol Antimicrob. 2004;3:6. doi: 10.1186/1476-0711-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiese M, Gruengreiff K, Guethoff W, Lafrenz M, Oesen U, Porst H. Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany-a 25-year multicenter study. J Hepatol. 2005;43:590–8. doi: 10.1016/j.jhep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–33. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 16.Seeff LB, Hollinger FB, Alter HJ, et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B, and type C hepatitis: A National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455–63. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 17.Vogt M, Lang T, Froesner G, et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. 1999;341:866–70. doi: 10.1056/NEJM199909163411202. [DOI] [PubMed] [Google Scholar]
- 18.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 19.Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–51. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- 20.Poordad F, McCone J, Jr, Bacon BR, et al. Bocepravir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeuzem S, Anderone P, Pol S, et al. Telapravir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–28. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 22.Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med. 2000;132:105–11. doi: 10.7326/0003-4819-132-2-200001180-00003. [DOI] [PubMed] [Google Scholar]