Abstract
Background.Enteroaggregative Escherichia coli (EAEC) are increasingly recognized as an important agent of inflammatory and often persistent diarrhea. Although previous studies report on the inflammatory aspects of EAEC pathogenesis, the mechanisms by which EAEC trigger these events are not well understood.
Methods.EAEC strains harboring mutations in known EAEC virulence determinants were tested in an in vitro model of transepithelial migration of polymorphonuclear neutrophils (PMNs) and in human intestinal xenografts in severe-combined immunodeficient (SCID-HU-INT) mice, a novel model for studying EAEC disease in vivo.
Results.Expression of aggregative adherence fimbriae (AAFs), the principal adhesins of EAEC, was required for EAEC-induced PMN transepithelial migration in vitro. Moreover, constructed plasmids encoding AAF gene clusters demonstrated that the AAF adhesins are sufficient for triggering this event in a nonpathogenic E. coli background. Furthermore, with use of the SCID-HU-INT mouse model, severe tissue damage and infiltration of inflammatory cells was observed in the human tissue after EAEC infection. These pathological marks were strongly related to AAF expression, thus clearly confirming our in vitro findings.
Conclusions.The present work establishes EAEC as an important inflammatory pathogen and the AAF adhesins as inducers of potentially detrimental immune responses.
Enteroaggregative Escherichia coli (EAEC) is increasingly recognized as a cause of severe diarrhea in developing countries and of outbreaks in industrialized countries [1, 2]. Of note, the recent major German outbreak, infecting >4000 individuals and resulting in 53 deaths, was caused by a Shiga toxin–producing EAEC strain [3]. EAEC pathogenesis is determined by the ability of the pathogen to colonize the intestinal tract, which in EAEC prototype strains is facilitated by aggregative adherence fimbriae (AAF). Four variants of the AAF major structural subunit are described to date: AggA (AAF/I), AafA (AAF/II), Agg3A (AAF/III), and Agg4A (AAF/IV) [4–7]. The AAFs are encoded on a large pAA virulence plasmid along with AggR, a transcription factor regulating AAF biogenesis, and other putative EAEC virulence factors [8, 9].
The AAF organelles appear to be involved in multiple aspects of EAEC pathogenesis. In addition to facilitating mucosal adherence, AAFs mediate biofilm formation to abiotic surfaces [7, 10]. They have also been implicated in pro-inflammatory properties of EAEC infection. Particularly, AAF/II is involved in eliciting basolateral release of the potent neutrophil chemoattractant interleukin (IL)–8 from polarized monolayers of T84 human colonic epithelial cells [11]. Moreover, EAEC prototype strains 042 and JM221 (expressing AAF/II and AAF/I, respectively) were both shown to induce AAF-dependent disruption of the intestinal epithelial barrier in vitro, caused by delocalization of the tight junction proteins occludin and claudin-1 [12]. Epithelial barrier disruption could be a contributing factor to infiltration of neutrophils, as observed with Salmonella Typhimurium [13].
We have recently shown that EAEC 042 induces transepithelial migration of polymorphonuclear neutrophils (PMNs) across polarized T84 cell monolayers and identified the host signaling cascade underlying this inflammatory event [14]. EAEC infection triggers activation of a conserved host cell pathway involving apical release of an eicosanoid-based PMN chemoattractant (presumably hepoxilin A3) generated through 12/15-lipoxygenase (12/15-LOX) activity. Once released from the apical surface, the eicosanoid forms a chemotactic gradient across the tight junctional complex guiding PMNs across the intestinal epithelium to the luminal surface [14–16].
Although in vitro model systems provide important insight into aspects of EAEC pathogenesis, they do not recapitulate the natural disease in humans. Several animal species have been used as models to study EAEC infection; however, most animal models exhibit only mild, if any, signs of disease [17–19]. It is likely that EAEC pathogenesis is highly adapted to human intestinal tissue. Thus, there is a need for additional in vivo model systems for investigation of EAEC virulence properties, ideally in the context of the complete human intestinal mucosa. Human intestinal xenografts in severe-combined immunodeficient (SCID-HU-INT) mice provide a model consistent with intact human intestinal tissue. These intestinal grafts become extensively vascularised, secrete mucus, and develop into morphologically normal human intestinal tissue [20, 21]. Use of SCID-HU-INT mice is a well-established model for studying innate immune responses to enteric pathogens, such as Salmonella Typhimurium, Shigella species, and strains of enterohemorrhagic E. coli (EHEC) [22–24]. This is the first time that SCID-HU-INT mice have been used to study EAEC disease.
In this study, we examined the mechanisms by which EAEC trigger PMN transepithelial migration, revealing a key role for AAFs. Our findings were confirmed in vivo using SCID-HU-INT mice to study innate immune responses to EAEC infection.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions
Bacterial strains used in this study are listed in Table 1. Bacteria were cultured in Luria-Bertani (LB) broth/agar containing antibiotics when appropriate: 100 μg/mL ampicillin, 30 μg/mL chloramphenicol, and 50 μg/mL kanamycin. Before infection, bacteria were subcultured in either Dulbecco's modified Eagle's medium (DMEM) with 0.45% glucose/L or in LB broth.
Table 1.
Bacterial Strains and Plasmids Used in This Study
| Strain or Plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| 042 | Wild-type EAEC strain expressing AAF/II | [25] |
| 042 ΔaggR | 042 with suicide plasmid pJP5603 inserted into the aggR gene. Km | [8] |
| 042 ΔaafA 3.4.14 | 042 with TnphoA inserted into the aafA gene encoding the major fimbrial subunit of AAF/II. Km | [5] |
| 042 ΔaafB | 042 with JP5603 inserted into the aafB gene encoding the minor pilin subunit of AAF/II. Km | [26] |
| 042 Δaap | 042 with JP5603 inserted into the aap gene encoding dispersin. Km | [27] |
| 042 Δpet-astA | 042 in which the genes encoding Pet and EAST1 (astA) have been deleted. Km | [12] |
| 042 ΔfliC | 042 harbouring suicide plasmid pJP5603 inserted into the fliC gene encoding flagellin. Km | [28] |
| 042 Δpic | 042 in which the gene encoding Pic has been deleted. Km | [17] |
| JM221 | Wild-type EAEC strain expressing AAF/I | [29] |
| JM221 ΔaggDCBA | JM221 in which a kanamycin cassette has been inserted into the aggDCBA gene cluster encoding AAF/I. Km | [12] |
| 55989 | Wild-type EAEC strain expressing AAF/III | [6] |
| C1010-00 | Wild-type EAEC strain expressing AAF/IV | [30] |
| C1010-00 Δagg4A | C1010-00 with TnphoA inserted into the agg4A gene encoding the major fimbrial subunit of AAF/IV. Km | [7] |
| HS | Human commensal E. coli strain | [31] |
| HS/pAA2 | HS transformed with pAA2 containing insertion into a silent locus. Km | [11] |
| HB101 | Non-fimbriated E. coli K-12 strain | [32] |
| Plasmids | ||
| pEJB01 | pUC18 harbouring the full aggDCBA gene cluster of EAEC JM221. Amp | This study |
| pEJB02 | pACYC184 harbouring the aafDA-IS1-aggR and aafCB gene clusters of EAEC 042 encoding AAF/II and AggR. Cm | This study |
| pEJB03 | pUC18 harbouring the full agg3DCBA gene cluster of EAEC 55989 encoding AAF/III. Amp | This study |
| pNBO1 | pUC18 harbouring the full agg4DCBA gene cluster of EAEC C1010-00 encoding AAF/IV. Amp | [7] |
Abbreviations: Amp, ampicillin resistance; Cm, chloramphenicol resistance; Km, kanamycin resistance.
Cell Cultures
Inverted polarized monolayers of the human colon cancer–derived cell line T84 were constructed as previously described [14].
PMN Transepithelial Migration Assay
The PMN transepithelial migration assay was performed as described in Supplementary Data.
Cloning of AAF Gene Clusters
Gene clusters responsible for AAF synthesis were constructed as described in Supplementary Data.
Adhesion and Biofilm Assays
Adherence to T84 monolayers and biofilm formation in microtiter plates was quantified as described in Supplementary Data.
Immunostaining for Detection of Fimbriae (AafA)
Detection of AAF/II expression was determined using primary AafA antibodies as described in Supplementary Data.
Transmission Electron Microscopy
Electron microscopy was performed as described by Struve et al [33]
Ethics Statement
The use of human volunteers for obtaining PMNs in this study was in accordance with appropriate guidelines and was approved by the University of Massachusetts Medical School Review Board for the Protection of Human Subjects (approval #13006). Written informed consent was provided by the study participants.
Human fetal small intestine was obtained from Bringham and Women's Hospital after therapeutic abortion. Procurement and procedures involving xenografting of human fetal tissues into C.B.-17 scid/scid mice were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments at Massachusetts General Hospital, Boston (Permit Number: P-003833).
Preparation and Infection of Xenografts
Fetal small intestinal tissues were washed in DMEM and implanted subcutaneously into the subscapular region of SCID mice as previously described [20]. Grafts were allowed to develop for at least 11 weeks before use. Human fetal small intestinal xenografts (3 per treatment) were infected by direct intraluminal inoculation with 100 μL of prepared suspensions containing sterile HBSS+ or 5 × 107 bacteria. 24 hours after infection, the animals were euthanized, and the grafts were removed for examination.
Histological Procedures
After experimentation, fetal intestinal xenograft tissues were removed from the mice, placed in cutting compound, and snap frozen in liquid nitrogen. Cryosections (5 μm) were mounted on glass slides before either hemotoxylin and eosin (HE) or immunofluorescent staining. HE staining was performed in accordance with the method of Chen et al [34]. Intestinal pathology was scored in a blinded fashion according to a modified 2-part histological scoring system previously described [35, 36]. Part 1 is the determination of the infiltration of inflammatory cells in the intestine, with scores ranging from 0 to 4 (0, normal cell pattern; 1, scattered inflammatory cells in the lamina propria; 2, increased numbers of inflammatory cells in the lamina propria; 3, confluence of inflammatory cells extending into the submucosa; and 4, transmural extension of the infiltrative inflammatory cells). Part 2 is the evaluation of intestine tissue damage, with scores also ranging from 0 to 4 (0, normal tissue pattern; 1, minimal inflammation and intestinal crypt hyperplasia; 2, mild intestinal crypt hyperplasia with or without focal invasion of epithelium; 3, obvious intestinal crypt hyperplasia, invasion of epithelium, and goblet cell depletion; and 4, extensive mucosal damage and extension through deeper structures of the bowel wall). The total intestine pathology score equals the inflammatory cell score plus the tissue damage score.
For immunofluorescent staining, slides were submerged in 4% formalin in phosphate-buffered saline (PBS) for 25 minutes, followed by washing. The slides were then submerged in blocking buffer (PBS with 1% bovine serum albumin and 0.1% Triton X-100) for 20 minutes, washed, and then stained overnight in a dark chamber using a fluorescein isothiocyanate (FITC)–conjugated rat-anti-mouse Ly-6G and Ly-6C (Gr-1) antibody, which recognizes PMNs and monocytes (BD Biosciences Pharmingen). After final washing, coverslips were mounted using Vectashield solution containing 4′,6′-diamidino-2-phenylindole (DAPI; Vector).
Statistical Analysis
PMN isolation was limited to repetitive donations by approximately 10 different donors over the course of the experiments. Because of variations in both PMNs and transepithelial resistance between monolayers (baseline resistance, 800–2000 Ω × cm2), data were analyzed in an individual experiment and not between experiments. However, the overall trends within an experiment were reproducible between experiments. All results are expressed as the mean ± standard deviation (SD) of an individual experiment performed in triplicate repeated at least 3 times. Student's t-test was used and P-values <.05 were considered to be statistically significant.
RESULTS
AAF/II Plays a Key Role in Triggering EAEC 042-induced PMN Transepithelial Migration
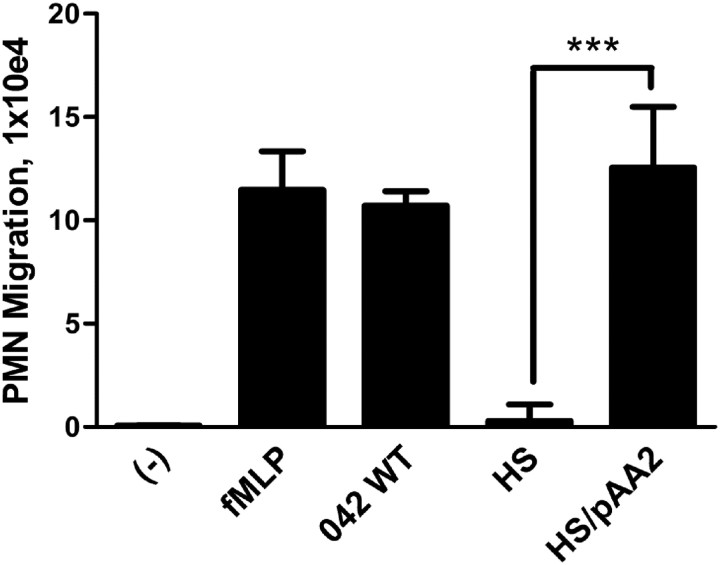
We have previously demonstrated that EAEC prototype strain 042 promotes PMN transepithelial migration [14]. In this study, the mechanisms by which EAEC 042 triggers this inflammatory event were assessed. The pAA2 virulence plasmid of 042 was shown to confer on nonpathogenic E. coli strain HS the ability to induce epithelial barrier disruption and basolateral release of IL-8 from polarized T84 monolayers [11, 12]. To determine whether pAA2 plays a similar role in triggering PMN transmigration, we tested HS carrying pAA2 in our in vitro model. We found that, unlike wild-type HS, HS/pAA2 induced PMN transmigration to the same extent as 042, demonstrating that EAEC-specific factors encoded on pAA2 are sufficient to induce this inflammatory response (Figure 1).
Figure 1.
Virulence plasmid pAA2 of EAEC strain 042 is sufficient for promoting PMN transepithelial migration across T84 cell monolayers. T84 cell monolayers were infected apically with EAEC 042, commensal E. coli strain HS, or HS carrying virulence plasmid pAA2 of 042. The ability to induce PMN transepithelial migration was assessed 90 minutes later (see Supplementary Data). HBSS+ buffer (-) and the potent PMN chemoattractant N-formylmethionyl-leucyl-phenylalanine (fMLP) were included as negative and positive controls, respectively. The data are expressed as means ± SD for at least triplicate samples and represent 1 of 3 independent experiments performed with similar results. ***P < .001.
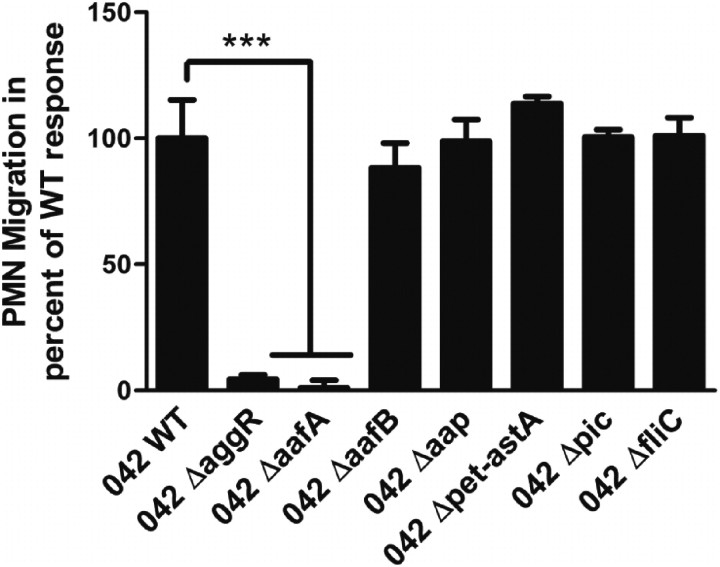
We next sought to identify the specific pAA2-encoded genes involved in promoting PMN transmigration by screening a bank of EAEC 042 strains harboring mutations in pAA2-encoded virulence genes in our in vitro model. We found that mutations in the genes encoding the AafA major pilin protein of AAF/II or transcription factor AggR almost entirely abolished 042-induced PMN transmigration. In contrast, 042 strains with mutations in the genes encoding the AafB minor pilin protein of AAF/II, the dispersin surface protein (aap), the toxins Pet or EAST1 (astA), or the chromosomally encoded Pic mucinase or flagellin (fliC) induced PMN transmigration to the same extent as wild-type 042 (Figure 2). These results suggest that the AAF/II organelle plays a key role in triggering EAEC 042–associated PMN transmigration, without the requirement for a functional AafB protein.
Figure 2.
EAEC 042-induced PMN transepithelial migration requires expression of AAF/II. PMN transmigration induced by wild-type 042 or 042 mutant strains (see Table 1). The data are expressed as means ± SD for at least triplicate samples and represent 1 of 3 independent experiments performed with similar results. ***P < .001.
To further address the role of AAF/II, we designed a plasmid construct, pEJB02, harboring the genes encoding AAF/II as well as AggR (See Supplementary Data) and transferred it to the nonfimbriated E. coli strain HB101. HB101 carrying pEJB02 adhered to T84 cell monolayers and produced biofilm in microtiter plates to the same extent as EAEC 042 (Figure 3A and 3B). Moreover, expression of the AafA major structural subunit was confirmed by immunostaining (Figure 3C). However, HB101/pEJB02 failed to induce PMN transmigration in our in vitro model (data not shown), suggesting that the AafA structural subunits on the surface of HB101 do not form a functional AAF/II organelle or that other factors are required to elicit AAF/II-dependent PMN transmigration in our in vitro model. These observations suggest that the fimbriae assembly and extension are important in inflammation.
Figure 3.
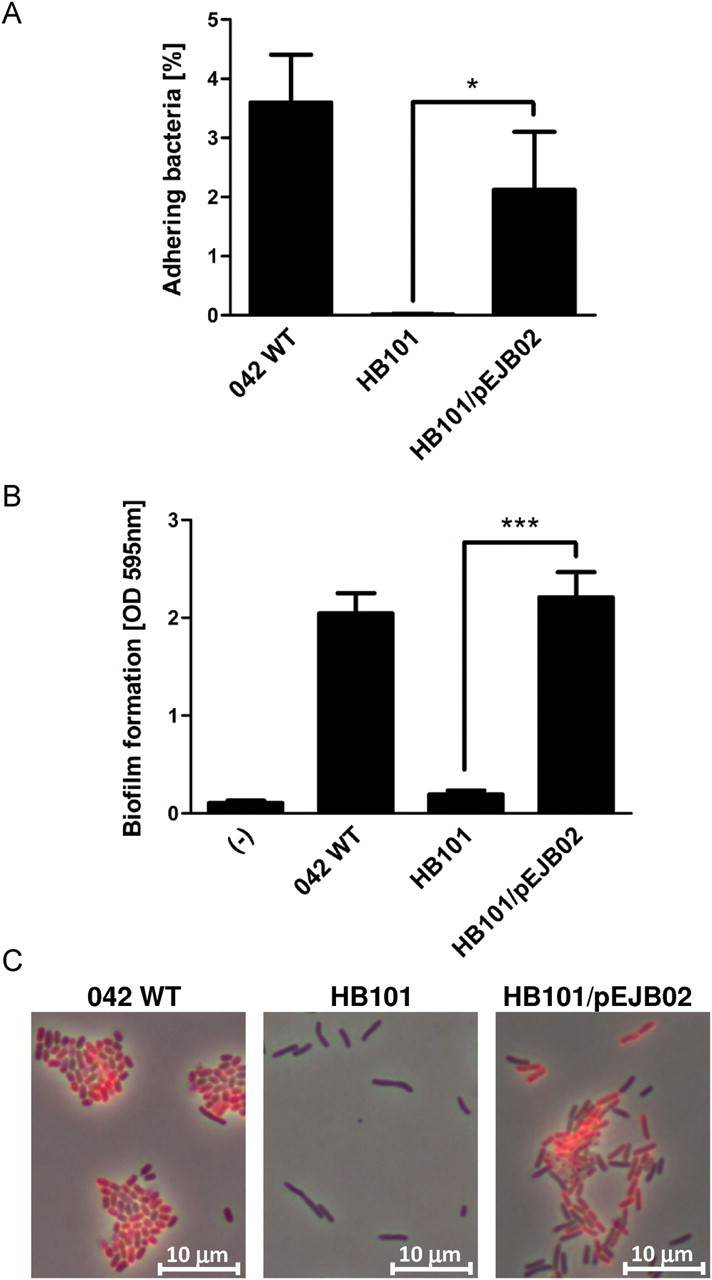
AAF/II-encoding plasmid pEJB02 facilitates adherence to T84 cell monolayers and abiotic surfaces and surface expression of AafA. (A) T84 cell monolayers were infected for 90 minutes with EAEC 042, commensal E. coli strain HB101, or HB101 carrying AAF/II-encoding plasmid pEJB02, followed by cell lysis and quantification of attaching bacteria by colony counting. (B) EAEC 042, HB101, and HB101/pEJB02 were grown in microtiter plates for 24 hours, after which biofilm formation was quantified. (C) Immunostaining of EAEC 042, HB101, and HB101/pEJB02 using an AafA polyclonal antibody (shown in red). See Supplementary Data for description of methods. *P < .05; ***P < .001.
Expression of AAF/I, -III, or –IV is Sufficient for PMN Transmigration Induced by Other EAEC Prototype Strains
We next examined whether other AAF variants play a similar role as AAF/II in triggering inflammatory responses, using EAEC prototype strains JM221 (AAF/I), 55989 (AAF/III), and C1010-00 (AAF/IV). All 3 EAEC strains induced extensive PMN transmigration (Figure 4). Moreover, mutations in the genes encoding AAF/I and AAF/IV strongly attenuated the ability of JM221 and C1010-00, respectively, to promote PMN transmigration (Figure 4A). An isogenic AAF/III mutant strain of 55989 could not be constructed because of the multidrug-resistant nature of this strain.
Figure 4.
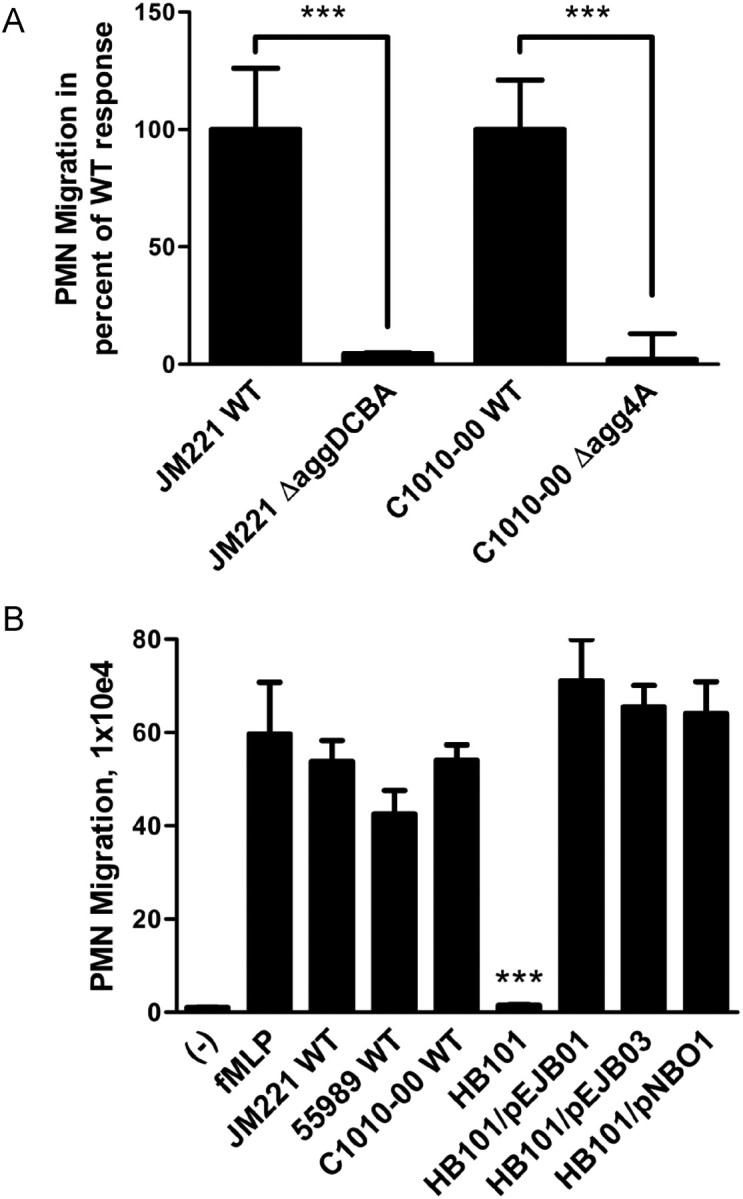
AAF variants I, III, and IV are required and sufficient for inducing in vitro PMN transepithelial migration by AAF-producing EAEC strains. (A) PMN transmigration induced by wild-type EAEC JM221, JM221 ΔaggDCBA, wild-type EAEC C1010-00, or C1010-00 Δagg4A. (B) PMN transmigration induced by EAEC strains JM221, 55989, or C1010-00; E. coli strain HB101; or HB101 carrying AAF/I-encoding plasmid pEJB01, AAF/III-encoding plasmid pEJB03, or AAF/IV-encoding plasmid pNBO1. fMLP was included as a positive control. The data are expressed as means ± SD for at least triplicate samples and represent 1 of 3 independent experiments performed with similar results. ***P < .001.
Next, plasmids pEJB01 and pEJB03 were constructed, encoding the 4 subunits of the AAF/I and AAF/III organelles, respectively (See Supplementary Data). Both plasmids conferred adherence to T84 cell monolayers in HB101 (data not shown). Of interest, HB101 carrying pEJB01, pEJB03, or pNBO1 (expressing AAF/IV) promoted PMN transmigration to the same extent as the respective EAEC strains (Figure 4B), inferring that the AAF adhesins themselves are sufficient for triggering this innate immune response. Collectively, these findings suggest that pro-inflammatory properties are conserved among AAF-producing EAEC strains and that a key contributing factor to these events are the AAF organelles.
EAEC 042 Elicits AAF-Dependent Inflammatory Responses In Vivo Using a Human Fetal Intestinal Xenograft Model
To verify the observed in vitro pro-inflammatory effects of EAEC infection, we used an in vivo model of HU-INT-SCID mice. Xenografts (3 grafts per treatment) were injected with wild-type EAEC 042, 042 ΔaafA, HB101, HB101/pEJB02, or buffer alone for 24 hours, after which the grafts were removed. Histological evaluation of HE-stained graft sections revealed extensive tissue damage and inflammation after infection with wild-type 042, as determined by PMN infiltration, goblet cell depletion, edema, and occasional breakage of the epithelium (Figure 5A; see Materials and Methods for scoring system). In contrast, the 042 ΔaafA-infected grafts showed significantly less inflammation, compared with the 042 wild-type–infected grafts, whereas the HB101-infected and buffer control grafts exhibited minimal signs of inflammation. Of interest, infection with HB101/pEJB02 caused the same extent of pathological effects as wild-type 042, strongly indicating expression of pro-inflammatory components of AAF/II, if not the complete organelle structure, in this in vivo model.
Figure 5.

AAF/II plays a key role in mediating EAEC 042–elicited inflammatory responses in a SCID-HU-INT mouse model of infection. (A) Human fetal small intestinal xenografts were infected for 24 hours with wild-type 042, 042 ΔaafA, E. coli strain HB101, HB101 carrying AAF/II-encoding plasmid pEJB02, or sterile HBSS+ (n = 3). After infection, intestinal histopathology of sections of the xenografts were quantified by using a combined pathology scoring system (scales 0 to 4), with 0 scored normal and a score of 4 showing the most substantial level of tissue inflammation and tissue damage, respectively (see Materials and Methods). The fraction of both scoring systems is denoted. Data represent means ± SD. Statistics are based on the combined histopathology scores. *P < .05, **P < .01. (B and C) Immunofluorescent-stained sections of the post-infected xenografts. Tissue sections (shown in blue) were stained with DAPI, and PMNs (shown in green) were stained using FITC-labeled antibody targeting a PMN surface marker (Ly-6G and Ly-6C). AafA (shown in red) was stained using Alexa Fluor 594-conjugated secondary antibody specific for an AafA polyclonal antibody. Photographs are representative tissues for each treatment.
To further substantiate differences in inflammation between treatments, we performed FITC staining targeting a PMN surface marker (reacts with a common epitope on Ly-6G and Ly-6C). Confirming our HE stain results, we observed extensive PMN infiltration in the xenografts infected with wild-type 042 or HB101/pEJB02, significantly less PMNs in the 042 ΔaafA-infected xenografts, and almost no PMNs present in the HB101-infected or buffer-treated xenografts (Figure 5B). Expression of the AafA subunit on the surface of wild-type 042 and HB101/pEJB02 adhering to the epithelial surface of the infected xenografts was verified by immunostaining (Figure 5C).
Taken together, these data clearly demonstrate the ability of EAEC 042 to promote PMN infiltration of the human fetal tissue and that AAF appears to play a key role in triggering this inflammatory event.
DISCUSSION
Several enteric pathogens elicit inflammatory diarrhea, the hallmark of which is infiltration of PMNs. Although studies have revealed inflammatory aspects of EAEC pathogenesis, the exact mechanism by which this diarrheagenic pathogen triggers innate immune responses is unclear. In this study, we demonstrate a key role for AAFs in mediating EAEC-induced PMN infiltration both in vitro and in vivo.
Having previously demonstrated that EAEC prototype strain 042 induces transmigration of PMNs across monolayers of polarized T84 epithelial cells [14], the initial objective of this study was to characterize the mechanism by which EAEC triggers this event. Focusing on AAF-producing EAEC prototype strains, we found that all of these strains required expression of AAF for promoting PMN transmigration. Moreover, transfer of plasmids harboring the gene clusters encoding AAF/I, -III, or -IV conferred on HB101 the ability to promote PMN transmigration, strongly suggesting that expression of AAF is sufficient for triggering this event. Remarkably, despite their genetic diversity [7], all 3 AAF variants exhibited these pro-inflammatory properties, thereby further emphasizing the contribution of AAFs to EAEC pathogenesis.
We also sought to characterize directly the pro-inflammatory role of AAF/II. Distinguishing it from other AAF variants, the genes required for expression of AAF/II map to 2 distinct clusters on the pAA2 virulence plasmid of EAEC 042. Surrounding the clusters are various insertion elements that may contribute to spontaneous mutations, offering an explanation as to why attempts to construct plasmids with stable expression of AAF/II have proven to be difficult [26]. Nevertheless, we constructed such a plasmid, designated pEJB02, and found that it conferred on HB101 the ability to adhere to T84 cell monolayers and to produce biofilm in microtiter plates, phenotypes characteristic of AAF-producing EAEC strains [7]. However, HB101 harboring the AAF/II-encoding pEJB02 plasmid failed to elicit PMN transmigration in our in vitro model. Although we could confirm expression of the AafA major structural subunit on the surface of HB101 carrying the plasmid, we failed to detect protruding fimbrial structures by electron microscopy, raising the possibility that our construct does not produce full-structure functional AAF/II. Moreover, the surface-coating protein dispersin secreted by EAEC 042 has been shown to play a key role in facilitating proper extension of AAF/II by preventing collapse of these positively charged structures on to the negatively charged lipopolysaccharides on the bacterial surface [27]. It is possible that the lack of dispersin coating on the surface of HB101 could be a contributing factor to hindering the functionality of AAF/II-like structures expressed on the surface of this strain, rendering them unable to trigger PMN transmigration in our in vitro model. However, mutations in the dispersin-encoding gene did not affect EAEC 042–induced PMN transmigration.
The in vitro pro-inflammatory properties of AAFs revealed here are supported by previous studies showing that AAFs play a key role in triggering EAEC-induced basolateral IL-8 secretion from intestinal epithelial cells and in promoting epithelial barrier disruption [11, 12]. Taken together, these findings suggest a key role for AAFs in triggering host signaling cascades underlying directed migration of PMNs toward and across epithelial mucosal surfaces.
It has previously been demonstrated that human intestinal tissues are susceptible to EAEC pathogenesis with use of an in vitro organ culture model [37]. Here, we established an in vivo EAEC infection model using chimeric SCID-HU-INT mice, in which the intestinally derived cells of the implanted tissue were of human origin, and the inflammatory cells were of murine origin [20]. These mice provide an opportunity to investigate molecular aspects of EAEC interactions with the human mucosa and, moreover, enable investigation of the functions of specific virulence factors in the native tissue. Distinguishing them from the native intestinal tract, the xenografts in this model are devoid of a normal microflora, likely resulting in more severe observed pathological effects of infection than would be expected in the native tissue. However, the lack of microflora in this model also offers the obvious advantage that the observed effects are directly related to the pathogen examined.
Using the SCID-HU-INT mouse model, we were able to reproduce the in vitro inflammatory effects of EAEC. We found that infection with wild-type 042 was associated with significant damage and inflammation in the xenografted intestinal tissue. In contrast, infection with an 042 AAF/II mutant strain caused significantly less PMN migration, compared with wild-type 042, suggesting a requirement of adherence to the mucosal surface by EAEC to elicit inflammation in vivo. The isogenic 042 AAF/II mutant strain did, however, cause tissue damage almost to the same extent as wild-type 042, which is likely attributable to toxin release. For instance, 042 expresses the serine protease autotransporter of Enterobacteriaceae (SPATE) Pet, which causes cytotoxic effects on human intestinal mucosa [37]. In the normal gastrointestinal tract, the lack of mucosal adherence would likely lead to flushing of this AAF/II mutant strain. However, the absence of peristalsis in this closed-loop model offers an explanation as to why toxins, such as Pet, may remain present and contribute to tissue damage of the xenografts, regardless of whether the pathogen is capable of mucosal adherence.
Of interest, although HB101 did not cause significant signs of pathology in the xenografts, infection with HB101 carrying the AAF/II-encoding plasmid caused PMN infiltration to the same extent as wild-type 042. This is in contrast with our findings from the in vitro model of PMN transmigration and could be a reflection of the host-pathogen–related physiological differences between these 2 model systems. For instance, environmental factors present in the xenografted tissue (eg, components of the mucus layer) may induce and favor AggR-regulated expression of AAF/II-like structures throughout the 24-hour infection period [10]. In comparison, any inflammatory response in our in vitro model would likely require expression of AAF/II-like structures in the bacterial culture before infection, which may be a hindering factor for this particular plasmid. Additional studies are needed to examine the structure and functionality of the proteins expressed through our AAF/II-encoding plasmid.
In addition to triggering PMN infiltration of the xenografted tissue, HB101 carrying the AAF/II-encoding plasmid caused significant tissue damage, compared with wild-type HB101. This observation likely reflects effects of the innate immune response on the intestinal mucosa (eg, neutrophil-derived elastases and other proteases causing damage to the tight junction barrier) [38]. Moreover, substantial loss of the intestinal barrier by induced apoptosis may results from sustained PMN transmigration [39]. Alternatively, the AAF organelle may be capable of inducing epithelial barrier disruption [12].
The crucial need for an increased understanding of the host-pathogen interactions underlying EAEC pathogenesis is emphasized by the recent major German outbreak caused by a Shiga toxin–producing EAEC strain expressing AAF/I and the SPATEs Pic, SepA, and SigA [3, 40]. A much larger percentage of ascertained cases resulted in hemolytic-uremic syndrome than was observed during outbreaks caused by other Shiga toxin–producing E. coli strains, suggesting that the EAEC background provides greater toxin delivery [3]. It is tempting to speculate that AAF-mediated host inflammatory responses play a key role in this regard, stressing the need for testing of this strain and future outbreak strains in our model systems.
In conclusion, we have established a key role for AAFs in mediating EAEC-induced PMN transmigration in vitro and recapitulated these findings in vivo with use of SCID-HU-INT mice, thus substantiating the physiological relevance of AAFs for inflammatory aspects of EAEC pathogenesis. Moreover, the implementation of the SCID-HU-INT mouse model provides an opportunity to study other aspects of EAEC pathogenesis, thereby potentially enhancing our understanding of this important pathogen.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr. Hai Ning Shi, for technical advice, expertise, and assistance with infection of the xenografts; The Xenograft Core Facility, Massachusetts General Hospital, for preparation of xenografts; Steen Stahlhut, for kind guidance in immunostaining of bacteria; and Nadia Boisen, for performing scanning electron microscopy.
Financial support. This work was supported by Danish Council for Strategic Research (grant 2101-07-0023 to K.A.K.), National Institutes of Health (grants DK56754 and DK33506 to B.A.Mc.C. and AI33096 to J.P.N.), and the Crohn's and Colitis Association of America (to B.A.Mc.C.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Harrington SM, Dudley EG, Nataro JP. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol Lett. 2006;254:12–8. doi: 10.1111/j.1574-6968.2005.00005.x. [DOI] [PubMed] [Google Scholar]
- 2.Itoh Y, Nagano I, Kunishima M, Ezaki T. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J Clin Microbiol. 1997;35:2546–50. doi: 10.1128/jcm.35.10.2546-2550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasko DA, Webster DR, Sahl JW, et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med. 2011;365:709–17. doi: 10.1056/NEJMoa1106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nataro JP, Deng YK, Maneval DR, German AL, Martin WC, Levine MM. Aggregative adherence fimbriae-I of enteroaggregative Escherichia coli mediate adherence to Hep-2 cells and hemagglutination of human erythrocytes. Infect Immun. 1992;60:2297–304. doi: 10.1128/iai.60.6.2297-2304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czeczulin JR, Balepur S, Hicks S, et al. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect Immun. 1997;65:4135–45. doi: 10.1128/iai.65.10.4135-4145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernier C, Gounon P, Le Bouguenec C. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-Encoding operon family. Infect Immun. 2002;70:4302–11. doi: 10.1128/IAI.70.8.4302-4311.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boisen N, Struve C, Scheutz F, Krogfelt KA, Nataro JP. New adhesin of enteroaggregative Escherichia coli related to the Afa/Dr/AAF family. Infect Immun. 2008;76:3281–92. doi: 10.1128/IAI.01646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nataro JP, Deng YK, Walker K. Aggr, a transcriptional activator of aggregative adherence fimbria-I expression in enteroaggregative Escherichia coli. J Bacteriol. 1994;176:4691–9. doi: 10.1128/jb.176.15.4691-4699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley EG, Thomson NR, Parkhill J, Morin NP, Nataro JP. Proteomic and microarray characterization of the AggR regulon identifies a pheU pathogenicity island in enteroaggregative Escherichia coli. Mol Microbiol. 2006;61:1267–82. doi: 10.1111/j.1365-2958.2006.05281.x. [DOI] [PubMed] [Google Scholar]
- 10.Sheikh J, Hicks S, Dall'Agnol M, Phillips AD, Nataro JP. Roles for Fis and YafK in biofilm formation by enteroaggregative Escherichia coli. Mol Microbiol. 2001;41:983–97. doi: 10.1046/j.1365-2958.2001.02512.x. [DOI] [PubMed] [Google Scholar]
- 11.Harrington SM, Strauman MC, Abe CM, Nataro JP. Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell Microbiol. 2005;7:1565–78. doi: 10.1111/j.1462-5822.2005.00588.x. [DOI] [PubMed] [Google Scholar]
- 12.Strauman MC, Harper JM, Harrington SM, Boll EJ, Nataro JP. Enteroaggregative Escherichia coli disrupts epithelial cell tight junctions. Infect Immun. 2010;78:4958–64. doi: 10.1128/IAI.00580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köhler H, Sakaguchi T, Hurley BP, Kase BJ, Reinecker HC, McCormick BA. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am J Physiol Gastrointest Liver Physiol. 2007;293:G178–87. doi: 10.1152/ajpgi.00535.2006. [DOI] [PubMed] [Google Scholar]
- 14.Boll EJ, Struve C, Sander A, Demma Z, Krogfelt KA, McCormick BA. Enteroaggregative Escherichia coli promotes transepithelial migration of neutrophils through a conserved 12-lipoxygenase pathway. Cell Microbiol. 2012;14:120–32. doi: 10.1111/j.1462-5822.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mumy KL, Bien JD, Pazos MA, Gronert K, Hurley BP, McCormick BA. Distinct isoforms of phospholipase A(2) mediate the ability of Salmonella enterica serotype Typhimurium and Shigella flexneri to induce the transepithelial migration of neutrophils. Infect Immun. 2008;76:3614–27. doi: 10.1128/IAI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pazos M, Siccardi D, Mumy KL, et al. Multidrug resistance-associated transporter 2 regulates mucosal inflammation by facilitating the synthesis of hepoxilin A(3) J Immunol. 2008;181:8044–52. doi: 10.4049/jimmunol.181.11.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington SM, Sheikh J, Henderson IR, Ruiz-Perez F, Cohen PS, Nataro JP. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect Immun. 2009;77:2465–73. doi: 10.1128/IAI.01494-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang G, Pulimood AB, Mathan MM, Mathan VI. Enteroaggregative Escherichia coli infection in a rabbit model. Pathology. 2001;33:341–6. [PubMed] [Google Scholar]
- 19.Tzipori S, Montanaro J, Robinsbrowne RM, Vial P, Gibson R, Levine MM. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect Immun. 1992;60:5302–6. doi: 10.1128/iai.60.12.5302-5306.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savidge TC, Morey AL, Ferguson DJP, Fleming KA, Shmakov AN, Philips AD. Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation. 1995;58:361–71. doi: 10.1046/j.1432-0436.1995.5850361.x. [DOI] [PubMed] [Google Scholar]
- 21.Seydel KB, Li E, Swanson PE, Stanley SL. Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–9. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertelsen LS, Paesold G, Eckmann L, Barrett KE. Salmonella infection induces a hypersecretory phenotype in human intestinal xenografts by inducing cyclooxygenase 2. Infect Immun. 2003;71:2102–9. doi: 10.1128/IAI.71.4.2102-2109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Jin LL, Champion G, Seydel KB, Stanley SL. Shigella infection in a SCID mouse-human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infect Immun. 2001;69:3240–7. doi: 10.1128/IAI.69.5.3240-3247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golan L, Gonen E, Yagel S, Rosenshine I, Shpigel NY. Enterohemorrhagic Escherichia coli induce attaching and effacing lesions and hemorrhagic colitis in human and bovine intestinal xenograft models. Dis Model Mech. 2011;4:86–94. doi: 10.1242/dmm.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nataro JP, Baldini MM, Kaper JB, Black RE, Bravo N, Levine MM. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis. 1985;152:560–5. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- 26.Elias WP, Czeczulin JR, Henderson IR, Trabulsi LR, Nataro JP. Organization of biogenesis genes for aggregative adherence fimbria II defines a virulence gene cluster in enteroaggregative Escherichia coli. J Bacteriol. 1999;181:1779–85. doi: 10.1128/jb.181.6.1779-1785.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheikh J, Czeczulin JR, Harrington S, et al. A novel dispersin protein in enteroaggregative Escherichia coli. J Clin Invest. 2002;110:1329–37. doi: 10.1172/JCI16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steiner TS, Nataro JP, Poteet-Smith CE, Smith JA, Guerrant RL. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J Clin Invest. 2000;105:1769–77. doi: 10.1172/JCI8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nataro JP, Deng YK, Cookson S, et al. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis. 1995;171:465–8. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 30.Olesen B, Neimann J, Bottiger B, et al. Etiology of diarrhea in young children in Denmark: a case-control study. J Clin Microbiol. 2005;43:3636–41. doi: 10.1128/JCM.43.8.3636-3641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine MM, Nalin DR, Hornick RB, et al. Escherichia coli strains that cause diarrhea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;1:1119–22. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 32.Boyer HW, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–72. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 33.Struve C, Bojer M, Krogfelt KA. Characterization of Klebsiella pneumoniae type 1 fimbriae by detection of phase variation during colonization and infection and impact on virulence. Infect Immun. 2008;76:4055–65. doi: 10.1128/IAI.00494-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CC, Louie S, Shi HN, Walker WA. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr Res. 2005;58:1185–91. doi: 10.1203/01.pdr.0000183660.39116.83. [DOI] [PubMed] [Google Scholar]
- 35.Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn's disease in mice. Gastroenterology. 2001;121:1428–36. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 36.Loher F, Schmall K, Freytag P, et al. The specific type-4 phosphodiesterase inhibitor mesopram alleviates experimental colitis in mice. J Pharmacol Exp Ther. 2003;305:549–56. doi: 10.1124/jpet.102.039529. [DOI] [PubMed] [Google Scholar]
- 37.Henderson IR, Hicks S, Navarro-Garcia F, Elias WP, Philips AD, Nataro JP. Involvement of the enteroaggregative Escherichia coli plasmid-encoded toxin in causing human intestinal damage. Infect Immun. 1999;67:5338–44. doi: 10.1128/iai.67.10.5338-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginzberg HH, Cherapanov V, Dong Q, et al. Neutrophil-mediated epithelial injury during transmigration: role of elastase. Am J Physiol Gastrointest Liver Physiol. 2001;281:G705–17. doi: 10.1152/ajpgi.2001.281.3.G705. [DOI] [PubMed] [Google Scholar]
- 39.Le'Negrate G, Selva E, Auberger P, Rossi B, Hofman P. Sustained polymorphonuclear leukocyte transmigration induces apoptosis in T84 intestinal epithelial cells. J Cell Biol. 2000;150:1479–88. doi: 10.1083/jcb.150.6.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheutz F, Nielsen EM, Frimodt-Moller J, et al. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104:H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Eurosurveillance. 2011;16:5–10. doi: 10.2807/ese.16.24.19889-en. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.