Abstract
Recent studies indicate that single-action-single-target agents are unlikely to cure central nervous system (CNS) disorders sharing a pathogenic triad consisting of vascular damage, neuronal injury/neurodegeneration and neuroinflammation. Here, we focus on a recent example of a multiple-action-multiple-target approach for CNS disorders based on newly discovered biological properties of activated protein C (APC), an endogenous plasma protease with antithrombotic, cytoprotective and anti-inflammatory activities in the CNS. We propose that APC-mediated signaling through the protease activated receptor-1 (PAR1) may favorably regulate multiple pathways within the neurovascular unit in non-neuronal cells and neurons during acute or chronic CNS insults, leading to stabilization of the blood-brain barrier, neuroprotection and control of neuroinflammation. Although much remains to be understood regarding the biology of APC, preclinical studies suggest that it has promising applications as disease-modifying therapy for ischemic stroke and other neuropathologies whose underlying pathology involves deficits in the vasculo-neuronal-inflammatory triad.
Introduction
Recent preclinical and clinical studies have revealed that stroke and many neurological disorders share a common pathogenic triad which includes: (i) vascular damage, (ii) neuronal injury and/or neurodegeneration, and (iii) the neuroinflammatory response (reviewed in 1-4). Moreover, it has became increasingly recognized that, in addition to neurons, different cell types comprising the neurovascular unit, i.e., vascular cells (endothelium, pericytes, vascular smooth muscle cells) and glia (astrocytes, microglia, oligodendroglia), may contribute to brain damage after an acute ischemic stroke (1,2) and/or during a chronic neurodegenerative process (3-6).
Despite years of research and pioneering clinical work, stroke remains a major public health concern. The current research in stroke has been directed at developing rational approaches for neuronal protection (1), advancing the existing acute reperfusion therapies (7,8), advancing various devices and strategies for mechanical revascularization (9), and developing treatments aimed to promote regeneration of brain tissue damaged by ischemia (10,11). Although the various concepts of neuroprotection are based on a sound scientific principle and data from preclinical studies, none of neuroprotectants has been successfully translated into a stroke therapy in humans (12). By analyzing methodological quality and efficacy of 1,026 stroke drugs tested in over 8,500 experiments in 3,500 publications, O’Collins et al. determined that only 5 drugs met the STAIR (Stroke Therapy Academic Industry Roundtable) criteria for drug development for stroke (13). We have also learned that adherence to the STAIR criteria does not guarantee success in clinical application in humans.
A recent failure of the Stroke-Acute Ischemic NXY Treatment (SAINT) II trial evaluating the free radical scavenger NXY-059 in acute ischemic stroke (14) has prompted discussions about the quality of preclinical and clinical stroke studies (12). Most experts generally agree that there is no evidence to suggest a biological barrier to translating stroke research from animals to humans, but the quality of preclinical research has been identified as a potential problem. It has been also argued that clinical trials may have underestimated the sample size needed to show an effect based on conventional outcome scales (15). Moreover, computed tomography and magnetic resonance imaging of infarct volumes fail to show the expected correlation with clinical outcomes (16-18) rendering these imaging biomarkers unacceptable to regulatory agencies as validated end points sufficient to grant approval of a neuroprotectant drug.
As a solution to these problems, it has been recommended that a candidate drug even before testing in animal models should have a clearly defined molecular mechanism of action, a valid molecular target, an acceptable toxicity profile, appropriate pharmacokinetics and pharmacodynamics, and a demonstrated ability to cross the blood-brain barrier (BBB) (19). As a subsequent step, a rigorous testing of neuroprotectants in animal models should follow a roadmap established by the STAIR committee (20,21).
Preclinical and clinical studies have clearly shown that so-called single-action-single-target agents are unlikely to provide a cure for stroke or for a variety of neurologic disorders (see Box 1). In this review, we focus on a recent example of a multiple-action-multiple-target approach for stroke and some central nervous system (CNS) disorders based on newly discovered biological properties of activated protein C (APC), an endogenous plasma protease with antithrombotic, cytoprotective, and anti-inflammatory activities in the brain and spinal cord. We discuss the cellular and molecular mechanisms as to why the APC approach could potentially offer a disease-modifying therapy for ischemic stroke and some neurological conditions sharing a vasculo-neuronal-inflammatory triad. We also review multiple pathological signaling pathways within injured non-neuronal cells and neurons that are regulated by APC. The beneficial effects of protein C and APC in patients and preclinical animal models of stroke and other chronic CNS disorders, as well as the potential serious side effects of APC are discussed. Furthermore, we examine the STAIR criteria for APC in relation to ischemic stroke, and discuss future research directions aimed at minimizing some of the current known side-effects of APC, in order to enhance the potential therapeutic benefits.
Vasculo-neuronal-inflammatory triad
Although many investigators of neurological disorders have assumed that damage within neuronal cells is the sole contributor to disease initiation and progression, recent studies have demonstrated that damage to the neurovascular unit, including BBB disruption, brain hypoperfusion and neuroinflammatory responses involving the activation of microglia and astrocytes, critically determine the magnitude of neuronal injury and loss after an acute ischemic stroke (1,2). Damage to the neurovascular unit also influences onset and/or progression of neurodegenerative processes in diseases such as Alzheimer’s disease (AD) (3), AD-related dementias (4) and amyotrophic lateral sclerosis (ALS) (6), to name a few. Hence, the term vasculo-neuronal-inflammatory triad has been coined to indicate that a pathological triad consisting of (1) vascular damage, (2) neuronal injury and/or neurodegeneration and (3) neuroinflammation is present in stroke and in several other neurological disorders at different stages of the disease process. In general support of this paradigm, large population-based epidemiological, neuroimaging, pathological and experimental studies have demonstrated that reductions in brain microcirculation and BBB dysfunction may precede cognitive decline in age-related dementias including AD, Parkinson’s disease and/or vascular dementia (reviewed in 2, 22). Recent experimental studies using an animal model of ALS that expresses mutant superoxide dismutase 1 (SOD1)have demonstrated that microglia and astrocytes, but not a primary injury to neuronal cells, drive disease progression (23,24), while the BBB disruption causing microhemorrhages is critical for disease initiation (25). Moreover, recent studies using different transgenic pericyte-deficient mouse lines have shown that degeneration of brain pericytes disrupts the BBB (26,27), which either alone or in combination with perfusion stress can lead to a secondary vascular-mediated neurodegeneration (27).
Based on the vasculo-neuronal-inflammatory paradigm for neurological injury, it should be conceivable to develop and evaluate future therapeutic strategies based on multiple-action-multiple-target agents that are directed at controlling vascular, neuronal and inflammatory components of the disease process. Agents with more than one action on more than one target have the potential for superior therapeutic effects in ischemic stroke and other CNS disorders compared to approaches involving only one action that is aimed at only one target (e.g., neurons).
Biology of Protein C and Activated Protein C
Protein C is a plasma zymogen of an active serine protease designated activated protein C (APC) which exerts potent anticoagulant activity (28). In addition to antithrombotic activity, APC can initiate cellular signaling via multiple receptors on a variety of cells including different cell types within the neurovascular unit (see below; 28-33).
Protein C is synthesized principally in the liver as a polypeptide precursor of 461 residues which has a 42 residue prepropeptide that signals for carboxylation of glutamic acid residues by a gamma-carboxylase which generates 9 γ-carboxyglutamic acid (Gla) residues in the mature glycoprotein containing 419 residues (28). Protein C mRNA is detectable at low levels in brain (34). The exact function of endogenous protein C in the CNS is still poorly understood. Protein C, APC and some APC analogs with reduced anticoagulant action can cross the BBB to achieve therapeutically effective concentrations in the CNS (35). The ability of a neuroprotectant drug to get access to the CNS is considered from a translational medicine perspective as an important step often missing in the STAIR profile of many tested stroke drugs (19). Transport of APC across the BBB is mediated by the endothelial protein C receptor (EPCR) (35). This transport pathway has been utilized for delivery of different forms of APC therapy to the brain and spinal cord in rodents (35-37).
The mature 62,000 Mr human protein C is cleaved by a furin-like endoprotease that releases Lys156-Arg157 prior to secretion from liver cells and it is thus a two-chain zymogen (Box 2). Circulating plasma protein C is a heterodimer comprised of a heavy and a light chain. The light chain has three separately folded domains, the N-terminal Gla domain and two epidermal growth-factor-like domains, EGF1 and EGF2 (Box 2). The heavy chain contains a serine protease zymogen homologous to other trypsin-like protease zymogens such as the prototype, chymotrypsinogen. Following proteolytic activation of protein C by thrombin (Figure 1), APC provides anticoagulant activity by highly specific protein-protein interactions with factors Va and VIIIa followed by cleavage of these coagulation cofactors at only a few Arg-containing peptide bonds. The stereo-specific interactions of APC with factors Va and VIIIa involve both the APC enzymatic active site region and also APC residues that are not part of the immediate APC active site. These residues are termed “exosites” on the APC active enzyme surface and can be mutated (eg. as in the 3K3A-APC and 5A-APC variants; Box 2) to diminish APC’s anticoagulant activity without altering APC’s cell signaling activity (38-41). The significance of such APC engineered mutations is that they provide APC variants for therapeutic purposes in which the risk of serious bleeding caused by APC’s anticoagulant activity is diminished while the cytoprotective activities of APC’s direct effects on cells and its pharmacologic benefits are preserved. In preclinical animal models of ALS (36), stroke (42,43), brain injury (44) and sepsis (45), these APC variants showed beneficial effects that were equivalent to, or sometimes greater than, the wild type (wt) recombinant APC (wt-APC). An APC variant with minimal cell signaling activity but with substantially increased anticoagulant activity, E149A-APC, has been useful for proof of concept studies and for antithrombotic indications (46). E149A-APC has superior antithrombotic activity in a mouse model of arterial thrombosis compared to wt-APC, but has no benefit in an endotoxemia sepsis model where wt-APC or the 5A-APC variant reduced mortality (45,46).
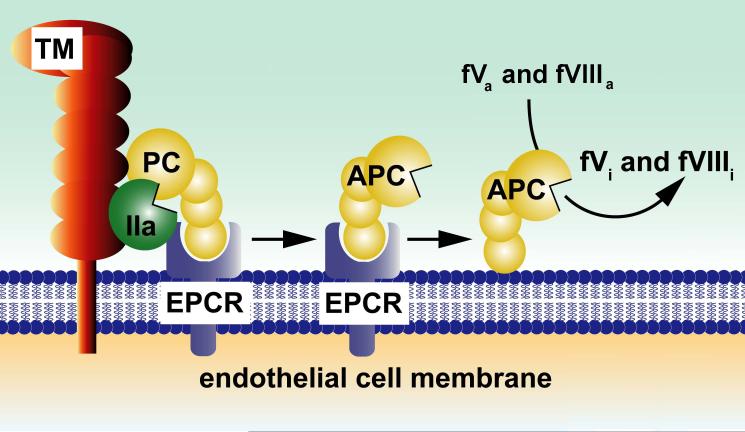
Figure 1. Pathways for protein C activation and expression of anticoagulant activity.
The endothelial cell receptors, thrombomodulin (TM) and EPCR, are required for efficient activation of protein C by alpha-thrombin (IIa). Dissociation of APC from EPCR allows for the expression of APC’s anticoagulant activity, whereas retention of APC bound to EPCR allows APC to express multiple direct cellular activities in the endothelium (see Figure 3a). APC conveys its anticoagulant activity when bound to cell membrane surfaces by cleaving the activated cofactors Va (fVa) and VIIIa (fVIIIa) to yield the inactivated cofactors, fVi and fVIIIi. Various proteins such as plasma protein S and factor V, high density lipoprotein particles, lipids (e.g., negatively charged phosphatidyl serine and cardiolipin, phosphatidyl ethanolamine) and certain glycosphingolipids (e.g., glycosyl ceramide, lactosyl ceramide, etc) provide APC-cofactor activities that accelerate proteolysis of factors Va and VIIIa by APC (not shown). Figure adapted from a review article originally published by L. Mosnier, B. Zlokovic and J.H. Griffin (2007) The cytoprotective protein C pathway Blood, 109, 3161-3172 [28].
APC signaling within the neurovascular unit
APC can regulate multiple cell signaling pathways in the neurovascular unit (Figure 2), with the net effect of blocking various pathological pathways during an acute or chronic CNS insult. Generally, these multiple actions of APC result in a suppression of the vasculo-neuronal-inflammatory triad’s pathological responses. Studies show that APC and its cell-signaling analogs exert direct vasculo-protective effects in the ischemic brain endothelium (47-51) and protect the BBB integrity (52,53), preventing accumulation in the CNS of serum proteins and reducing vasculotoxic and neurotoxic blood-derived deposits (e.g., fibrin, hemosiderin, thrombin, plasmin) (25,27,36). Moreover, APC and its analogs can cross an intact BBB (35-37) and exert in parallel direct neuronal protective effects (36,42,55). By inhibiting transport of leukocytes across the BBB (56) and by suppressing microglia activation (36), APC therapy also exerts significant anti-inflammatory activities. These multiple cell-signaling effects of APC on various cell types within the neurovascular unit are discussed below.
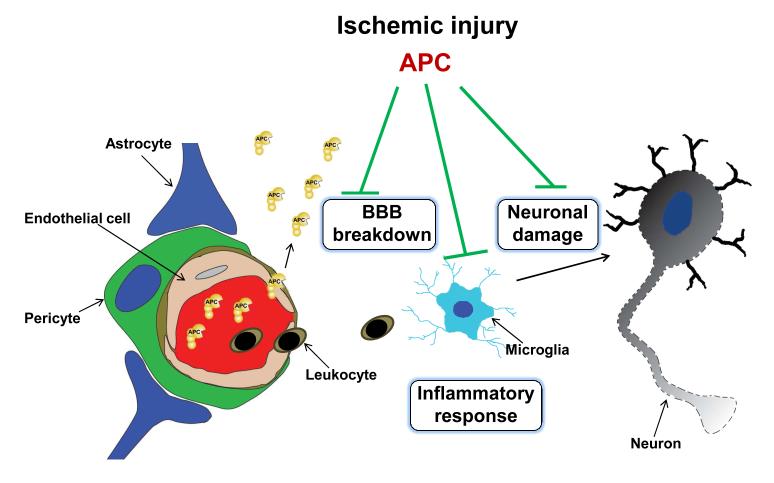
Figure 2. Control of ischemic injury to the neurovascular unit by APC and/or its cell-signaling analogs.
APC protects the BBB integrity and ameliorates post-ischemic BBB breakdown thus preventing secondary neuronal damage mediated by entry of several blood-derived neurotoxic and vasculotoxic molecules. It crosses an intact BBB via EPCR-dependent transport to reach its neuronal targets in brain and expresses its direct neuronal protective activity to prevent neuronal damage. APC also expresses anti-inflammatory activities by blocking early post-ischemic infiltration of brain by neutrophils. It also suppresses microglia activation. The key membrane signaling receptor mediating beneficial effects of APC on different cell types within the neurovascular unit is PAR1, as illustrated in Figure 3.
Effects on endothelium: stabilization of the BBB and vasculoprotection
APC exerts pleiotropic cytoprotective activities in the endothelium, including downregulation of pro-apoptotic and pro-inflammatory mRNA transcripts (47,48), direct protection of cells from divergent inducers of apoptosis (49-51) and enhancement of the endothelial barriers (52,53). The main known substrate for the cytoprotective activity of APC in the endothelium is protease activated receptor 1 (PAR1). PAR1 was originally discovered as a receptor for thrombin (57). PAR1 and the other three PARs (i.e., PAR2, 3 and 4) are G-protein-coupled receptors (GPCRs) which are activated by proteolytic cleavage of an extracellular N-terminal tail that generates an intramolecular tethered ligand, which subsequently triggers intracellular signaling (58). PAR1 activation by APC and thrombin results in the activation of distinct intracellular signaling cascades (59). A specific exosite on APC that is required for normal APC signaling on cells via PAR1 has been indentified (60), enabling genetic manipulations of APC’s cytoprotective activity.
APC enhances the endothelial barrier via EPCR-dependent PAR1-mediated activation of sphingosine kinase-1 (Figure 3a), which generates sphingosine-1-phosphate (S1P) (52,53), a biologically active sphingolipid that signals via GPCRs belonging to the endothelial differentiation gene family (61). S1P in turn cross-activates sphingosine-1-phosphate receptor-1 (S1P1) resulting in the activation of Rac, a member of the Rho family of GTPases, and the rearrangement of the cellular cytoskeleton, which subsequently leads to enhancement of the endothelial barrier (52,53). APC also downregulates nuclear translocation of nuclear factor kappa B (NF-κB), resulting in blockade of NF-κB-dependent activation of matrix metalloproteinase-9 (MMP9) which, in turn, reduces post-ischemic degradation by this proteinase of the basement membrane proteins of the BBB and the associated intracerebral bleeding, an effect induced by tissue plasminogen activator (tPA) (54) (Figure 3a right). By suppressing NF-κB-dependent expression of several pro-inflammatory cytokines [e.g., tumor necrosis factor-α (TNFα), interleukin 1β, interleukin-6] and adhesion molecules (47), APC exerts an in vivo anti-inflammatory activity which blocks post-ischemic brain infiltration by neutrophils (56).
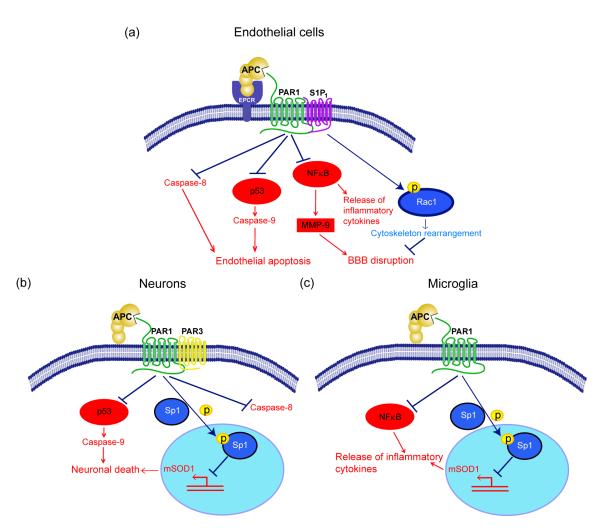
Figure 3. APC protective signaling activities in different cell types within the neurovascular unit.
a. BBB ‘sealing’ effect and direct vasculoprotection: Beneficial cytoprotective activities of APC and its cytoprotective-selective variants involve direct effects on endothelial cells that require the cellular receptors EPCR and PAR-1 and EPCR-dependent PAR1-mediated cross-activation of sphingosine 1-phosphate receptor 1 (S1P1). Cross-activation of S1P1 activates Rac1 leading to Rac1-dependent stabilization of the cytoskeleton, which results in enhanced integrity of the endothelial membranes (right). APC also suppresses NF-kB-dependent transcriptional activation of MMP-9 which in turn blocks degradation of the BBB basement membrane, thereby preventing intracerebral bleeding (middle right). By controlling Nf-κB nuclear translocation, APC blocks expression of pro-inflammatory cytokines, thereby limiting neuroinflammation (middle left). By activating PAR-1 in a EPCR-dependent manner, APC suppresses the pro-apoptotic p53 transcription factor and p53-dependent transcription of Bax (not shown) and directly upregulates anti-apoptotic Bcl-2 (not shown), which results in blockade of caspase-9 activation and controls the intrinsic apoptotic pathway (middle left). APC can also block the extrinsic apoptotic pathway and caspase-8 activation (left). b. Direct neuronal protection: APC and its cytoprotective cell signaling variants act through PAR-1 and PAR-3 to inhibit p53 activation in injured neurons resulting in blockade of caspase-9-dependent intrinsic apoptotic pathway (left) and caspase-8-dependent extrinsic apoptotic pathway (right). APC also blocks nuclear transport of the transcription factor, Sp1, through phosphorylation of cytoplasmic Sp1 resulting in transcriptional suppression of mutant SOD1 (mSOD1) expression in motor neurons in a mouse ALS model (middle). c. Anti-inflammatory activity: In SOD1 mutant mice, APC and its cell signaling variants downregulate mSOD1 expression though PAR-1 and the downstream mechanisms as noted in panel b. This suppresses microglia activation resulting in reduced number of activated microglia as well as blockade of inflammatory cytokine production from microglia (right). On the left is shown that APC blocks NF-κB activation in microglia providing another anti-inflammatory pathway mediated by a blockade of NF-kB-dependent transcriptional expression of different pro-inflammatory cytokines as noted above in a. for endothelial cells.
APC inhibits mitochondria-mediated caspase-9-dependent and death receptor-mediated caspase-8-dependent apoptotic pathways in brain endothelial cells, both in vitro and in vivo (49,54,62). APC upregulates antiapoptotic Bcl-2 genes (47,49) and suppresses the p53 and Bax pro-apoptotic pathway (49,62). These effects require the enzymatic active site of APC as well as EPCR and PAR1 (Figure 3a left). Reduction of brain endothelial apoptosis by APC in vivo is associated with vasculoprotection and neuroprotection after ischemic stroke (49,62). The 3K3A-APC variant with reduced anticoagulant activity appears to exert even greater vasculoprotection of ischemic brain endothelial cells than wt-APC (42,43). Wt-APC and its analogs with reduced anticoagulant activity can also prevent microhemorrhages and BBB disruption in the spinal cord of an animal model of ALS (36). These beneficial APC effects are likely mediated by APC’s barrier-enhancing properties and/or direct vasculoprotection.
APC stimulates angiogenesis from endothelial cells in vitro through EPCR and PAR1 (63) and promotes brain angiogenesis in vivo after an acute ischemic stroke (64) and traumatic brain injury (TBI) (65). APC-mediated post-ischemic brain angiogenesis results in an increased number of new replicating endothelial cells and the formation of new brain capillaries (64).
Direct neuronal protection
wt-APC and 3K3A-APC act via PAR1 and PAR3 on mouse cortical neurons to protect neuronal cells from glutamate excitotoxicity in vitro and from NMDA receptor-mediated excitotoxic brain lesions in vivo (36,42,55) (Figure 3b). APC can inhibit both the intrinsic, caspase-9-dependent and p53-mediated apoptotic pathways in injured neurons as well as the extrinsic, caspase-8-dependent apoptotic pathway in neurons in vitro and in vivo (36,42,55,62,66). Although EPCR was below detection limits in mouse neurons (36), it was detectable in rat hippocampal neurons, where it was required for neuronal protection mediated by PAR1 signaling(67).
Inhibition of SOD1 synthesis in neuronal cells by APC requires PAR1 and PAR3, and APC inhibits nuclear transport of the Sp1 transcription factor that acts as a positive regulator of SOD1 transcription (36). Both, wt-APC and variants of APC with reduced anticoagulant activity diminish mutant SOD1 synthesis in motor neurons in vivo, and this effect likely contributes to the beneficial effects of APC therapy in mouse models of ALS that express mutant SOD1 (36).
APC treatment stimulates post-ischemic neurogenesis if initiated during the repair phase of ischemic stroke, for example when given at 72 h after transient brain ischemia in rodents (64). The pro-neurogenic effects of APC are independent of its direct neuronal protective activity and are mediated through increased proliferation of neuronal progenitor cells in the subventricular zone (SVZ) and through increased migration of neuroblasts from the SVZ towards the ischemic border (64). Similar to other neuroprotective actions of APC, these pro-neurogenic effects require PAR1 (64). APC treatment also promotes neurogenesis after an acute TBI (65).
APC Anti-inflammatory activities
The multiple anti-inflammatory effects of APC include its vascular effects on endothelial cells and its effects on leucocytes (e.g., neutrophils, monocytes) and microglia. APC anti-inflammatory effects on endothelial cells include the inhibition of the release of inflammatory mediators, combined with down-regulation of the expression and release of vascular adhesion molecules. This results in a reduction of leukocyte adhesion and infiltration of tissues, thereby limiting damage to underlying tissue (Figure 3a).
In a mouse model of focal ischemic stroke, APC was shown to downregulate adhesion molecules at the BBB, which blocked the transport of neutrophils from the blood to the brain, thereby limiting neutrophil-induced post-ischemic brain damage (56). In a rat model of compression-induced spinal cord injury (SCI), APC reduces neutrophil accumulation and cytokine levels (e.g., TNFα) and improves motor outcome (68).
APC reduces the chemotactic effects of several potent chemotactic agents including E. coli-derived lipopolysaccharide (LPS) on leukocytes and neutrophils (69,70). By diminishing cytokine release from leukocytes, APC attenuates initiation of systemic inflammatory responses and can alleviate the ‘cytokine storm” that is associated with sepsis and LPS-induced production of pro-inflammatory mediators by monocytes (28). Although the exact mechanisms for APC’s anti-inflammatory effects on leukocytes and microglia are not completely clear, most studies suggest that APC therapy can reduce the expression of pro-inflammatory cytokines in monocytes and microglia via PAR1-mediated downregulation of NF-kB (28,47) (Figure 3c). The anti-inflammatory actions of APC may also involve its interactions with integrins (71) or its direct effects on dendritic-type cells mediated by EPCR (72).
APC therapy for stroke and clinical implications
Several preclinical studies using different types of rodent models of stroke have demonstrated beneficial effects on neuropathological and neurological outcomes for APC and for APC mutants with reduced anticoagulant activity, as discussed in a greater detail below. Recent research on the biology of APC has established APC’spleiotropic activities, especially its ability to have multiple effects on different cell types within the neurovascular unit. Thus, in contrast to many previously studied single-action-single-target agents for stroke, APC is a candidate as a multiple-action-multiple-target agent for stroke. Although not observed in preclinical studies of neurological injury models, clinical trials of wt-APC showed that continuous APC infusion over four days increased the incidence of serious bleeding in sepsis patients (73,74), raising a serious concern about potential bleeding risks for APC-based therapies. Subsequent efforts have thus been focused on the development of APC mutants with reduced anticoagulant activity, in the hope that such a strategy might address the risks for serious bleeding associated with current APC therapies. Below, we will briefly discuss the current uses of APC and protein C in patients, and elaborate in a greater detail on the potential of APC as a stand-alone therapy for stroke, as well as its use as a combination therapy together with recombinant tPA.
The current FDA-approved indication for purified plasma-derived protein C concentrate is as a replacement therapy for humans who have hereditary protein C severe deficiency (see review 28). As this is a rare condition, there are currently limited data available regarding this therapy. However, there is a large amount of clinical experience with the use of a recombinant version of wt-APC that has mildly increased anticoagulant activity (DrotAA) relative to plasma-derived APC and which is FDA-approved for adult patients with severe sepsis (73). Over 9,000 adults with severe sepsis have been enrolled in multiple different clinical studies of APC therapy (reviewed in [74]). Two such clinical trials have demonstrated that wt-APC reduced mortality in severe sepsis patients who have a high risk of death, whereas other trials failed to show that APC reduced mortality in adult or pediatric patients with sepsis and a lower risk of death. Notably, the major complication of wt-APC infusion therapy (over four days) is serious bleeding, including an increased risk for intracranial hemorrhage (73,74). To date, no studies have compared bleeding risks for infusion APC therapy versus APC bolus dosing therapies, which have been more commonly used for animal preclinical studies [36,42-46,49,54,56,62,64,65,68-72,75-78,88-90]. Nonetheless, this very serious bleeding risk complication might limit wt-APC use in patients with stroke or other neurological disorders, as discussed below.
APC therapy is neuroprotective in mice and rat models of transient brain ischemia (49,54,56,62,64), permanent distal middle cerebral artery occlusion (MCAO) (42,43), embolic stroke (75) and neonatal hypoxic/ischemic brain injury (76). According to the recommended STAIR criteria and a modified scoring STAIR system with a focus on the scope of testing across experimental models from 0 to 10 (with 10 being the highest beneficial score) (13), the STAIR quality score for APC is 8 (Table 1). For comparison, recombinant tPA has a score of 9 (13). Moreover, only about 20% of all drugs that have been developed so far for stroke trials in humans have had scores of 8 or higher on this experimental scale (13). Although the criteria given in Table 1 largely reflect the STAIR recommendations (20,21) some departures have also been made, as suggested (13). For example, testing in nonhuman primates was not included as a criterion in Table 1 because the superior validity of primate models is not well established and has not been included in STAIR analysis of other stroke drugs (13). It is of note that APC’s beneficial effects in nonhuman primates have been shown in models of sepsis (77) and arterial thrombosis (78). APC therapy with DrotAA is currently being tested in a phase I/IIa trial involving patients with acute ischemic stroke (clinical trial identifier NCT00533546; www.clinicaltrials.gov).
Table 1.
STAIR quality of APC using Experimental Stroke Scale Modified by O’Collins et al. (ref. 13)
| *STAIR criterion | Description | Score | Refs |
|---|---|---|---|
| Laboratory setting | Focal model tested in two or more laboratories | YES | 42,49,75,76 |
| Animal species | Focal model tested in two or more species | YES | 49,75,76 |
| Health of animals | Focal model tested in old or diseased animals | NO | |
| Sex of animals | Focal model tested in male and female animals | YES | 49,54,62 |
| Reperfusion | Tested in temporary and permanent models of focal ischemia | YES | 42,43,49,54,75 |
| Time window | Drug administered at least 1 hour after occlusion in focal model | YES | 42,43,64 |
| Dose response | Drug administered using at least two doses in focal model | YES | 49,54 |
| Route of delivery | Tested using a feasible mode of delivery (eg, not intracisternal or intraventricular, cortical transplant or graft only) |
YES | 42,43,54,64,75 |
| Endpoint | Both behavioral and histological outcome measured | YES | 42,43,54,64,65 |
| Long-term effect | Outcome measured at 4 weeks after occlusion in focal models | NO |
STAIR criteria are not given in order of priority
Based on preclinical studies, one can expect that when APC is administered as a neuroprotectant, the APC therapeutic window for stroke should be wider than that for tPA. For example, data in preclinical models suggests that APC therapy is neuroprotective even when given 12 h after permanent MCAO (42,43) or 24 h after a transient MCAO in mice (64). It has also been shown that the 3K3A-APC mutant provides significant advantages over recombinant wt-APC, including reduced risk for bleeding. This is especially the case when the different APCs were compared following their administration at later time points after stroke (42,43). In terms of possible future clinical trials involving APC therapies, we speculate that the current protocol involving recombinant wt-APC with a time window of 6 h (ie. clinical trial identifier NCT00533546; www.clinicaltrials.gov can potentially be extended with APC analogs with reduced anticoagulant activity. Preclinical studies in rodent models of stroke (42,43,64) suggest that APC analogs with reduced anticoagulant activity might be able to be given to stroke patients using a multiple-dose bolus regimen beginning as late as 72 h after stroke. Clearly, these various speculative strategies for APC therapies that are based on animal models remain to be determined by the future clinical studies.
If the primary objective of APC therapy is to modify hemostasis through APC’s antithrombotic effects and prevent spreading of post-ischemic thrombosis (56), then APC molecules with either normal or increased anticoagulant activity (46) might be considered for administration within a narrow post-stroke therapeutic window (e.g., less than 4.5 h), comparable to that of tPA.
In terms of a combined tPA/APC therapy, it is clear that APC blocks tPA-mediated vascular and neuronal toxicities in a mouse model of ischemic stroke, as well as in models of ischemia in brain endothelium and neurons in vitro (62). APC inhibits tPA-induced caspase-8 activation of caspase-3 in brain endothelium and caspase-3-dependent nuclear translocation of apoptosis-inducing factor in NMDA-treated neurons, and reduces tPA-mediated cerebral ischemic injury in mice. On the other hand, it has been reported that tPA shifts the apoptotic signal in stressed brain cells (e.g. in endothelial cells as well as neurons) from the intrinsic to the extrinsic pathway, which requires caspase-8 (62). This demonstrates that APC blocks tPA neurovascular toxicity and suggests that APC may add substantially to the effectiveness of tPA therapy for stroke. Furthermore, APC therapy blocks tPA-mediated brain hemorrhage after transient brain ischemia in mice and embolic stroke in rats (54). The cellular mechanism for this has been demonstrated to be due to the inhibition of a pro-hemorrhagic tPA-induced, NF-κB-dependent MMP9 pathway in ischemic brain endothelium in vivo and in vitro by acting through PAR1 (54). This rodent stroke study clearly implies that APC may improve tPA thrombolytic therapy for stroke, at least in part, by reducing tPA-induced hemorrhage. APC may also help prevent vascular leakage by acting directly on endothelium to stabilize endothelial cell-cell junctions. Moreover, potential APC/tPA combination therapy using mutant APC variants with reduced anticoagulant activity might further extend the narrow temporal therapeutic window of tPA, increase the safety of tPA as a thrombolytic agent, and reduce the risk of tPA-mediated brain hemorrhage and neurotoxicity.
Plasmin is a direct-acting thrombolytic agent which has been claimed to have a much higher hemostatic safety level, compared to plasminogen activators such as tPA. Specifically,, plasmin has been reported to be well-tolerated without bleeding at several-fold higher amounts than those needed for thrombolysis in animal models of thrombosis (79,80), in contrast to plasminogen activators which risk bleeding at effective thrombolytic doses. A more detailed comparison between tPA and plasmin in a model of thrombin-induced MCAO in rabbits using angiographic documentation of vascular patency and recanalization (81) has indicated that plasmin delivered by an intra-arterial catheter induces early recanalization of MCAO within 10 min of administration and does not produce bleeding at up to 4-fold increased therapeutic dose, whereas tPA produces bleeding at all doses in proportion to its thrombolytic potential (82). Plasmin has been reported as being safe in a current clinical trial in patients with peripheral arterial or bypass graft occlusion (NCT00418483; www.clinicaltrials.gov). In addition, phase I/IIa clinical trials of plasmin in human MCA ischemic stroke have also been initiated (NCT01014975; www.clinicaltrials.gov). Of potential concern is that some studies in animal models have shown that if plasmin gets into the brain it can degrade neuronal laminin (an extracellular matrix protein), leading to neuronal injury and death due to detachment from the extracellular matrix (83). This could potentially be a limitation if a similar mechanism exits in humans in the presence of an open BBB, where plasmin could readily access the brain. Whether a combination therapy with APC analogs and plasmin might extend the therapeutic efficacy and/or temporal window of plasmin is currently unknown and might be considered in future preclinical studies or clinical trials.
Another possibility is combining APC with mechanical devices, such as, devices used for endovascular thrombectomy (eg. a flexible wire with coil loops that is used in conjunction with a microcatheter for intracranial clot retrieval), or devices used for mechanical thrombus disruption, as reviewed in detail elsewhere [9]. However, preclinical data comparing mechanical devices alone versus devices in combination with APC therapy are not currently available.
APC beneficial effects for other neurological and systemic disorders
Besides stroke, APC therapy has been shown to have favorable pharmacological effects for a number of different disorders and injuries throughout the body. There is good evidence that APC can exert significant protection in preclinical models of ischemia/reperfusion injury in heart (84), liver (85) and kidney (69). Furthermore, APC treatment is beneficial for inflammatory lung injury (70), reduces mortality caused by P. Aeruginosa in mice (86), and greatly reduces nephropathy in diabetic mice by inhibiting endothelial and podocytes apoptosis (87) The remainder of this section will focus on discussing neurological disorders for which APC therapies have been studied. Early studies had demonstrated that plasma-derived APC was neuroprotective in a rabbit model of compression-induced SCI (68). In this model, APC, but not inactive mutants of APC, reduced the amount of TNFα at the site of injury, which inhibited neutrophils accumulation in the spinal cord and ameliorated damage to the endothelial cells. This resulted in decreased nervous tissue injury and improved the motor neurological outcome [68]. APC was also protective in a rabbit model of ischemia/reperfusion-induced SCI through inhibition of neutrophil activation and suppression of TNFα expression in the injured spinal cord tissue (88,89). Although these studies indicated that the primary mechanism of APC action in SCI animal models of is suppression of leucocyte activation, the direct neuronal protective and vasculoprotective effects of APC cannot be excluded as contributory mechanisms.
More recent studies have shown that APC may hold a significant therapeutic potential for TBI. APC treatment improved functional outcome and was neuroprotective in a mouse model of TBI (65). In addition to its direct vasculoprotective and neuronal protective effects, APC increased post-traumatic angiogenesis and neurogenesis suggesting its potential as a brain restorative therapy. Moreover, a delayed treatment of mice subjected to TBI indicated that the 3K3A-APC analog with reduced anticoagulant activity has superior neuroprotective effects compared to recombinant wt-APC and was a safer therapy for TBI with no risk for bleeding (44).
Wt-APC and APC variants with reduced anticoagulant activity (i.e., 3K3A-APC, 5A-APC), but not proteolytically inactive APC, had strikingly beneficial disease-modifying effects in mouse models of ALS (36). Besides its well-documented direct neuronal protective and anti-inflammatory activities, APC preserved endothelial capillary integrity in SOD1 mutant mice and blocked the early appearance of microhemorrhages, which is likely related to a delayed disease onset. Importantly, APC therapy transcriptionally downregulated mutant SOD1 expression in several cell types within the neurovascular unit, including endothelial cells, neurons, microglia and astrocytes (36). APC-mediated downregulation of mutant SOD1 expression within motor neurons, microglia and astrocytes may represent a therapeutic approach aimed directly at disease mechanisms. With the recognition that accumulation of aberrant SOD1 species has been linked to sporadic ALS, strategies based on activation of the endogenous protein C cellular pathway within the neurovascular unit may also provide promising directions for treating patients with familial ALS and possibly also sporadic ALS (36). Because APC crosses the BBB and thus could be given with a simple peripheral administration, APC’s ability to slow ALS disease progression may offer therapeutic advantages for some forms of ALS compared to other SOD1 gene-silencing strategies, which typically require invasive surgery and delivery by direct infusion into the spinal cord (e.g., antisense DNA oligonucleotides or retroviral delivery of transcription-mediated shRNA).
A recent proteomic analysis of multiple sclerosis (MS) active lesions isolated by laser-capture microdissection from human brain autopsy samples identified protein C inhibitor as present in lesions, suggesting that suppression of the protein C pathway might contribute to MS (90). It was subsequently shown that daily APC treatments reduced disease severity in a mouse experimental autoimmune encephalomyelitis (EAE) model of MS, and suppressed Th1 and Th17 cytokines in astrocytes and immune cells (90). Furthermore, it was reported that both anticoagulant and cell-signaling activities of APC are required for optimal amelioration of the disease in this mouse model (90). Although one can envisage that APC anti-inflammatory activity might alter expression of adhesion molecules and chemokines expressed at the luminal membrane of the BBB and modulate chemokine GPCRs on leucocytes, thereby suppressing the immune response during EAE, it is less clear why the anticoagulant activity of APC was also required in this experimental model of MS.
In spite of favorable effects of chronic APC administration in experimental mouse models of MS and ALS, there may be a variety of potential side-effects of continuous therapy with APC in humans. Such side-effects remain unknown at this stage, but would need to be considered carefully in future studies.
Conclusions and future perspectives
For two decades following the discovery of human hereditary protein C deficiency linked to venous thrombosis and neonatal purpura fulminans (28), researchers assumed that the major and possibly only physiological role for plasma protein C and APC involved the downregulation o blood coagulation. This viewpoint stimulated the development and subsequent preclinical and clinical trials of protein C/APC for replacement therapy, as well as for antithrombotic therapy, the treatment of sepsis-related disseminated intravascular coagulation, and for the treatment of different forms of arterial thrombosis, including stroke (28). However, subsequent findings have since revealed that APC has anti-inflammatory activities that are independent of its anticoagulant action, and studies in the CNS have demonstrated that inhibition of neutrophil infiltration by APC diminishes tissue damage in animal models of SCI (68,88,89) and stroke (56).
More recent efforts to elucidate the mechanisms for the beneficial pharmacological actions of APC show that it exerts direct cell signaling effects in systemic and brain endothelium that mediate expression of antiapoptotic and anti-inflammatory pathways and suppression of of pro-apopoptotic and pro-inflammatory genes, through its interaction with EPCR and PAR1 (47-51). Specifically, in models of stroke, these receptors and pathways were shown to be critical in mediating APC’s beneficial effects (49). Another important discovery was that APC exerts direct neuronal protective effects in different in vivo and in vitro models of neuronal injury and that these effects require PAR1 and PAR3 (55). It is now evident that APC can positively modulate pathological signaling pathways in different injured cell types in that brain by mechanisms that go beyond its anticoagulant and anti-inflammatory actions.
In parallel to advances in APC biology and pharmacology, we have learned that the long-standing, prevailing “neurocentric” dogma which posits that damage within neuronal cells is the sole contributor to initiation and progression of many neurological disorders is inadequate. It is becoming evident that a newer concept of the neurovascular unit with different cell types contributing to CNS damage will enable greater insights and promote greater understanding of various neuropathologies (1-6, 23-27). It has became apparent that the CNS damaged not only by stroke but also by a chronic insult mounts a complex response reflecting the vasculo-neuronal-inflammatory triad that affects CNS injury. Given the role for this pathological triad and the fact that numerous single-target-single-action agents have failed in past clinical trials for stroke and other acute and chronic neurological disorders, such as SCI, TBI, AD, ALS and MS, to name a few, the field is logically moving in a direction of considering multiple-action-multiple-target agents, as outlined in this review using APC as one such example..
Although recent research on the biology of APC and the positive initial results obtained using APC administration in animal models of stroke, ALS, TBI and MS puts it in the spotlight as a potential therapeutic approach for these maladies, multiple obstacles must be overcome before APC therapy can be routinely used to treat patients with stroke and other CNS disorders. Among these, first is the fact that therapies that have been successful in animal models more often than not fail to be translated to humans for a variety of reasons, as discussed above. Furthermore, with respect to chronic neurodegenerative and autoimmune neurological disorders, it is of note that the side-effects of long-term therapy with any form of APC in humans are currently unknown and need to be carefully explored in future clinical trials. Second, the major complication of recombinant wt-APC therapy in clinical trials of APC for patients with severe sepsis is serious bleeding, including an increased risk for intracerebral bleeding (73,74). APC variants with reduced anticoagulant activity may help reduce this risk. Third, the absence of a demonstrable benefit of recombinant wt-APC on mortality in adult patients with sepsis with a low risk of death and in pediatric sepsis patients implies that the currently used APC sepsis therapeutic regimen likely has significant limitations for other indications (74). The FDA-approved protocol limits APC therapy to four days of relatively low dose continuous infusion of APC because the drug may increase risk of bleeding complications. To date, there are no clinical trials involving multidose bolus therapies using APC in humans, although this has been very successful in animals. Taken together, a significant number of additional clinical studies are needed to improve APC dosing regimens and to determine which APC analogs are most suitable for a particular CNS disorder (see Box 3) before any successful translation to patients with stroke and neurological disorders can be achieved.
Box 1. Treatments of some CNS disorders that involve the vasculo-neuronal-inflammatory triad.
Tissue plasminogen activator (tPA) is currently the only approved therapy for acute ischemic stroke. Still, the rate of recombinant tPA use is unfortunately < 4% of stroke patients (91). Problems with tPA include BBB disruption causing intracerebral bleeding (54,92,93), a narrow therapeutic window of use (94) and neuronal and neurovascular toxicity (62). Stroke also increases the rate of cognitive decline which requires treatment for dementia (95). Thus, targeting multiple disease mechanisms early after stroke and during repair phase may provide new therapeutic roads for improving short-term and long-term neurological outcomes after stroke.
Traumatic brain injury frequently results in permanent functional deficits that have been linked to Alzheimer’s disease (AD) (96). Neurologic impairment is due to an immediate BBB and CNS tissue disruption (primary injury), and post-traumatic cellular and molecular events mediating secondary BBB breakdown which worsens the original neurologic insult (97). Pharmacological agents targeting a single injury mechanism have been explored, but without much success (98).
Research into AD has not yet succeeded in terms of developing a disease-modifying therapy (99). The amyloid hypothesis that has been the cornerstone for most work on AD pathogenesis has recently been critically reappraised (100). A two hit vascular hypothesis for AD has been proposed (101), whereby an initial vascular brain damage mediated by hypoxia, perfusion stress and/or disruption of the BBB (hit 1) precedes accumulation of the cytotoxic Alzheimer’s amyloid β-peptide (Aβ) around vasculature and in the brain, which amplifies neurovascular dysfunction and neurodegeneration (hit 2). Given the complex pathogenesis of AD, a single-target therapy is unlikely to provide significant and long-term therapeutic benefits for sporadic AD (99). Thus, the modulation of multiple disease mechanisms may be of potential benefit in delaying the onset and/or in controlling AD progression.
There is currently no curative treatment for common motor neuron diseases such asALS (102). Riluzole, a glutamate antagonist, remains the only medication with demonstrated mild efficacy and regulatory approval for the treatment of ALS (103). The pathogenesis of familial ALS involves at least eight proposed mechanisms including important roles for non-neuronal cells such as astrocytes and microglia (104) and for BBB disruption (25,36). The pathogenesis of sporadic ALS is likely to be even more complex than of familial ALS. Like in AD, one can envisage targeting multiple mechanisms that mediate disease onset and/or ALS progression.
Box 2. Mutation of APC exosites and APC variants with reduced anticoagulant activity.
Replacement of a cluster of five positively charged residues by alanine residues (ie. 5A-APC) on the top surface of the APC heavy chain protease domain (40) (Figure I), restructures this critical positively charged exosite, causing loss of > 98 % of human APC’s anticoagulant activity (Figure 1) while leaving intact APC’s cell signaling activities on different cell types within the neurovascular unit (Figure 3). Replacement of three of these five residues (i.e., lysine residues 191-193), by three alanine residues produces the 3K3A-APC variant (38), which has a similar effect to the 5A-APC variant, causing loss of > 92 % of APC’s anticoagulant activity.
Box 3. Key Questions for developing APC therapies for CNS disorders.
Is a higher dose regimen of APC variants safe and efficacious for stroke patients? Currently, low expected-to-be safe doses of the FDA-approved version of wt-APC (DrotAA), far below an optimized beneficial dose in animal models of stroke, is being tested in stroke patients. One potential concern, however, is whether such a low DrotAA dose can provide the needed therapeutic benefit. Another concern is that increasing DrotAA dose in stroke patients may cause a serious intracerebral bleeding that has been a major complication of DrotAA in a sepsis trial. Determining the highest, but still safe, dose of an APC variant with reduced anticoagulant action (ie. 3K3A- and 5A-APC mutants) in toxicity studies in animals (mice, monkeys) and in Phase I (safety) trial in humans should define the safe higher beneficial dose of APC therapy for Phase 2 trials in stroke patients.
Which APC variants should be developed for brain and/or spinal cord trauma? The most successful APC therapies for neurotrauma will likely involve the use of APC variants that completely lack any anticoagulant activity. Such therapies would have the best chance of mitigating brain damage without the risk of bleeding, which is especially important after neurotrauma where significant BBB breakdown and intracerebral bleeding commonly occurs. APC variants that are valuable for therapeutic purposes after a neurotrauma might be different from the lead APC variants currently being tested for stroke, since in the latter case, the ability to performsome levels of antithrombotic activity would be of considerable benefit to control thrombosis inside the occluded cerebral arteries.
What is the APC therapy of choice for various chronic neurodegenerative disorders? To address this question, several APC variants with varying degrees of anticoagulant to cytoprotective activities ratios should be tested in animal models of neurodegeneration to optimize future APC treatments. As in the case for brain injury discussed above, it is likely that an APC mutant with no anticoagulant activity, but with preserved or enhanced cell signaling activity, could offer the greatest functional benefit and the safest approach in models of ALS and MS.
Can CNS-‘tailored’ therapies with APC variants be extended to systemic diseases? The limitations of the current APC therapy for sepsis with DrotAA are likely related to the use of a restricted low-dose protocol, with the valid concern being that increasing doses of APC might lead to unwanted side-effects, including serious bleeding. Therapies with APC variants with greatly reduced (or ablated) anticoagulant activity, but with preserved or enhanced cytoprotective activities that are currently being developed for stroke and other CNS applications might overcome the bleeding complications of DrotAA.
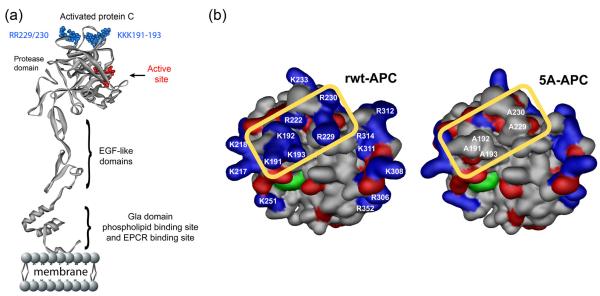
Figure I. APC exosite structures responsible for APC activities.
a. The model of full-length APC is based on the serine protease domain crystal structure of APC (105,106; Protein Data Bank entry 1AUT; http://www.rcsb.org/pdb/explore/explore.do?structureId=1AUT). In the ribbon diagram of the polypeptide structure of APC, the folded serine protease domain at the top of panel, the two EGF-like domains and the N-terminal Gla-domain (which mediates binding to phospholipid membranes and EPCR) are shown in gray. The active serine protease site is in red. The positively charged lysine residues within the so-called 37 loop (KKK191-193) and the arginine residues in the calcium binding-binding loop (RR229/230) determine APC specificity for factors Va and VIIIa.
b. Amino acid residues that determine exosite specificity of the recombinant wild-type APC (wt-APC) protease domain are schematically identified in the space filling model where the close apposition of the 37-loop and the calcium-binding loop positively charged residues (blue) comprising lysine residues K191-193, and arginine residues R229 and R230 are seen inside the yellow rectangle. The active site triad of serine, histidine and aspartic acid residues characteristic of serine proteases is shown in green while red indicates negative side chains. These five basic residues are critical for recognition of the coagulation factor Va and thus for anticoagulant activity; however, they are not required for normal anti-apoptotic activity. The 5A-APC mutant (containing 5 Ala substitutions at residues 191-193 and 229-230) is depicted on the right side inside the yellow rectangle showing a remarkable loss of the positively charged amino acid side chain cluster. The model of wt-APC is based on the serine protease domain structure of APC 1AUT (107) and was generated using Modeller (107).
Acknowledgments
Our work on APC biology and on the preclinical development of APC therapy for stroke has been supported by the US Public Health service grants HL63290 and HL81528 to BVZ, and HL031950, HL052246, and HL104074 to JHG. We thank Dr. Laurent Mosnier and Dr. Abhay Sagare for their help with preparation of figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement/conflict-of-interest B.V.Z. is a scientific founder and Chief Scientific Officer of ZZ Biotech L.L.C., a biotechnology company with a mission to develop recombinant APC variant for stroke. J.H. G. serves on the Scientific Advisory Board of ZZ Biotech L.L.C. and is an inventor on patents covering different applications of APC mutants with reduced anticoagulant activity.
References
- 1.Moskowitz MA, et al. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009;40:S4–S7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlokovic BV. Neurodegeneration: are we overlooking the role of neurovascular unit? Nat. Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- 6.Boillée S, et al. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Alexandrov AV. Current and future recanalization strategies for acute ischemic stroke. J. Intern. Med. 2010;267:209–219. doi: 10.1111/j.1365-2796.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 8.Lees KR, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 9.Nogueira RG, et al. Endovascular approaches to acute stroke, part 1: Drugs, devices, and data. AJNR Am. J. Neuroradiol. 2009;30:649–661. doi: 10.3174/ajnr.A1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arai K, et al. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009;276:4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer SC, Riley JD. Neuroplasticity and brain repair after stroke. Curr. Opin. Neurol. 2008;21:76–82. doi: 10.1097/WCO.0b013e3282f36cb6. [DOI] [PubMed] [Google Scholar]
- 12.Tymianski M. Can molecular and cellular neuroprotection be translated into therapies for patients? Yes, but not the way we tried it before. Stroke. 2010;41:S87–S90. doi: 10.1161/STROKEAHA.110.595496. [DOI] [PubMed] [Google Scholar]
- 13.O’Collins VE, et al. 1,026 experimental treatments in acute stroke. Ann. Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 14.Shuaib A, et al. NXY-059 for the treatment of acute ischemic stroke. N. Eng. J. Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 15.Fisher M, et al. Enhancing the development and approval of acute stroke therapies: Stroke Therapy Academic Industry roundtable. Stroke. 2005;36:1808–1813. doi: 10.1161/01.STR.0000173403.60553.27. [DOI] [PubMed] [Google Scholar]
- 16.Saver JL, et al. Infarct volume as a surrogate or axilliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke. 1999;30:293–298. doi: 10.1161/01.str.30.2.293. [DOI] [PubMed] [Google Scholar]
- 17.Johnston KC, et al. Validation of an acute ischemic stroke model: does diffusion-weighted imaging lesion volume offer a clinically significant improvement in prediction of outcome? Stroke. 2007;38:1820–1825. doi: 10.1161/STROKEAHA.106.479154. [DOI] [PubMed] [Google Scholar]
- 18.Hand PJ, et al. MR diffusion-weighted imaging and outcome prediction after ischemic stroke. Neurology. 2006;66:1159–1163. doi: 10.1212/01.wnl.0000202524.43850.81. [DOI] [PubMed] [Google Scholar]
- 19.Feuerstein GZ, et al. Missing steps in the STAIR case: a translational medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J. Cereb. Blood Flow Metab. 2008;28:217–219. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- 20.Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 21.Saver JL, et al. Stroke therapy academic industry roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009;40:2594–2600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchesi VT. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011;25:5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- 23.Boillée S, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 24.Yamanaka K, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong Z, et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armulik A, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 27.Bell RD, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosnier LO, et al. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 29.Griffin JH, et al. Activated protein C: potential therapy for severe sepsis, thrombosis, and stroke. Semin. Hematol. 2002;39:197–205. doi: 10.1053/shem.2002.34093. [DOI] [PubMed] [Google Scholar]
- 30.Griffin JH, et al. The promise of protein C. Blood Cells Mol. Dis. 2006;36:211–216. doi: 10.1016/j.bcmd.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin. Thromb. Hemost. 2006;32(Suppl 1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 32.Danese S, et al. The protein C pathway in tissue inflammation and injury: pathogenic role and therapeutic implications. Blood. 2010;115:1121–1130. doi: 10.1182/blood-2009-09-201616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr. Med. Chem. 2010;17:2059–2069. doi: 10.2174/092986710791233706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto K, Loskutoff DJ. Extrahepatic expression and regulation of protein C in the mouse. Am. J. Pathol. 1998;153:547–555. doi: 10.1016/S0002-9440(10)65597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deane R, et al. Endothelial protein C receptor-assisted transport of activated protein C across the mouse blood-brain barrier. J. Cereb. Blood Flow Metab. 2009;29:25–33. doi: 10.1038/jcbfm.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong Z, et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J. Clin. Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esmon CT, Glass JD. The APCs of neuroprotection. J. Clin. Invest. 2009;119:3205–3207. doi: 10.1172/JCI40682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gale AJ, et al. Molecular characterization of an extended binding site for coagulation factor Va in the positive exosite of activated protein C. J. Biol. Chem. 2002;277:28836–28840. doi: 10.1074/jbc.M204363200. [DOI] [PubMed] [Google Scholar]
- 39.Mosnier LO, et al. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740–1744. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 40.Mosnier LO, et al. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activatable fibrinolysis inhibitor-dependent cytoprotective functions. J. Biol. Chem. 2007;282:33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 41.Bae JS, et al. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J. Biol. Chem. 2007;282:9251–9259. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 42.Guo H, et al. Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity. Eur. J. Neurosci. 2009;29:1119–1130. doi: 10.1111/j.1460-9568.2009.06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, et al. Differential neuroprotection and risk for bleeding from activated protein C with varying degrees of anticoagulant activity. Stroke. 2009;40:1864–1869. doi: 10.1161/STROKEAHA.108.536680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker CT, et al. Activated protein C analog with reduced anticoagulant activity improves functional recovery and reduces bleeding risk following controlled cortical impact. Brain Res. 2010;1347C:125–131. doi: 10.1016/j.brainres.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerschen EJ, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J. Exp. Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosnier LO, et al. Hyperantithrombotic, noncytoprotective Glu149Ala-activated protein C mutant. Blood. 2009;113:5970–5978. doi: 10.1182/blood-2008-10-183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit. Care Med. 2002;30:S288–S293. doi: 10.1097/00003246-200205001-00019. [DOI] [PubMed] [Google Scholar]
- 48.Riewald M, et al. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 49.Cheng T, et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 50.Dömötör E, et al. Activated protein C alters cytosolic calcium flux in human brain endothelium via binding to endothelial protein C receptor and activation of protease activated receptor-1. Blood. 2003;101:4797–4801. doi: 10.1182/blood-2002-12-3680. [DOI] [PubMed] [Google Scholar]
- 51.Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem. J. 2003;373:65–70. doi: 10.1042/BJ20030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 53.Finigan JH, et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J. Biol. Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 54.Cheng T, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat. Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 55.Guo H, et al. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 56.Shibata M, et al. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–1805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- 57.Vu TK, et al. Domains specifying thrombin-receptor interaction. Nature. 1991;353:674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- 58.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 59.Riewald M, Ruf W. Protease-activated Receptor-1 signaling by activated protein C in cytokine perturbed endothelial cells is distinct from thrombin signaling. J. Biol. Chem. 2005;280:19808–19814. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 60.Yang L, et al. Identification of a specific exosite on activated protein C for interaction with protease-activated receptor 1. J. Biol. Chem. 2007;282:25493–25500. doi: 10.1074/jbc.M702131200. [DOI] [PubMed] [Google Scholar]
- 61.Gardell SE, et al. Emerging medicinal roles for lysophospholipid signaling. Trends Mol. Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Liu D, et al. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat. Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 63.Uchiba M, et al. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ. Res. 2004;95:34–41. doi: 10.1161/01.RES.0000133680.87668.FA. [DOI] [PubMed] [Google Scholar]
- 64.Thiyagarajan M, et al. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J. Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petraglia AL, et al. Activated protein C is neuroprotective and mediates new blood vessel formation and neurogenesis after controlled cortical impact. Neurosurgery. 2010;66:165–171. doi: 10.1227/01.NEU.0000363148.49779.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorbacheva L, et al. Activated protein C prevents glutamate- and thrombin-induced activation of nuclear factor-κB in cultured hippocampal neurons. Neuroscience. 2010;165:1138–1146. doi: 10.1016/j.neuroscience.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 67.Gorbacheva L, et al. Endothelial protein C receptor is expressed in rat cortical and hippocampal neurons and is necessary for protective effect of activated protein C at glutamate excitotoxicity. J. Neurochem. 2009;111:967–975. doi: 10.1111/j.1471-4159.2009.06380.x. [DOI] [PubMed] [Google Scholar]
- 68.Taoka Y, et al. Activated protein C reduces the severity of compression-induced spinal cord injury in rats by inhibiting activation of leukocytes. J. Neurosci. 1998;18:1393–1398. doi: 10.1523/JNEUROSCI.18-04-01393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Mizutani A, et al. Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood. 2000;95:3781–3787. [PubMed] [Google Scholar]
- 70.Nick JA, et al. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood. 2004;104:3878–3885. doi: 10.1182/blood-2004-06-2140. [DOI] [PubMed] [Google Scholar]
- 71.Cao C, et al. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J. Clin. Invest. 2010;120:1971–1980. doi: 10.1172/JCI40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kerschen E, et al. Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice. J. Clin. Invest. 2010;120:3167–3178. doi: 10.1172/JCI42629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernard GR, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 74.Short MA, et al. From bench to bedside: a review of clinical trial development plan of drotrecogin alfa (activated) Curr. Med. Res. Opin. 2006;22:2525–2540. doi: 10.1185/030079906x154060. [DOI] [PubMed] [Google Scholar]
- 75.Zlokovic BV, et al. Functional recovery after embolic stroke in rodents by activated protein C. Ann. Neurol. 2005;58:474–477. doi: 10.1002/ana.20602. [DOI] [PubMed] [Google Scholar]
- 76.Yesilirmak DC, et al. Effects of activated protein C on neonatal hypoxic ischemic brain injury. Brain Res. 2008;1210:56–62. doi: 10.1016/j.brainres.2008.02.088. [DOI] [PubMed] [Google Scholar]
- 77.Taylor FB, et al. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the Baboon. J. Clin. Invest. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gruber A, et al. Inhibition of thrombus formation by activated recombinant protein C in a primate model of arterial thrombosis. Circulation. 1990;82:578–585. doi: 10.1161/01.cir.82.2.578. [DOI] [PubMed] [Google Scholar]
- 79.Marder VJ, Novokhatny V. Direct fibrinolytic agents: biochemical attributes, preclinical foundation and clinical potential. J. Thromb. Haemost. 2009;8:433–444. doi: 10.1111/j.1538-7836.2009.03701.x. [DOI] [PubMed] [Google Scholar]
- 80.Stewart D, et al. Distinct dose-dependent effects of plasmin and t-PA on coagulation and hemorrhage. Blood. 2003;101:3002–3007. doi: 10.1182/blood-2002-08-2546. [DOI] [PubMed] [Google Scholar]
- 81.Jahan R, et al. Middle cerebral artery occlusion in the rabbit using selective angiography: application for assessment of thrombolysis. Stroke. 2008;39:1613–1615. doi: 10.1161/STROKEAHA.107.507376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marder VJ, et al. Thrombolysis with plasmin: implications for stroke treatment. Stroke. 2010;41:S45–S49. doi: 10.1161/STROKEAHA.110.595157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen ZL, Strickland S. Neuronal death in hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 84.Loubele ST, et al. Activated protein C protects against myocardial ischemia/ reperfusion injury via inhibition of apoptosis and inflammation. Arterioscler. Thromb. Vasc. Biol. 2009;29:1087–1092. doi: 10.1161/ATVBAHA.109.188656. [DOI] [PubMed] [Google Scholar]
- 85.Kuriyama N, et al. The cytoprotective effects of addition of activated protein C into preservation solution on small-for-size grafts in rats. Liver Transpl. 2009;16:1–11. doi: 10.1002/lt.21923. [DOI] [PubMed] [Google Scholar]
- 86.Bir, et al. Cytoprotective-selective Activated Protein C Attenuates P. aeruginosa-induced Lung Injury in Mice. Am. J. Respir. Cell Mol. Biol. 2011 doi: 10.1165/rcmb.2010-0397OC. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iserman B, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat. Med. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 88.Hirose K, et al. Activated protein C reduces the ischemia/reperfusion-induced spinal cord injury in rats by inhibiting neutrophil activation. Ann. Surg. 2000;232:272–280. doi: 10.1097/00000658-200008000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamauchi T, et al. Neuroprotective effects of activated protein C through induction of insulin-like growth factor-1 (IGF-1), IGF-1 receptor, and its downstream signal phosphorylated serine-threonine kinase after spinal cord ischemia in rabbits. Stroke. 2006;37:1081–1086. doi: 10.1161/01.STR.0000206280.30972.21. [DOI] [PubMed] [Google Scholar]
- 90.Han MH, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 91.Kleindorfer D, et al. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke. 2008;39:924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 92.NINDS t-PA Stroke Study Group Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 93.Carpenter CR, et al. Thrombolytic therapy for acute ischemic stroke beyond three hours. J. Emerg. Med. 2010 doi: 10.1016/j.jemermed.2010.05.009. DOI: 10.1016/j.jemermed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saver JL, Levine SR. Alteplase for ischaemic stroke-much sooner is much better. Lancet. 2010;375:1667–1668. doi: 10.1016/S0140-6736(10)60634-4. [DOI] [PubMed] [Google Scholar]
- 95.Savva GM, et al. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–e46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- 96.Johnson VE, et al. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease? Nat. Rev. Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shlosberg D, et al. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tolis CM, Bullock MR. Critical appraisal of neuroprotection trials in head injury: what have we learned? NeuroRx. 2004;1:71–79. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mangialasche F, et al. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 100.Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J. Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 101.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 102.Orrell RW. Motor neuron disease: systematic reviews of treatment for ALS and SMA. Br. Med. Bull. 2010;93:145–159. doi: 10.1093/bmb/ldp049. [DOI] [PubMed] [Google Scholar]
- 103.Bensimon G, et al. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 104.Ilieva H, et al. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pellequer JL, et al. Three-dimensional model of coagulation factor Va bound to activated protein C. Thromb. Haemost. 2000;84:849–857. [PubMed] [Google Scholar]
- 106.Mather T, et al. The 2.8 Å crystal structure of Gla-domainless activated protein C. EMBO J. 1996;15:6822–6831. [PMC free article] [PubMed] [Google Scholar]
- 107.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]