Abstract
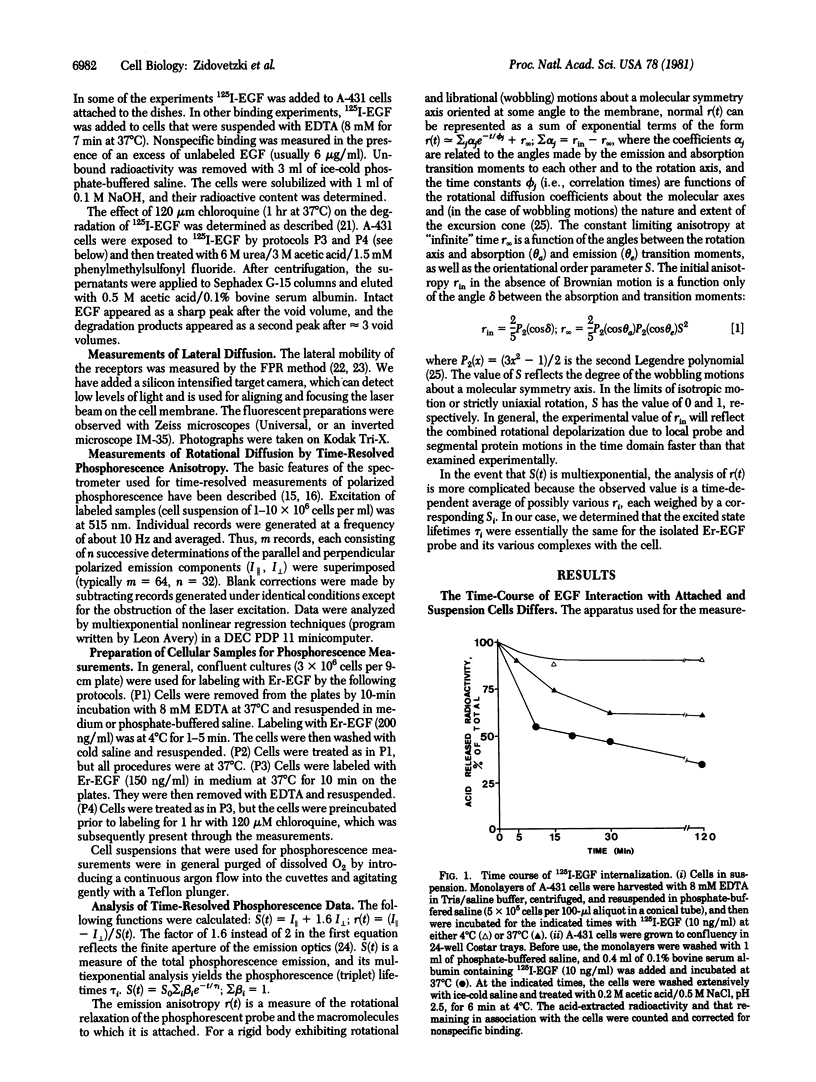
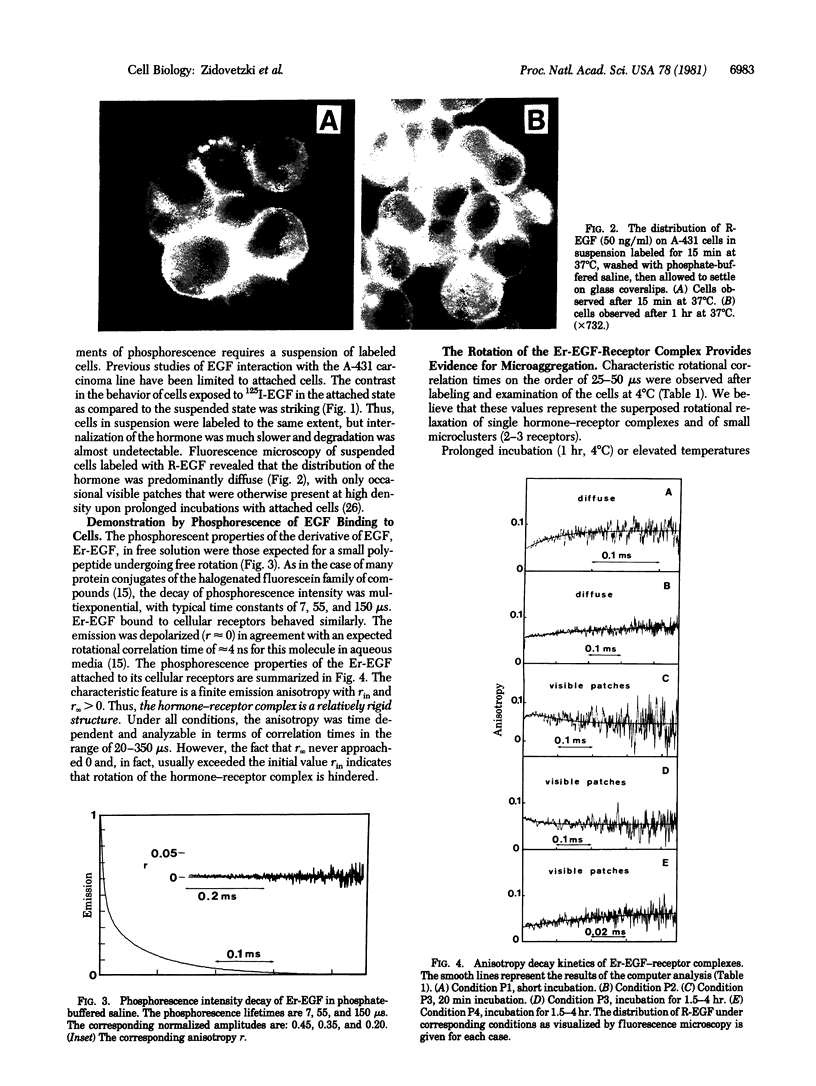
The rotational diffusion of epidermal growth factor (EGF)--receptor complexes on living human epidermoid carcinoma cells (A-431) has been measured by phosphorescence emission and anisotropy in the mu s time domain. A biologically active phosphorescent conjugate of EGF, erythrosin-EGF, was applied to living cells. The hormone--receptor complexes were mobile with rotational correlation times in the range 25--90 mu s when labeled and measured at 4 degrees C. Prolonged incubation and exposure to higher temperatures (23 and 37 degrees C) resulted in longer times up to 350 mu s, indicative of the progressive formation of microclusters, estimated to contain 10-50 receptors. Upon internalization of the hormone--receptor complexes, visible patches were observed by fluorescence microscopy, and the rotational correlation times were shorter, indicating a decrease in size of the dynamic unit. The sign of the rotational relaxation also varied with localization and processing of the hormones. The rate of lateral diffusion of EGF--receptor complexes, measured under similar conditions by fluorescence photobleaching recovery, increased with temperature in contrast to the rotational motion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. H., Chan S. S., Jovin T. M. Rotational diffusion of cell surface components by time-resolved phosphorescence anisotropy. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5650–5654. doi: 10.1073/pnas.76.11.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Measurement of protein rotational diffusion in membranes by flash photolysis. Methods Enzymol. 1978;54:47–61. doi: 10.1016/s0076-6879(78)54007-x. [DOI] [PubMed] [Google Scholar]
- Chinkers M., McKanna J. A., Cohen S. Rapid induction of morphological changes in human carcinoma cells A-431 by epidermal growth factors. J Cell Biol. 1979 Oct;83(1):260–265. doi: 10.1083/jcb.83.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Cohen S., Orci L. Epidermal growth factor: morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H., Ash J. F., Singer S. J., Cohen S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R. A., Nexø E., Hollenberg M. D. Isolation of the human placenta receptor for epidermal growth factor-urogastrone. Nature. 1979 Feb 1;277(5695):403–405. doi: 10.1038/277403a0. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Bartholdi M., Vaz W. L., Austin R. H. Rotational diffusion of biological macromolecules by time-resolved delayed luminescence (phosphorescence, fluorescence) anisotropy. Ann N Y Acad Sci. 1981;366:176–196. doi: 10.1111/j.1749-6632.1981.tb20753.x. [DOI] [PubMed] [Google Scholar]
- King A. C., Hernaez-Davis L., Cuatrecasas P. Lysomotropic amines cause intracellular accumulation of receptors for epidermal growth factor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3283–3287. doi: 10.1073/pnas.77.6.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipari G., Szabo A. Effect of librational motion on fluorescence depolarization and nuclear magnetic resonance relaxation in macromolecules and membranes. Biophys J. 1980 Jun;30(3):489–506. doi: 10.1016/S0006-3495(80)85109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. H., Garland P. B. Synthesis of erythrosin isothiocyanate and its use as a phosphorescent depolarization probe for slow rotational mobility of membrane proteins [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):945–946. doi: 10.1042/bst0070945. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Cuatrecasas P., Willingham M. C., Pastan I. Quantitative determination of the lateral diffusion coefficients of the hormone-receptor complexes of insulin and epidermal growth factor on the plasma membrane of cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5353–5357. doi: 10.1073/pnas.75.11.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Schlessinger J., Jacobs S., Chang K. J., Cuatrecasas P. Fluorescent labeling of hormone receptors in viable cells: preparation and properties of highly fluorescent derivatives of epidermal growth factor and insulin. Proc Natl Acad Sci U S A. 1978 May;75(5):2135–2139. doi: 10.1073/pnas.75.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol. 1974 Jan 5;85(4):603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- Sonne O., Gliemann J. Insulin receptors of cultured human lymphocytes (IM-9). Lack of receptor-mediated degradation. J Biol Chem. 1980 Aug 10;255(15):7449–7454. [PubMed] [Google Scholar]
- Träuble H., Sackmann E. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4499–4510. doi: 10.1021/ja00768a015. [DOI] [PubMed] [Google Scholar]
- Wrann M. M., Fox C. F. Identification of epidermal growth factor receptors in a hyperproducing human epidermoid carcinoma cell line. J Biol Chem. 1979 Sep 10;254(17):8083–8086. [PubMed] [Google Scholar]
- Yarden Y., Gabbay M., Schlessinger J. Primary amines do not prevent the endocytosis of epidermal growth factor into 3T3 fibroblasts. Biochim Biophys Acta. 1981 May 5;674(2):188–203. doi: 10.1016/0304-4165(81)90377-9. [DOI] [PubMed] [Google Scholar]




