The 2010 World Health Organization pediatric guidelines for nonvirological treatment monitoring have high misclassification rates and limited accuracy, even among children with extensive drug resistance. Affordable virological monitoring suitable for resource-limited settings is desperately needed.
Abstract
Background. Antiretroviral therapy (ART) in resource-limited settings (RLSs) is monitored clinically and immunologically, according to World Health Organization (WHO) or national guidelines. Revised WHO pediatric guidelines were published in 2010, but their ability to accurately identify virological failure is unclear.
Methods. We evaluated performance of WHO 2010 guidelines and compared them with WHO 2006 and Cambodia 2011 guidelines among children on ≥6 months of first-line ART at Angkor Hospital for Children between January 2005 and September 2010. We determined sensitivity, specificity, positive and negative predictive values, and accuracy using bootstrap resampling to account for multiple tests per child. Human immunodeficiency virus (HIV) resistance was compared between those correctly and incorrectly identified by each guideline.
Results. Among 457 children with 1079 viral loads (VLs), 20% had >400 copies/mL. For children with WHO stage 1/2 HIV, misclassification as failure (met CD4 failure criteria, but VL undetectable) was 64% for WHO 2006 guidelines, 33% for WHO 2010 guidelines, and 81% for Cambodia 2011 guidelines; misclassification as success (did not meet CD4 failure, but VL detectable) was 11%, 12%, and 12%, respectively. For children with WHO stage 3/4 HIV, misclassification as failure was 35% for WHO 2006 guidelines, 40% for WHO 2010 guidelines, and 43% for Cambodia 2011 guidelines; misclassification as success was 13%, 24%, and 21%, respectively. Compared with WHO 2006 guidelines, WHO 2010 guidelines significantly increased the risk of misclassification as success in stage 3/4 HIV (P < .05). The WHO 2010 guidelines failed to identify 98% of children with extensive reverse-transcriptase resistance.
Conclusions. In our cohort, lack of virological monitoring would result in unacceptable treatment failure misclassification, leading to premature ART switch and resistance accumulation. Affordable virological monitoring suitable for use in RLSs is desperately needed.
Of 33.3 million people living with human immunodeficiency virus (HIV), 2.5 million (8%) are children aged <15 years [1]. Cambodia, among the poorest Southeast Asian nations [2], has been successful in controlling its HIV epidemic through aggressive testing, treatment, and prevention. The HIV prevalence in the population aged 15–49 years decreased from 2% in 1998 to 0.7% in 2010. Among the country's 13.4 million inhabitants, 56 000–80 000 live with HIV/AIDS [3, 4], among them 5600 children, 4000 (71%) of whom are on antiretroviral therapy (ART) [3].
Pediatric ART success varies in Cambodia [5, 6] and elsewhere [7–9], and treatment monitoring remains a global challenge. Due to rapid ART roll-out, cost-effective treatment monitoring is increasingly important. Accurate treatment failure identification is important to prevent resistance accumulation [10, 11] and avoid an inappropriate second-line therapy switch, which is costly and complicated by lack of fixed-dose combinations, poor palatability, and cold-chain requirements. However, as in most resource-limited settings (RLSs), viral load (VL) testing is not widely available.
World Health Organization (WHO) guidelines direct national programs in identifying ART failure where VL testing is limited. Accordingly, Cambodian pediatric ART guidelines recommend clinical staging and immunological monitoring every 6 months [12, 13]. Immunological and clinical criteria poorly predict virological failure in adults, with unacceptable rates of failure misclassification [14–19]. Performance of such criteria in children is not well described, and data on resistance consequences are scarce. In a previous Cambodian study, WHO 2006 guidelines poorly predicted virological failure in 22 children treated for 24 months [5].
In 2010, the WHO published revised guidelines that simplified the immunological failure definition in children aged ≥2 years and significantly restricted CD4 thresholds used to define failure [20]. Recently revised Cambodian guidelines [21] incorporated these thresholds but included a 30% CD4 decrease over 6 months in an effort to increase early treatment failure detection. Performance of these new guidelines has not been systematically evaluated.
The objectives of this study were to evaluate the performance of the 2010 WHO pediatric guidelines in identifying treatment failure; compare performance between the 2010 WHO, 2006 WHO, and 2011 Cambodian guidelines; and examine the association between treatment failure misclassification and resistance in a cohort of HIV-infected Cambodian children.
MATERIALS AND METHODS
Study Setting
This retrospective cohort study was performed at Angkor Hospital for Children (AHC) in Siem Reap, Cambodia, 1 of 32 pediatric AIDS care sites recognized by the National Center for HIV/AIDS, Dermatology, and STIs (NCHADS). A nongovernmental organization–funded pediatric referral center, AHC had provided free care to 913 HIV-infected children through September 2010. Antiretroviral therapy became available in 2003, and as of January 2011, 502 children were receiving ART.
Children are seen every 8 weeks; WHO stage and medications are recorded electronically at each visit. The CD4 count and/or percentage are determined every 6 months, and, unlike in the Cambodian public sector, VL testing is performed annually and when immunological and/or clinical failure is suspected based on national guidelines. Second-line regimens are based on reverse-transcriptase (RT) genotype and consist of lopinavir/ritonavir with ≥2 nucleoside/nucleotide RT inhibitors (NRTIs).
Patient Cohort
Children were included if they met the following 4 criteria: (1) were HIV-infected; (2) were aged 6 months to 15 years at time of VL testing; (3) had received ≥180 days of first-line ART (zidovudine/stavudine+lamivudine+nevirapine/efavirenz) between 1 January 2005 and 30 September 2010; and (4) had ≥1 VL. Electronic databases and treatment records were reviewed for date of birth, sex, HIV diagnosis date, WHO staging, ART initiation, ART regimens, tuberculosis treatment, CD4 counts/percentages, and VLs. The study was approved by AHC and Lifespan Institutional Review Boards.
Data Analysis
The WHO guidelines provide clinical and immunological thresholds defining treatment failure and treatment recommendations based on these definitions. We defined immunological failure by age-related thresholds used in each guideline evaluated: WHO 2010, WHO 2006, and Cambodia 2011 (Table 1). A single CD4 count/percentage below age-related immunological threshold was considered indicative of immunological failure. Because treatment recommendations depend on clinical staging (Table 2), we compared guideline performance by WHO stage. Virological failure cutoffs were defined as either >400 copies/mL (detection limit) or >5000 copies/mL (WHO threshold). Sensitivity, specificity, positive predictive values (PPVs), negative predictive values (NPVs), misclassification rates, and overall accuracy were calculated for each guideline using virological failure as the treatment failure gold-standard.
Table 1.
Age-Related CD4 Criteria Defining Immunological Failure in Children According to World Health Organization (WHO) and Cambodia Guidelines
| WHO 2006 Guidelines |
WHO 2010 Guidelines |
Cambodia 2011 Guidelines |
|||
|---|---|---|---|---|---|
| Age (years) | Age-Related CD4 Threshold | Age (years) | Age-Related CD4 Threshold | Age (years) | Age-Related CD4 Threshold |
| 1 to <3 | CD4 percentage <20 or CD4 count <750 | <2 | No threshold given | <2 | No threshold given |
| 3 to <5 | CD4 percentage <20 or CD4 count <350 | 2 to <5 | CD4 percentage <10 or CD4 count<200 | 2 to <5 | CD4 percentage <10 or CD4 count <200 or CD4 count or percentage decrease >30% in previous 6 months |
| ≥5 | CD4 percentage <15 or CD4 count <200 | ≥5 | CD4 <100 | ≥5 | CD4 count <100 or CD4 count decrease >30% in previous 6 months |
CD4 counts in cells/mm3.
Abbreviation: WHO, World Health Organization.
Table 2.
World Health Organization 2010 Treatment Recommendations Based on Clinical Stage and CD4
| WHO Stage | Age-Related Immunological Failure | Treatment Recommendation |
|---|---|---|
| Stage 1 or 2 | No | Continue current regimen |
| Intensify follow-up if CD4 falls to near age-related threshold | ||
| Yes | Consider switch only if ≥2 CD4 measurements are below age-related threshold | |
| Stage 3 | No | Intensify follow-up if CD4 falls to near age-related threshold |
| Yes | Switch regimen, especially if good initial immune response to ART | |
| Stage 4 | No | Switching may not be necessary |
| Yes | Switch regimen, especially if good initial immune response to ART |
Abbreviations: ART, antiretroviral therapy; WHO, World Health Organization.
Each analyzed time point included VL and linked (within 3 months) CD4 measurements. To evaluate CD4 decline as a measure of immunological failure, a CD4 value from 6 months ± 3 months prior to VL testing was used. To account for imprecise timing of CD4 testing, we assumed CD4 decline to be linear and used the estimated CD4 count at exactly 6 months prior to the linked CD4-VL date to calculate the 6-month CD4 change.
Misclassification was defined as VL below cutoff and either CD4 count or percentage predicting treatment failure (misclassification as failure), or VL above cutoff and CD4 count and percentage predicting treatment success (misclassification as success). When CD4 counts and percentages were discordant, a single value falling below age-related threshold was considered predictive of failure.
Sequence alignment and quality assurance were performed by SQUAT [22]. Subtyping was done by Rega [23]. Resistance mutations were interpreted with the International AIDS Society-USA list [24].
Laboratory Methods
Over the study period, CD4 measurements were performed at various laboratories, including the Cambodian Ministry of Health Reference Laboratory (Kampong Cham), the National Institute of Public Health (Phnom Penh), and the Cambodia Pasteur Institute (IPC, Phnom Penh), by Becton Dickinson FACSCount (BD Biosciences). Biannual external quality control was performed by United Kingdom National External Quality Assessment Service; Quality Assurance Systems International, Canada; and Centre of Excellence, Thailand.
Viral load testing was performed at IPC using Agence Nationale de Recherche sur le Sida (ANRS) second-generation real-time RT polymerase chain reaction (Biocentric) [25, 26]. The lower limit of detection was decreased from 400 to 250 copies/mL during the study. To account for this, detectable VL was defined as >400 for all analyses.
The RT genotyping was performed at IPC in viremic children with confirmed adherence whose clinicians were considering second-line ART [5]. Viral load testing and resistance external quality control were performed biannually by the virology quality assurance program of the National Institutes of Health and annually by ANRS.
Statistical Analysis
The estimation of guidelines’ performance used all observed data, including multiple VLs per child. The most recent VL was used to analyze association of clinical measures and misclassification rates. For resistance analysis, data from genotype-linked visits were used. We calculated sensitivity, specificity, PPV, NPV, misclassification rates, and diagnostic accuracy and estimated 95% confidence intervals (CIs) using bootstrap resampling, with child being the primary resampling unit. Children were considered misclassified as failure if VL was below the cutoff for virological failure but CD4 count or percentage was below the guideline-defined threshold for immunological failure (1-PPV). Children were considered misclassified as nonfailure if VL was above the cutoff for virological failure but CD4 count and percentage were above the guideline-defined threshold for immunological failure (1-NPV). To limit the number of hypothesis tests performed, bootstrap P values were estimated for overall accuracy and misclassification rates. Age, gender, ART duration, highest WHO stage in prior 6 months (1/2 vs 3/4), tuberculosis treatment, and age at HIV diagnosis were compared between misclassified and correctly classified children. Comparisons were made at recent VL using chi-square tests for categorical variables and Student t tests for continuous variables.
When >1 genotype was available, the earliest was used. Numbers of NRTI (0–1 or 2 or ≥3) and NNRTI mutations were compared by misclassification status using ordinal logistic regression after confirming acceptability of proportional odds assumption. Analyses were performed using R 2.13.1 [27]; P < .05 was considered statistically significant.
RESULTS
Patient Cohort
Among 913 children, 648 (71%) received ART, 557 (61%) of whom received ART for ≥180 days, and 496 (54%) of whom had VL data available. Four hundred sixty-eight (51%) were on first-line ART; 457 (50%) were aged <15 years and were included in the study. Demographic, clinical, and laboratory characteristics are summarized in Table 3. The median age at diagnosis was 4.9 years. At most recent VL testing, most children (89%; 407 of 457) were aged >5 years (median, 8.8 years); 50% were male; most (87%; 398 of 457) were on stavudine/lamivudine/nevirapine for a median of 3.1 years; and 16% were exposed to tuberculosis medications. The cohort was clinically advanced, with 58% reaching WHO stage 3/4 before ART initiation, and 76% ever reaching WHO stage 3/4. The median nadir CD4 percentage was 11 (range, 0%–45%), and the most recent CD4 percentage was 27 (range, 1%–53%). Of 457 children, 49 (11%) had no CD4 percentage and 9 (2%) no CD4 count at most recent VL testing. A total of 1079 VLs were available for the 457 children (median, 2/child; range, 1–8): 7 VLs were from children aged <2 years, 124 were from children aged 2–5 years, and 948 were from children aged >5 years. At most recent VL testing, 20% (92 of 457) were viremic (>400 copies/mL), and the majority (80%) had >5000 copies/mL.
Table 3.
Demographic, Clinical, and Laboratory Data at Most Recent Viral Load Measurement Among 457 Cambodian Children on First-line Antiretroviral Therapy
| All | <2 Years | 2–5 Years | >5 Years | |
|---|---|---|---|---|
| Number of children | 457 | 4 | 46 | 407 |
| Age in years at HIV diagnosis | 4.9 (0.1–13) | 0.6 (0.3–1) | 1.6 (0.1–3) | 5.4 (0.4–13) |
| Age in years at most recent VL testing | 8.8 (1.4–14.9) | 1.6 (1.4–1.9) | 3.9 (2.1–4.98) | 9.3 (5.1–14.9) |
| Gender: male | 231/457 (50) | 1/4 (25) | 22/46 (48) | 208/407 (51) |
| Regimen | ||||
| D4T, 3TC, NVP | 398/457 (87) | 4/4 (100) | 42/46 (91) | 352/407 (87) |
| D4T, 3TC, EFV | 52/457 (11) | 0/4 (0) | 4/46 (9) | 48/407 (12) |
| 3TC, NVP, AZT | 5/457 (1) | 0/4 (0) | 0/46 (0) | 5/407 (1) |
| 3TC, EFV, AZT | 2/457 (0.4) | 0/4 (0) | 0/46 (0) | 2/407 (0.5) |
| Years on HAART | 3.1 (0.6–7.3) | 1 (1–1) | 1.9 (1–4.2) | 3.2 (0.6–7.3) |
| Ever treated for tuberculosis | 74/457 (16) | 0/4 (0) | 7/46 (15) | 67/407 (17) |
| Highest pre-HAART WHO stage | ||||
| 1 | 40/450 (9) | 1/4 (25) | 8/45 (18) | 31/401 (8) |
| 2 | 146/450 (32) | 1/4 (25) | 8/45 (18) | 137/401 (34) |
| 3 | 225/450 (49) | 1/4 (25) | 27/45 (60) | 197/401 (49) |
| 4 | 39/450 (9) | 1/4 (25) | 2/45 (4) | 36/401 (9) |
| Highest ever WHO stage | ||||
| 1 | 26/457 (6) | 1/4 (25) | 7/46 (15) | 18/407 (4) |
| 2 | 87/457 (19) | 1/4 (25) | 4/46 (9) | 82/407 (20) |
| 3 | 268/457 (59) | 1/4 (25) | 30/46 (65) | 237/407 (58) |
| 4 | 76/457 (17) | 1/4 (25) | 5/46 (11) | 70/407 (17) |
| Nadir CD4 count | 220 (1–2000) | 1228 (41–2000) | 731 (12–2000) | 193 (1–1428) |
| Nadir CD4 percentage | 11 (0–45) | 32 (9–39) | 15 (1–37) | 10 (0–45) |
| Most recent CD4 count | 856 (11–4060) | … | 1420 (16–2304) | 805 (11–4060) |
| Most recent CD4 percentage | 27 (0.5–53) | … | 32 (16–47) | 26 (0.5–53) |
| Most recent VL >400 | 92/457 (20) | 1/4 (25) | 15/46 (33) | 76/407 (19) |
| 400< VL ≤1000 | 8/92 (9) | 0/1 (0) | 1/15 (7) | 7/76 (9) |
| 1000< VL ≤5000 | 10/92 (11) | 0/1 (0) | 4/15 (27) | 6/76 (8) |
| VL >5000 | 74/92 (80) | 1/1 (100) | 10/15 (67) | 63/76 (83) |
| Number of VL/child | 2 (1–8) | 3 (1–3) | 2 (1–8) | 3 (1–6) |
Results are shown as median (range) or number (%). Viral load results are given in copies/mL.
Abbreviations: 3TC, lamivudine; AZT, zidovudine; D4T, stavudine; EFV, efavirenz; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; NVP, nevirapine; VL, viral load; WHO, World Health Organization.
WHO 2010 Guideline Performance
Table 4 demonstrates the performance of the WHO 2010 guidelines compared with the WHO 2006 and the Cambodian 2011 guidelines. Changing the definition of virological failure from 400 copies/mL (shown in Table 4) to 5000 copies/mL (not shown) had minimal effect on results.
Table 4.
Performance of 3 Different HIV Guidelines in Predicting Virological Failure (>400 copies/mL) Among 457 Cambodian Children on First-line Antiretroviral Therapy
| WHO 2006 Guidelines |
WHO 2010 Guidelines |
Cambodia 2011 Guidelines |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| WHO 1 or 2 | WHO 3 or 4 | All Stages | WHO 1 or 2 | WHO 3 or 4 | All Stages | WHO 1 or 2 | WHO 3 or 4 | All Stages | |
| Proportion with virological failure | |||||||||
| n/N (%) | 121/969 (12) | 21/80 (26) | 142/1049 (14) | 117/937 (12) | 20/76 (26) | 137/1013 (14) | 104/805 (13) | 16/65 (25) | 120/870 (14) |
| Sensitivity: probability guidelines classify as failing those with virological failure | |||||||||
| n/N (%) | 27/121 (22) | 13/21 (62) | 40/142 (28) | 4/117 (3) | 3/20 (15) | 7/137 (5) | 27/104 (26) | 4/16 (25) | 31/120 (26) |
| Bootstrap 95% CI | (14%–31%) | (40%–82%) | (20%–37%) | (1%–7%) | (0%–31%) | (2%–9%) | (17%–35%) | (6%–47%) | (17%–34%) |
| Specificity: probability guidelines classify as not failing those without virological failure | |||||||||
| n/N (%) | 799/848 (94) | 52/59 (88) | 851/907 (94) | 818/820 (100) | 54/56 (96) | 872/876 (100) | 587/701 (84) | 46/49 (94) | 633/750 (84) |
| Bootstrap 95% CI | (92%–96%) | (79%–97%) | (92%–95%) | (99%–100%) | (92%–100%) | (99%–100%) | (81%–87%) | (88%–100%) | (82%–87%) |
| Positive predictive value: probability of having virological failure among those with guideline predicted failure | |||||||||
| n/N (%) | 27/76 (36) | 13/20 (65) | 40/96 (42) | 4/6 (67) | 3/5 (60) | 7/11 (64) | 27/141 (19) | 4/7 (57) | 31/148 (21) |
| Bootstrap 95% CI | (23%–48%) | (42%–89%) | (29%–52%) | (20%–100%) | (0%–100%) | (30%–89%) | (12%–26%) | (17%–100%) | (14%–28%) |
| Negative predictive value: probability of not having virological failure among those without guideline predicted failure | |||||||||
| n/N (%) | 799/893 (89) | 52/60 (87) | 851/953 (89) | 818/931 (88) | 54/71 (76) | 872/1002 (87) | 587/664 (88) | 46/58 (79) | 633/722 (88) |
| Bootstrap 95% CI | (87%–92%) | (77%–95%) | (87%–92%) | (85%–90%) | (64%–86%) | (84%–90%) | (85%–91%) | (67%–88%) | (84%–91%) |
| Misclassification as failure: probability of not having virological failure among those with guideline predicted failure | |||||||||
| n/N (%) | 49/76 (64) | 7/20 (35) | 56/96 (58) | 2/6 (33) | 2/5 (40) | 4/11 (36) | 114/141 (81) | 3/7 (43) | 117/148 (79) |
| Bootstrap 95% CI | (52%–77%) | (11%–58%) | (48%–71%) | (0%–80%) | (0%–100%) | (11%–70%) | (74%–88%) | (0%–83%) | (72%–86%) |
| Misclassification as success: probability of having virological failure among those without guideline predicted failure | |||||||||
| n/N (%) | 94/893 (11) | 8/60 (13) | 102/953 (11) | 113/931 (12) | 17/71 (24) | 130/1002 (13) | 77/664 (12) | 12/58 (21) | 89/722 (12) |
| Bootstrap 95% CI | (8%–13%) | (5%–23%) | (8%–13%) | (10%–15%) | (14%–36%) | (10%–16%) | (9%–15%) | (12%–33%) | (9%–16%) |
| Accuracy: probability guidelines correctly characterize those with and without virological failure | |||||||||
| n/N (%) | 826/969 (85) | 65/80 (81) | 891/1049 (85) | 822/937 (88) | 57/76 (75) | 879/1013 (87) | 614/805 (76) | 50/65 (77) | 664/870 (76) |
| Bootstrap 95% CI | (83%–88%) | (72%–90%) | (82%–88%) | (85%–90%) | (64%–84%) | (84%–89%) | (73%–79%) | (66%–87%) | (73%–79%) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; WHO, World Health Organization.
Of 1079 VLs, 1049 (97%) had CD4 values available for evaluation of WHO 2006 guidelines, 1013 (94%) had CD4 values available for WHO 2010 guidelines, and 870 (81%) had CD4 values available for Cambodia 2011 guidelines. Reduction in the number of evaluable VLs for the WHO 2010 guidelines is due to the guidelines’ lack of criteria for children aged 1–2 years and the provision to use only CD4 count cutoffs (but not CD4 percentages) for children aged >5 years; the reduction in the number of evaluable VLs for the Cambodia 2011 guidelines is due to the need for data to analyze a 6-month CD4 trend.
The accuracy of the WHO 2010 guidelines for predicting virological failure was 87%. One hundred one of 457 (22%) children at 134 of 1013 (13%) visits were misclassified. Thirty-six percent of visits were misclassified as failure (4 of 11) and 13% were misclassified as success (130 of 1002); the latter was more common with a higher WHO stage. Though specificity was high (99.6%), sensitivity for virological failure detection was very low (5%); among 117 observations with WHO stage 1/2 classification and detectable VL, only 4 were correctly identified by the 2010 guidelines.
Guidelines Comparison
The WHO 2010 guidelines demonstrated slightly improved accuracy compared with the WHO 2006 guidelines (87% vs 85%; P = .05), stable high misclassification as failure (36% vs 58%; P = .14) and slightly worse misclassification as success (13% vs 11%; P < .001). The addition of 6-month 30% CD4 decrease as required by the Cambodian 2011 guidelines resulted in lower accuracy (76%; P = .001), higher misclassification as failure (79%; P = .01), and similar misclassification as success (12%; P = .37).
Examination of demographic, clinical, and laboratory measurements with misclassification demonstrated that shorter ART duration (12 vs 43 months; P < .001) and older age at HIV diagnosis (7 vs 5 years; P = .05) were associated with misclassification as failure by the WHO 2010 guidelines. A WHO stage 3/4 classification was associated with lower misclassification as success in 2006, but this association was lost in 2010. Age, gender, and tuberculosis treatment history were not predictive of failure misclassification by any guideline. No covariates were associated with misclassification by the Cambodian guidelines.
Guideline Detection of Resistance
Fifty-six RT genotypes were available; 5 did not associate with VL and were excluded. All but 1 sequence (subtype B) were CRF01_AE. Ninety-eight percent (50 of 51) of children had evidence of ≥1 resistance mutation. Twenty-one of 51 (41%) children had 1 mutation to NRTIs; 15 of 51 (29%) had 2 mutations to NRTIs; and 13 of 51 (25%) had ≥3 mutations to NRTIs. M184V/I was present in 45 of 51 (88%) children, and thymidine analog mutation was present in 18 of 51 (35%) children. Only 1 child had no NNRTI mutations; 14 of 51 (27%) had 1 NNRTI mutation; 31 of 51 (61%) had 2 NNRTI mutations; 4 of 51 (8%) had 3 NNRTI mutations; and 1 of 51 (2%) had 4 NNRTI mutations. A detailed analysis of the drug resistance patterns is ongoing.
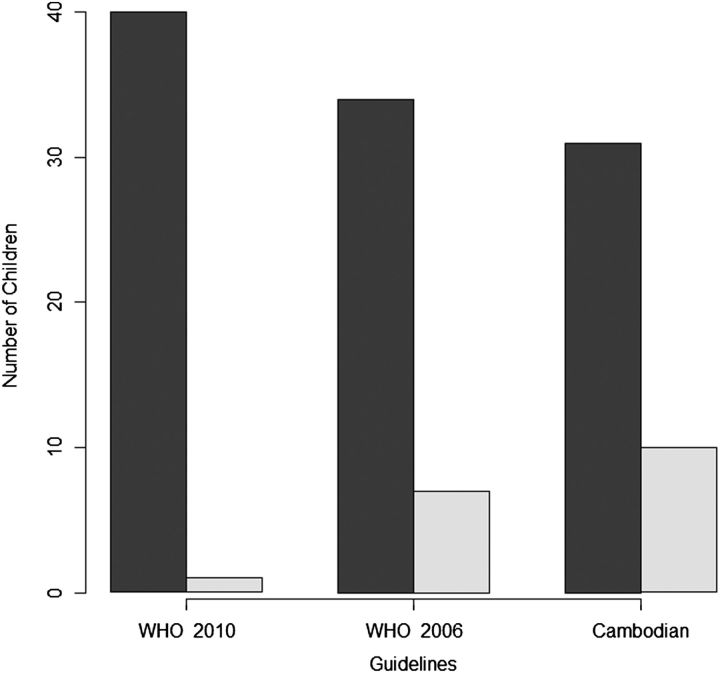
Forty-one of 51 children with available genotypes had CD4 data available to evaluate all 3 guidelines. The vast majority were not identified as ART failures by the guidelines’ criteria (Figure 1), with the WHO 2010 guidelines missing a significantly larger percentage (P < .05). Ninety-eight percent (40 of 41) were missed using WHO 2010 guidelines (95% CI, 83%–100%); 83% (34 of 41) were missed using WHO 2006 guidelines (95% CI, 68%–93%); and 76% (31 of 41) were missed using Cambodian 2011 guidelines (95% CI, 60%–88%). Only 1 of 51 children with virological failure and available genotype was identified as failing by WHO 2010 guidelines. All other children with available genotypes had CD4 counts >100 cells/mL if aged >5 years or CD4 counts >200 cells/mL and CD4 percentages >10% if aged <5 years.
Figure 1.
Prediction of treatment failure among 41 Cambodian children with available genotypes and linked data. The bars depict the number of children with available genotypes and linked CD4 and viral load data who were predicted as not failing (misclassified; dark bars) or as failing (correctly classified; light bars) antiretroviral therapy based on the World Health Organization 2010, WHO 2006, and Cambodia 2011 guidelines. Abbreviation: WHO, World Health Organization.
Children predicted to be failing ART based on WHO 2006 guidelines had significantly more NRTI mutations (odds ratio [OR], 5.5; 95% CI, 1.3–23.8; P = .02 by ordinal regression). This association was not observed with the WHO 2006 guidelines for NNRTI mutations (OR, 1.5; 95% CI, .36–6.4; P = .60 by ordinal regression) or with Cambodian 2011 guidelines (NRTI: OR, 0.70; 95% CI, .21–2.67; NNRTI: OR, 1.1; 95% CI, .4–7.5). Because only 1 child was identified with immunological failure based on the WHO 2010 guidelines, this association was not examined.
DISCUSSION
We evaluated the performance of the recently published WHO 2010 pediatric guidelines in predicting ART failure in 457 Cambodian children (median age at diagnosis 4.9 years) who were treated with first-line ART for at least 6 months. Compared with the WHO 2006 guidelines, we found that the nonvirological ART monitoring strategies presented in the WHO 2010 guidelines still have high rates of treatment failure misclassification, with children misclassified at 13% of clinic visits. In our data, 134 of 1013 visits with VL data were misclassified among 22% (101 of 457) of children. All but 4 of 134 visits were misclassified as not failing ART, thereby delaying identification of virological failure and increasing risk for resistance accumulation. Using the WHO 2010 guidelines, high (24%) misclassification was seen even in children with WHO stage 3/4 events and true virological failure. In comparison with the WHO 2006 guidelines, the WHO 2010 guidelines had slightly better accuracy, with less misclassification as failure but greater misclassification as success. Higher misclassification rates were seen in older children with shorter ART duration. The addition of a 6-month 30% drop in CD4 counts or CD4 percentages, incorporated in the Cambodian 2011 guidelines, did not improve accuracy.
Immunological and clinical surrogates of virological failure poorly predict treatment failure in adults and result in high misclassification rates [14–19]. This evidence led the WHO to strongly recommend use of VL to confirm treatment failure where available [28]. Using the WHO 2006 guidelines, high specificity (92%–100%) but low sensitivity (3.5%) for detection of treatment failure was described in Tanzanian [29] and Kenyan [30] children. Our data extend prior South African [31] and Ugandan [10] reports and show that use of the WHO 2010 guidelines without virological monitoring would also result in unacceptably poor detection of virological failure, identifying only 4 of 117 (3%) with WHO stage 1/2 disease and VL >400 copies/mL.
Of 41 children with available CD4 and resistance data, only 1 was identified by the WHO 2010 guidelines as failing ART due to their new, strict immunological thresholds. The patient was a 12-year-old male, who was diagnosed with HIV at 9.3 years, was on stavudine, lamivudine, and nevirapine for 2 years, and had WHO stage 1 disease, a CD4 count of 94 (5%), a VL of 64 621 copies/mL, and 4 NRTI (A62V, M184V, L210W, T215F) and 2 NNRTI (V90I, Y181C) mutations. It is unclear why development of resistance occurs without sufficient immunological failure. Possible explanations include strict guideline immunologic criteria and rapid resistance evolution; low nonadherence, supported by aggressive adherence counseling at the site, which may have resulted in enough drug selection pressure to allow resistance evolution; and annual VL monitoring, which may allow identification of failure early enough to minimize immunological decline. Prospective studies should further evaluate these hypotheses.
Early virological failure detection prevents accumulation of resistance and allows more effective second-line ART [32, 33]. It is unclear whether nonvirological monitoring can be sensitive enough to detect viral replication before substantial resistance accumulates or whether early failure detection allows sufficiently rapid regimen switch. In a large South African cohort, only 38% of virologically failing children were switched to second-line therapy, with a median of 10 months between detection of first elevated VL and switch [7]. Our data support an association between late identification of failure and resistance accumulation [34], further suggesting a benefit from virological monitoring [35, 36]. Although the WHO guidelines do not claim to detect resistance, our finding of almost complete lack of identification of children with extensive resistance by the WHO 2010 guidelines is concerning and justifies further research.
Interpreting WHO guidelines can be extremely difficult for busy clinicians as well as national guideline committees. Particularly challenging are CD4 percentage and count thresholds that differ between age groups and vague statements such as “rapid rate of decline” [13]. Criteria simplifications in the WHO 2010 guidelines are an improvement, but they now leave clinicians and national programs without guidance for children aged <2 years or for those aged >5 years but with discordant low CD4 percentages. This lack of clarity may lead to inaction with regard to treatment failure identification or an inappropriately prompt switch to second-line therapy. Global guidelines should provide clear directions for children of all ages because attempts at local or national levels to overcome these limitations may lead to suboptimal treatment monitoring strategies or even inappropriate use of ART regimens. Research is urgently needed to provide guideline committees with such data.
There are several limitations to our study. First, participation of few children aged <5 years, and particularly aged <2 years, limited evaluation of this critical age group. This is a reflection of the struggle to link HIV-exposed infants to routine testing and care in RLSs. Second, confirmatory VL/CD4 testing was unavailable, and CD4 count/percentage testing concordance was at times absent. These reflect real-world limitations in RLSs and likely mirror current practice in much of the developing world. Moreover, confirmatory CD4 would most likely further decrease sensitivity and increase specificity because fewer children would be classified as failing immunologically. Confirmatory CD4 testing (within 3 months of VL testing) was performed on 9 of 80 (11%) and 2 of 11 (18%) children with immunological failure based on WHO 2006 and WHO 2010 guidelines, respectively. Of the 9 of 80, the immunological status of 7 would not have changed; 1 who was misclassified as failing would have been correctly classified; and 1 who was correctly classified as failing would have been misclassified as success. Both of the 2 of 11 would not have changed classification. Third, this was a retrospective study with VL testing performed yearly and at the clinicians’ discretion. Prospective studies with real-time and frequent detection of virological failure are needed. Finally, ART adherence data were lacking, although believed to be high [5, 37], and only a few (15 of 142; 11%) VL resuppression instances on first-line ART were observed.
In summary, ART monitoring using clinical and immunological criteria is problematic in children, and misclassification rates using the WHO 2010 pediatric guidelines remain high. Finding better tools for treatment monitoring in RLSs, which is currently ongoing by our group [15] and others [38–40], is challenging from both statistical/analytical and host/assay diversity aspects. Despite shortcomings presented here and elsewhere, guidelines should be continuously followed and used to derive data that will drive their continued improvement. Further evaluations of virological vs nonvirological monitoring on clinical outcome, second-line therapy effectiveness, and programmatic costs are essential. Incorporation of novel VL testing technologies into current monitoring strategies should also be of high research priority. One thing remains clear: affordable VL testing suitable for use in RLSs is urgently needed.
Notes
Acknowledgments. We thank Drs Seitaboth Soeung, Ngoun Chanpheaktra, Varun Kumar, Chheng Kheng, and William Houseworth and Ms Kazumi Akao for their leadership and support; Mr Im Uom, Mr Sivpheng Seng, Mr Sopheary Sun, Ms Ken Sreymom, Ms Nouhin Janin, and Ms Ngin Sopheak for their technical assistance; and Drs Timothy Flanigan, Charles Carpenter, Kenneth Mayer, and Susan Cu-Uvin for their vision to deliver the highest level of clinical care and research to the developing world.
Financial support. This work was supported by the Brown University Framework in Global Health Program (National Institutes of Health [NIH] grant R25 TW008102). R. K., A. K. D., and L. S. are funded by an NIH/RO1 grant (AI66922). R. K. and A. K. D. are also funded by the National Institute of Allergy And Infectious Diseases (grant P30AI042853).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.UNAIDS. Global report. Available at: http://www.unaids.org/globalreport/ Accessed 23 August 2011. [Google Scholar]
- 2.National Institute of Statistics of. Cambodia: Available at: www.nis.gov.kh . Accessed 19 January 2011. [Google Scholar]
- 3.Ministry of Health, Kingdom of Cambodia. Third quarterly comprehensive report, 2010. Phnom Penh, Cambodia: HIV/AIDS & STI Prevention and Care Programme. 2010 [Google Scholar]
- 4.Charpentier KP, Wolf F, Noble L, Winn B, Resnick M, Dupuy DE. Irreversible electroporation of the liver and liver hilum in swine. HPB (Oxford) 2011;13:168–73. doi: 10.1111/j.1477-2574.2010.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isaakidis P, Raguenaud ME, Te V, et al. High survival and treatment success sustained after two and three years of first-line ART for children in Cambodia. J Int AIDS Soc. 2010;13:11. doi: 10.1186/1758-2652-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raguenaud ME, Isaakidis P, Zachariah R, et al. Excellent outcomes among HIV+ children on ART, but unacceptably high pre-ART mortality and losses to follow-up: a cohort study from Cambodia. BMC Pediatr. 2009;9:54. doi: 10.1186/1471-2431-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa—the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011;56:270–8. doi: 10.1097/QAI.0b013e3182060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charpentier C, Gody JC, Mbitikon O, et al. Virological response and resistance profiles after 18 to 30 months of first- or second/third-line antiretroviral treatment: a cross-sectional evaluation in HIV-1-infected children living in the Central African Republic. AIDS Res Hum Retroviruses. 2011;28:87–94. doi: 10.1089/aid.2011.0035. [DOI] [PubMed] [Google Scholar]
- 9.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–20. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruel TD, Kamya MR, Li P, et al. Early virologic failure and the development of antiretroviral drug resistance mutations in HIV-infected Ugandan children. J Acquir Immune Defic Syndr. 2011;56:44–50. doi: 10.1097/QAI.0b013e3181fbcbf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantor R, Shafer RW, Follansbee S, et al. Evolution of resistance to drugs in HIV-1-infected patients failing antiretroviral therapy. AIDS. 2004;18:1503–11. doi: 10.1097/01.aids.0000131358.29586.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministry of Health, Kingdom of Cambodia. National guidelines for the use of pediatric antiretroviral therapy in Cambodia. Phnom Penh, Cambodia: Ministry of Health. 2007. [Google Scholar]
- 13.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access, recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2006. [PubMed] [Google Scholar]
- 14.Mee P, Fielding KL, Charalambous S, Churchyard GJ, Grant AD. Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. AIDS. 2008;22:1971–7. doi: 10.1097/QAD.0b013e32830e4cd8. [DOI] [PubMed] [Google Scholar]
- 15.Kantor R, Diero L, Delong A, et al. Misclassification of first-line antiretroviral treatment failure based on immunological monitoring of HIV infection in resource-limited settings. Clin Iinfect Dis. 2009;49:454–62. doi: 10.1086/600396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaiwarith R, Wachirakaphan C, Kotarathititum W, Praparatanaphan J, Sirisanthana T, Supparatpinyo K. Sensitivity and specificity of using CD4+ measurement and clinical evaluation to determine antiretroviral treatment failure in Thailand. Int J Infect Dis. 2007;11:413–6. doi: 10.1016/j.ijid.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Meya D, Spacek LA, Tibenderana H, et al. Development and evaluation of a clinical algorithm to monitor patients on antiretrovirals in resource-limited settings using adherence, clinical and CD4 cell count criteria. J Int AIDS Soc. 2009;12:3. doi: 10.1186/1758-2652-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds SJ, Nakigozi G, Newell K, et al. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. AIDS. 2009;23:697–700. doi: 10.1097/QAD.0b013e3283262a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Oosterhout JJ, Brown L, Weigel R, et al. Diagnosis of antiretroviral therapy failure in Malawi: poor performance of clinical and immunological WHO criteria. Trop Med Int Health. 2009;14:856–61. doi: 10.1111/j.1365-3156.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access, recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 21.Ministry of Health, Kingdom of Cambodia. National guidelines for the use of pediatric antiretroviral therapy in Cambodia. Phnom Penh, Cambodia: Ministry of Health. 2011. [Google Scholar]
- 22.Delong AK, Wu M, Bennett D, et al. Sequence quality analysis tool for HIV type 1 protease and reverse transcriptase. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/aid.2011.0120. PMID: 21916749. doi:10.1089/aid.2011.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Oliveira T, Deforche K, Cassol S, et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics. 2005;21:3797–800. doi: 10.1093/bioinformatics/bti607. [DOI] [PubMed] [Google Scholar]
- 24.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2010. Top HIV Med. 2010;18:156–63. [PubMed] [Google Scholar]
- 25.Rouet F, Chaix ML, Nerrienet E, et al. Impact of HIV-1 genetic diversity on plasma HIV-1 RNA quantification: usefulness of the Agence Nationale de Recherches sur le SIDA second-generation long terminal repeat-based real-time reverse transcriptase polymerase chain reaction test. J Acquir Immune Defic Syndr. 2007;45:380–8. doi: 10.1097/QAI.0b013e3180640cf5. [DOI] [PubMed] [Google Scholar]
- 26.Lan NT, Recordon-Pinson P, Hung PV, et al. HIV type 1 isolates from 200 untreated individuals in Ho Chi Minh City (Vietnam): ANRS 1257 study. Large predominance of CRF01_AE and presence of major resistance mutations to antiretroviral drugs. AIDS Res Hum Retroviruses. 2003;19:925–8. doi: 10.1089/088922203322493111. [DOI] [PubMed] [Google Scholar]
- 27.Team RDC. R: A language and environment for statistical computing. Available at: http://www.R-project.org/ Accessed 15 August 2011. [Google Scholar]
- 28.World Health Organization. Antiretroviral therapy for HIV infection. in adults and adolescents. Recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 29.Emmett SD, Cunningham CK, Mmbaga BT, et al. Predicting virologic failure among HIV-1–infected children receiving antiretroviral therapy in Tanzania: a cross-sectional study. J Acquir Immune Defic Syndr. 2010;54:368–75. doi: 10.1097/QAI.0b013e3181cf4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dufort E, Nyandiko W, DeLong A, et al. Misclassification of treatment failure using immunologic and clinical monitoring in HIV infected children and adolescents in western Kenya. 2010 doi: 10.1093/jpids/piw018. Program and abstracts of the 48th annual meeting of IDSA (Vancouver, BC, Canada). 2010. Arlington, VA: IDSA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies MA, Boulle A, Eley B, et al. Accuracy of immunological criteria for identifying virological failure in children on antiretroviral therapy—the IeDEA Southern Africa collaboration. Trop Med Int Health. 2011 doi: 10.1111/j.1365-3156.2011.02854.x. doi:10.1111/j.1365-3156.2011.02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigaloff KC, Hamers RL, Wallis CL, et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr. 2011;58:23–31. doi: 10.1097/QAI.0b013e318227fc34. [DOI] [PubMed] [Google Scholar]
- 33.Barth RE, Tempelman HA, Smelt E, Wensing AM, Hoepelman AI, Geelen SP. Long-term outcome of children receiving antiretroviral treatment in rural South Africa: substantial virologic failure on first-line treatment. Pediatr Infect Dis J. 2011;30:52–6. doi: 10.1097/INF.0b013e3181ed2af3. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Mu W, Harwell J, et al. Drug resistance profiles among HIV-1–infected children experiencing delayed switch and 12-month efficacy after using second-line antiretroviral therapy: an observational cohort study in rural China. J Acquir Immune Defic Syndr. 2011;58:47–53. doi: 10.1097/QAI.0b013e318229f2a2. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira R, Krauss M, Essama-Bibi S, et al. Viral load predicts new World Health Organization stage 3 and 4 events in HIV-infected children receiving highly active antiretroviral therapy, independent of CD4 T lymphocyte value. Clin Infect Dis. 2010;51:1325–33. doi: 10.1086/657119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider K, Puthanakit T, Kerr S, et al. Economic evaluation of monitoring virologic responses to antiretroviral therapy in HIV-infected children in resource-limited settings. AIDS. 2011;25:1143–51. doi: 10.1097/QAD.0b013e3283466fab. [DOI] [PubMed] [Google Scholar]
- 37.Janssens B, Raleigh B, Soeung S, et al. Effectiveness of highly active antiretroviral therapy in HIV-positive children: evaluation at 12 months in a routine program in Cambodia. Pediatrics. 2007;120:e1134–40. doi: 10.1542/peds.2006-3503. [DOI] [PubMed] [Google Scholar]
- 38.Abouyannis M, Menten J, Kiragga A, et al. Development and validation of systems for rational use of viral load testing in adults receiving first-line ART in sub–Saharan Africa. AIDS. 2011;25:1627–35. doi: 10.1097/QAD.0b013e328349a414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Zyl GU, Preiser W, Potschka S, Lundershausen AT, Haubrich R, Smith D. Pooling strategies to reduce the cost of HIV-1 RNA load monitoring in a resource-limited setting. Clin Infect Dis. 2011;52:264–70. doi: 10.1093/cid/ciq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips AN, Pillay D, Garnett G, et al. Effect on transmission of HIV-1 resistance of timing of implementation of viral load monitoring to determine switches from first to second-line antiretroviral regimens in resource-limited settings. AIDS. 2011;25:843–50. doi: 10.1097/QAD.0b013e328344037a. [DOI] [PubMed] [Google Scholar]