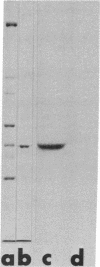
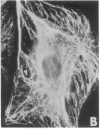
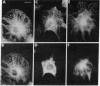
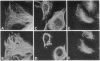
Abstract
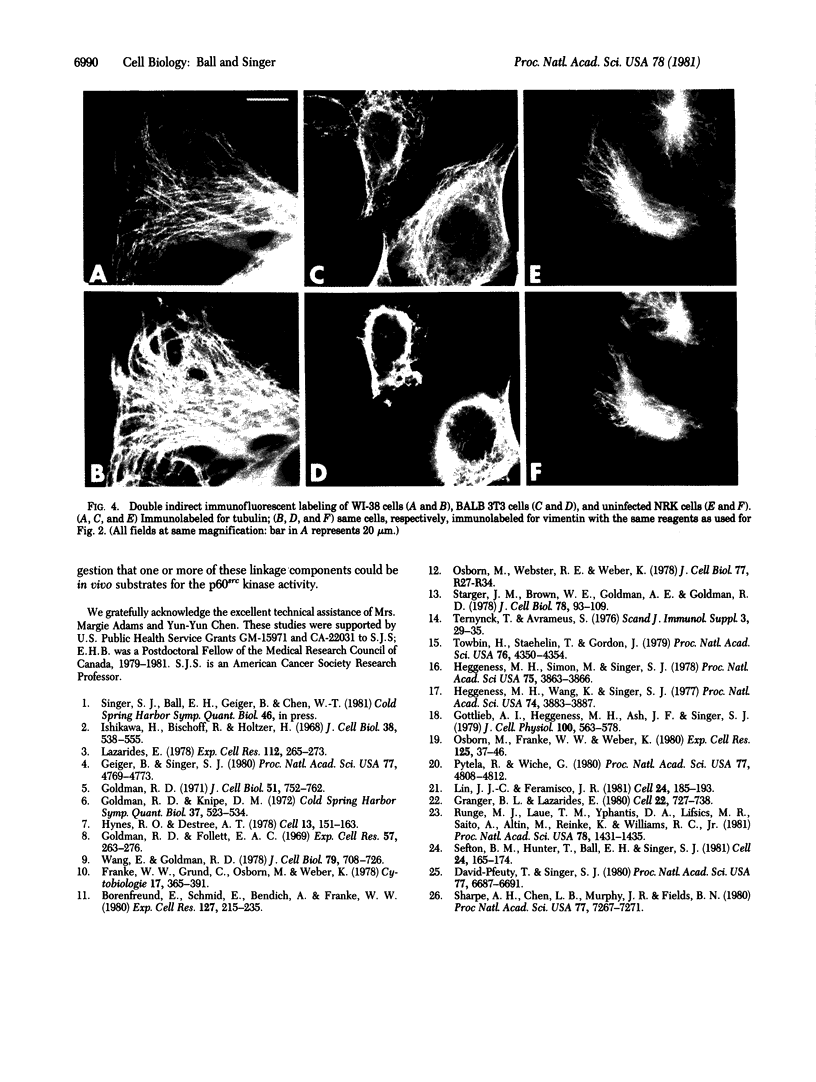
By double indirect immunofluorescence, using primary rabbit antibodies to tubulin and guinea pig antibodies to vimentin, we have simultaneously labeled microtubules and intermediate filaments in several types of cultured normal fibroblasts. With well-spread interphase cells there was an extensive but not complete correspondence of the labeling patterns for the two filamentous structures out to the cell periphery. This correspondence existed both at a gross level, where parallel but not coincident arrays of thickly labeled strands of the two types of filaments were observed, and at a fine level, where thinly labeled strands of the two were superimposed. The results suggest that there may be some type(s) of molecular linkages between microtubules and vimentin intermediate filaments that is under metabolic control. With NRK fibroblasts infected with a temperature-sensitive mutant (LA23) of Rous sarcoma virus, cells grown at the nonpermissive temperature (39 degrees C) showed the correspondence of the distributions of the microtubules and intermediate filaments characteristic of the normal phenotype but within 1 hr after a shift to the permissive temperature (33 degrees C) there was an extensive retraction of the intermediate filaments around the cell nucleus whereas the microtubules remained dispersed into the cell periphery. These results suggest that one of the functions carried out by p60src, the protein kinase responsible for transformation by Rous sarcoma virus, may be to modify the component(s) involved in the putative linkages between microtubules and intermediate filaments in the normal cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borenfreund E., Schmid E., Bendich A., Franke W. W. Constitutive aggregates of intermediate-sized filaments of the vimentin and cytokeratin type in cultured hepatoma cells and their dispersal by butyrate. Exp Cell Res. 1980 May;127(1):215–235. doi: 10.1016/0014-4827(80)90428-0. [DOI] [PubMed] [Google Scholar]
- David-Pfeuty T., Singer S. J. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Osborn M., Weber K. The intermediate-sized filaments in rat kangaroo PtK2 cells. I. Morphology in situ. Cytobiologie. 1978 Aug;17(2):365–391. [PubMed] [Google Scholar]
- Geiger B., Singer S. J. Association of microtubules and intermediate filaments in chicken gizzard cells as detected by double immunofluorescence. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4769–4773. doi: 10.1073/pnas.77.8.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D., Follett E. A. The structure of the major cell processes of isolated BHK21 fibroblasts. Exp Cell Res. 1969 Oct;57(2):263–276. doi: 10.1016/0014-4827(69)90150-5. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb A. I., Heggeness M. H., Ash J. F., Singer S. J. Mechanochemical proteins, cell motility and cell-cell contacts: the localization of mechanochemical proteins inside cultured cells at the edge of an in vitro "wound". J Cell Physiol. 1979 Sep;100(3):563–578. doi: 10.1002/jcp.1041000318. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Synemin: a new high molecular weight protein associated with desmin and vimentin filaments in muscle. Cell. 1980 Dec;22(3):727–738. doi: 10.1016/0092-8674(80)90549-8. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Simon M., Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Wang K., Singer S. J. Intracellular distributions of mechanochemical proteins in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3883–3887. doi: 10.1073/pnas.74.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. The distribution of desmin (100 A) filaments in primary cultures of embryonic chick cardiac cells. Exp Cell Res. 1978 Mar 15;112(2):265–273. doi: 10.1016/0014-4827(78)90209-4. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Feramisco J. R. Disruption of the in vivo distribution of the intermediate filaments in fibroblasts through the microinjection of a specific monoclonal antibody. Cell. 1981 Apr;24(1):185–193. doi: 10.1016/0092-8674(81)90514-6. [DOI] [PubMed] [Google Scholar]
- Osborn M., Franke W., Weber K. Direct demonstration of the presence of two immunologically distinct intermediate-sized filament systems in the same cell by double immunofluorescence microscopy. Vimentin and cytokeratin fibers in cultured epithelial cells. Exp Cell Res. 1980 Jan;125(1):37–46. doi: 10.1016/0014-4827(80)90186-x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Webster R. E., Weber K. Individual microtubules viewed by immunofluorescence and electron microscopy in the same PtK2 cell. J Cell Biol. 1978 Jun;77(3):R27–R34. doi: 10.1083/jcb.77.3.r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Wiche G. High molecular weight polypeptides (270,000-340,000) from cultured cells are related to hog brain microtubule-associated proteins but copurify with intermediate filaments. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4808–4812. doi: 10.1073/pnas.77.8.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge M. S., Laue T. M., Yphantis D. A., Lifsics M. R., Saito A., Altin M., Reinke K., Williams R. C., Jr ATP-induced formation of an associated complex between microtubules and neurofilaments. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1431–1435. doi: 10.1073/pnas.78.3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Chen L. B., Murphy J. R., Fields B. N. Specific disruption of vimentin filament organization in monkey kidney CV-1 cells by diphtheria toxin, exotoxin A, and cycloheximide. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7267–7271. doi: 10.1073/pnas.77.12.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Goldman R. D. Functions of cytoplasmic fibers in intracellular movements in BHK-21 cells. J Cell Biol. 1978 Dec;79(3):708–726. doi: 10.1083/jcb.79.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]