Abstract
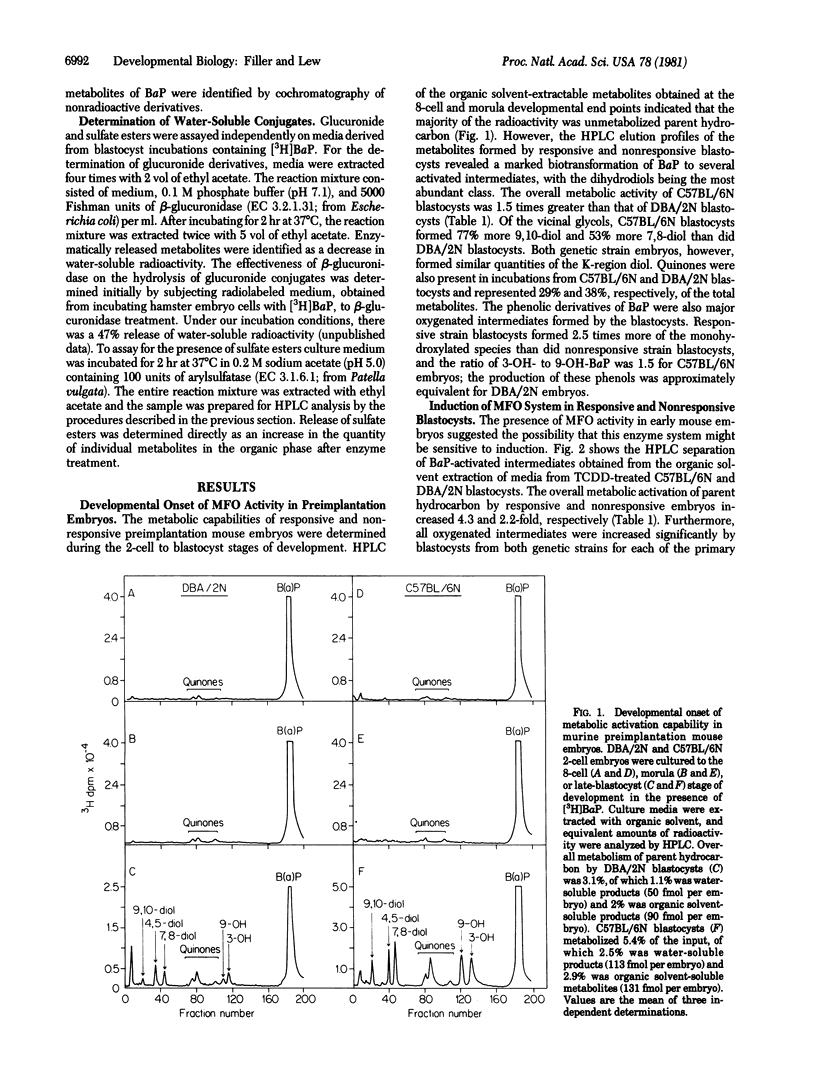
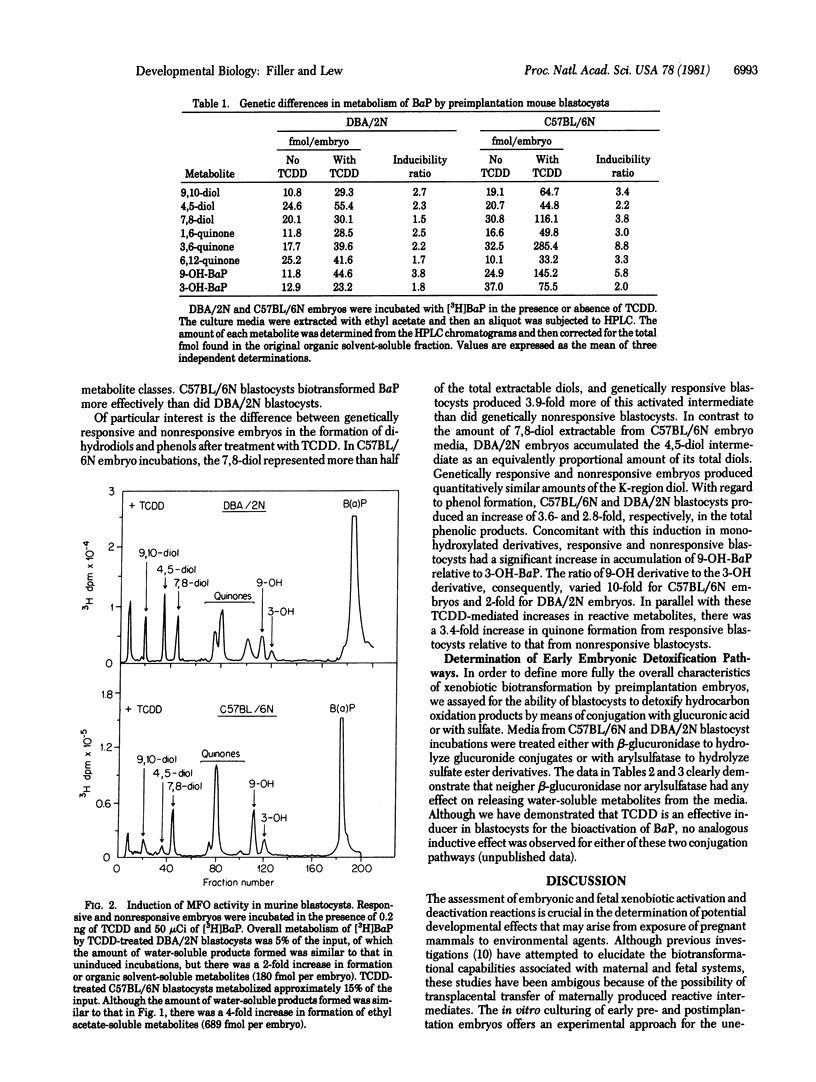
Two-cell embryos, obtained from the C57BL/6N and DBA/2N strains, were cultured in media that supported in vitro differentiation and that contained [3H]benzo[a]pyrene. High-pressure liquid chromatography of the activated intermediates formed during in vitro early embryonic development indicated that the onset of polynuclear aromatic hydrocarbon activation coincided with blastocyst formation. Comparison of individual oxygenated intermediates metabolically formed from embryos genetically "responsive" or "nonresponsive" to aromatic hydrocarbons revealed significant quantitative differences in the production of dihydrodiol, quinone, and phenolic derivatives. In addition to exhibiting basal mixed-function oxidase activity, blastocysts were also responsive to enzymatic induction when exposed to 2,-3,7,8-tetrachlorodibenzo-p-dioxin. The presence of operative metabolite-detoxifying pathways was also assayed. Enzymatic treatment of water-soluble metabolites with beta-glucuronidase or arylsulfatase revealed that neither glucuronic acid conjugates nor sulfate ester derivatives were present. These data, therefore, provide direct evidence that late preimplantation mouse embryos (day 3 1/2 of gestation) are similar to later developmental stages in having the enzymatic capability for xenobiotic activation and enzyme induction but are dissimilar with respect to their detoxification mechanism(s). Moreover, the ability of preimplantation embryos to activate directly polynuclear aromatic hydrocarbon to bioreactive intermediates may be of importance in assessing the ontological susceptibility of the developing embryo to carcinogenic or teratogenic chemicals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS C. E., HAY M. F., LUTWAK-MANN C. The action of various agents upon the rabbit embryo. J Embryol Exp Morphol. 1961 Sep;9:468–491. [PubMed] [Google Scholar]
- CHANG M. C. EFFECTS OF CERTAIN ANTIFERTILITY AGENTS ON THE DEVELOPMENT OF RABBIT OVA. Fertil Steril. 1964 Jan-Feb;15:97–106. doi: 10.1016/s0015-0282(16)35113-5. [DOI] [PubMed] [Google Scholar]
- Dewey M. J., Filler R., Mintz B. Protein patterns of developmentally totipotent mouse teratocarcinoma cells and normal early embryo cells. Dev Biol. 1978 Jul;65(1):171–182. doi: 10.1016/0012-1606(78)90188-4. [DOI] [PubMed] [Google Scholar]
- Filler R., Garner-Shinpock S. Metabolism of benzo(a)pyrene by murine embryonal carcinoma cells. Cancer Res. 1981 Mar;41(3):1104–1109. [PubMed] [Google Scholar]
- Galloway S. M., Perry P. E., Meneses J., Nebert D. W., Pedersen R. A. Cultured mouse embryos metabolize benzo[a]pyrene during early gestation: genetic differences detectable by sister chromatid exchange. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3524–3528. doi: 10.1073/pnas.77.6.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- Lambert G. H., Nebert D. W. Genetically mediated induction of drug-metabolizing enzymes associated with congenital defects in the mouse. Teratology. 1977 Oct;16(2):147–153. doi: 10.1002/tera.1420160206. [DOI] [PubMed] [Google Scholar]
- Lucier G. W., Lui E. M., Lamartiniere C. A. Metabolic activation/deactivation reactions during perinatal development. Environ Health Perspect. 1979 Apr;29:7–16. doi: 10.1289/ehp.79297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Bausserman L. L. Genetic differences in the extent of aryl hydrocarbon hydroxylase induction in mouse fetal cell cultures. J Biol Chem. 1970 Dec 10;245(23):6373–6382. [PubMed] [Google Scholar]
- Nebert D. W., Robinson J. R., Niwa A., Kumaki K., Poland A. P. Genetic expression of aryl hydrocarbon hydroxylase activity in the mouse. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):393–414. doi: 10.1002/jcp.1040850407. [DOI] [PubMed] [Google Scholar]
- Poland A. P., Glover E., Robinson J. R., Nebert D. W. Genetic expression of aryl hydrocarbon hydroxylase activity. Induction of monooxygenase activities and cytochrome P1-450 formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice genetically "nonresponsive" to other aromatic hydrocarbons. J Biol Chem. 1974 Sep 10;249(17):5599–5606. [PubMed] [Google Scholar]
- Russell L. B. Sensitivity patterns for the induction of homeotic shifts in a favorable strain of mice. Teratology. 1979 Aug;20(1):115–125. doi: 10.1002/tera.1420200115. [DOI] [PubMed] [Google Scholar]
- Sherman M. I. Developmental biochemistry of preimplantation mammalian embryos. Annu Rev Biochem. 1979;48:443–470. doi: 10.1146/annurev.bi.48.070179.002303. [DOI] [PubMed] [Google Scholar]
- Shum S., Jensen N. M., Nebert D. W. The murine Ah locus: in utero toxicity and teratogenesis associated with genetic differences in benzo[a]pyrene metabolism. Teratology. 1979 Dec;20(3):365–376. doi: 10.1002/tera.1420200307. [DOI] [PubMed] [Google Scholar]
- Takahashi G., Yasuhira K. Chromatographic analyses of 3-methylcholanthrene metabolism in adult and fetal mice and the occurrence of conjugating enzymes in the fetus. Cancer Res. 1975 Mar;35(3):613–620. [PubMed] [Google Scholar]
- Wang I. Y., Rasmussen R. E., Crocker T. T. Metabolism of benzo(a)pyrene by microsomes from tissues of pregnant and fetal hamsters. Life Sci. 1974 Oct 1;15(7):1291–1300. doi: 10.1016/0024-3205(74)90310-5. [DOI] [PubMed] [Google Scholar]


