Abstract
Addition of membrane-permeable cyclic GMP (cGMP) and cyclic AMP (cAMP) were shown to cause elevation of cytosolic Ca2+ concentration ([Ca2+]cyt) in tobacco (Nicotiana plumbaginofolia) protoplasts. Under the same conditions these cyclic nucleotides were shown to provoke a physiological swelling response in the protoplasts. Nonmembrane-permeable cAMP and cGMP were unable to trigger a detectable [Ca2+]cyt response. Cyclic-nucleotide-mediated elevations in [Ca2+]cyt involved both internal and external Ca2+ stores. Both cAMP- and cGMP-mediated [Ca2+]cyt elevations could be inhibited by the Ca2+-channel blocker verapamil. Addition of inhibitors of phosphodiesterases (isobutylmethylxanthine and zaprinast) and the adenylate cyclase agonist forskolin to the protoplasts (predicted to elevate in vivo cyclic-nucleotide concentrations) caused elevations in [Ca2+]cyt. Addition of the adenylate cyclase inhibitor 2′,5′-dideoxyadenosine before forskolin significantly inhibited the forskolin-induced [Ca2+]cyt elevation. Taken together, these data suggest that a potential communication point for cross-talk between signal transduction pathways using cyclic nucleotides in plants is at the level of Ca2+ signaling.
Environmental and hormonal signals regulate various physiological processes in plants via signal transduction pathways. Essential components of signal transduction pathways include second messengers. Vital roles for the second messengers Ca2+, cAMP, cGMP, IP3, and 1,2-diacylglycerol were first discovered in animal systems (Berridge and Irvine, 1984; Nishizuka, 1984). The last decade has been marked by substantial progress in elucidating the mechanisms of intracellular signaling in plants (Gilroy and Trewavas, 1994). Attempts to draw comparisons with mammalian systems have been successful in showing that Ca2+-calmodulin, cGMP (Neuhaus et al., 1993), and phosphoinositide signal mechanisms (Alexandre et al., 1990; Allen at al., 1995) also exist in plants, although cAMP involvement in transduction pathways has remained controversial (Assmann, 1995; Bolwell, 1995).
It is known that a number of chemical and physical stimuli mediate their effects via transient increases in the concentration of intracellular free Ca2+ (Gilroy and Trewavas, 1994). Ca2+ influx into the cytosol can occur because of the opening of Ca2+ channels in plasma membrane, with Ca2+ entering the cell down a concentration gradient. Receptor activation, at least in mammalian cells, can trigger the phosphoinositide cascade, which leads to the production of IP3 and 1,2-diacylglycerol (Berridge and Irvine, 1984). IP3 can then provoke the release from the internal Ca2+ stores of plants cells by opening intracellular Ca2+ channels (Alexandre et al., 1990; Gilroy et al., 1990; Allen et al., 1995). Increases in [Ca2+]cyt produced either by influx or release from internal Ca2+ stores stimulate the phosphorylation of proteins within the cell (Poovaiah and Reddy, 1990). An increase in [Ca2+]cyt detected by Ca2+ microelectrodes, Ca2+-sensitive fluorescence probes, and extrinsic and intrinsic aequorin (Cobbold and Rink, 1987; Chae et al., 1990; Knight et al., 1991; Shacklock et al., 1992), followed by the alteration of the activities of intercellular targets, enzymes, genes, pumps, and channels, have all been demonstrated in plants (Fallon et al., 1993; Neuhaus et al., 1993; Bowler et al., 1994a). In some circumstances activated plant cells can display repeated Ca2+ spikes or oscillations (McAinsh et al., 1995) and spreading Ca2+ waves (Shacklock et al., 1992; Campbell et al., 1996). Such elevations in [Ca2+]cyt have been shown to be involved in several signaling pathways triggered, for example, by light via phytochrome (Neuhaus et al., 1993; Bowler et al., 1994a, 1994b), by phytohormones (Felle, 1988; Gehring et al., 1990), and even by mechanical signals and cold shock (Knight et al., 1991, 1992; Haley et al., 1995; Campbell et al., 1996). The multifunctional role of Ca2+ suggests that it participates in many signaling pathways in the plant cell. A simple but effective method for measuring changes in [Ca2+]cyt in whole plants has been developed (Knight et al., 1991). This involves the expression of the Ca2+-activated photoprotein aequorin in transgenic plants (Knight et al., 1993; Knight and Knight, 1995). This approach can be adapted for use in protoplasts that provide a superlative system for calibration of the Ca2+ signal (Haley et al., 1995; Knight et al., 1996).
The data presented in this paper show that increases in intracellular concentrations of the second messengers cGMP and cAMP (Tepper et al., 1995) provoke increases in [Ca2+]cyt in plant cells. This suggests that a potential communication point for cross-talk between signal transduction pathways using these second messengers (Bowler et al., 1994b) is the release of intracellular and extracellular Ca2+ stores.
MATERIALS AND METHODS
Plant Material and Protoplast Isolation
Tobacco (Nicotiana plumbaginifolia) genetically transformed to express apoaequorin under the control of the constitutive 35S promoter of cauliflower mosaic virus was used in this study (transgenic line MAQ2.4; Knight et al., 1991). Protoplasts were isolated from the leaves of 8-week-old plants grown in white light (150 μmol m−2 s−1) for 15 h/d at 20°C according to standard methods by Shillito and Potrykus (1985), with slight modifications. The epidermal layer was stripped from the leaves and they were incubated for 3.5 to 5.5 h with regular shaking (30 min) in 20 mm Mes (Sigma) buffer solution adjusted to pH 5.5 and containing 2% (w/v) cellulase Onozuka R-10 (Serva, Heidelberg, Germany), 400 mm mannitol (Chemapol, Bratislava, Slovakia), 100 mm Gly (Merck, Darmstadt, Germany), and 5 mm CaCl2. After incubation, the protoplast suspension was filtered through a nylon filter with a 100-μm pore size and the protoplasts were washed several times in 20 mm Hepes (Sigma), pH 7.0, 500 mm mannitol, 2 mm MgCl2, and 0.1 mm EGTA (Sigma), followed by centrifugation at 200g for 3 min. After isolation, the protoplast concentration was adjusted to 2 × 105 cells mL−1 in the same solution that was used for washing. Protoplasts were placed in this medium for all in vivo Ca2+ measurements. Protoplast viability was tested according to the procedure of Rudenok et al. (1973) with the exclusion dye methylene blue (0.3 mm, Sigma).
Aequorin Reconstitution and [Ca2+]cyt Monitoring
For in vivo aequorin reconstitution (Knight and Knight, 1995), the protoplasts were incubated for 4 h at room temperature with 2 μm coelentrazine (Molecular Probes, Eugene, OR) diluted in methanol (Merck). After washing, chemiluminescence measurements were performed with a digital chemiluminometer (model PCHL-01, Biopribor, Moscow, Russia) equipped with a PEU-84 photomultiplier and chart recorder. The total sample volume in the luminometer cuvettes was 0.5 mL. All additions (usually not more than 0.2 mL) were added to the cuvette using a micropipette with a thin plastic tube. At the end of each measurement protoplasts were discharged by mixing with an equal volume of 100 mm CaCl2/0.1% (v/v) Triton X-100. The peak value of the stimuli-induced [Ca2+]cyt transient was calculated as described by Cobbold and Rink (1987), with some modifications, to take into account the specific isoform used and the experimental temperature (Knight et al., 1996).
Protoplast Swelling
Protoplast swelling was used as a simple indicator of plant cell physiological response (Kim et al., 1986; Bossen et al., 1988; Shacklock et al., 1992). After cAMP/cGMP addition, protoplasts (5 × 105 in 0.5 mL) were kept in the dark for different time intervals at 22°C. Then, protoplasts were placed on a hemocytometer and photographed. The diameters of 100 protoplasts for each incubation time were determined and the mean volume was evaluated, making the assumption that the cells were spherical. All measurements of the changes in protoplast volume were made in the same medium that was used for Ca2+ measurements.
RESULTS
All in vivo [Ca2+]cyt measurement experiments were repeated at least three times. The traces presented were taken from the replicates and represent luminescence over time. The size of the luminescence peak and the luminescence kinetics are governed not only by the rate and concentration of [Ca2+]cyt, but also by the absolute amount of aequorin present in the cells. Higher or lower amounts of aequorin will give larger or smaller peaks in response to the same changes in Ca2+. If the consumption of aequorin becomes significant, then a smaller-than-expected peak is obtained. All of these factors are taken into account when the peak [Ca2+]cyt values are calibrated. Thus, these calibrated [Ca2+]cyt values may be compared between treatments in the same experiment. For the reasons outlined above, however, no qualitative comparisons can be made between experiments or treatments based on magnitude and kinetics of luminescence alone. Addition of 2 mm extracellular CaCl2 to the protoplasts (Fig. 1, A and B) provoked increases of [Ca2+]cyt of about 0.2 to 0.3 μm, indicating successful in vivo reconstitution of aequorin. Regardless of whether this extracellular Ca2+ was added before or after second messengers, its addition produced maximum [Ca2+]cyt values that were quite similar (data not shown).
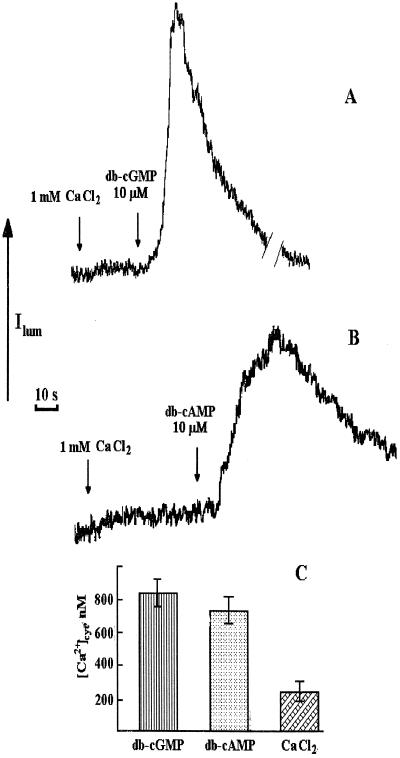
Figure 1.
Influence of db-cGMP (A) and db-cAMP (B) on Ca2+-dependent chemiluminescence of aequorin in protoplasts isolated from transgenic tobacco leaves (traces from chart recorder). Applications of stimuli are shown by arrows. Aliquots (200 μL) of cyclic mononucleotides diluted in buffer to a final concentration of 10 μm, were added to the suspension (0.5 mL) of protoplasts (2 × 105 cells mL−1) in 0.5 m mannitol, 20 mm Hepes, pH 7.0, 2 mm MgCl2, and 0.1 mm EGTA. Before the addition of cyclic mononucleotides, CaCl2 solution (200 μL) was added to give a final CaCl2 concentration of 1 mm (shown by arrows). C, Peak [Ca2+]cyt values after the addition of cyclic mononucleotides and Ca2+ to protoplasts were calculated after chemiluminescence discharge under the action of 0.1% Triton X-100/100 mm CaCl2, as described previously (Cobbold and Rink, 1987; Knight and Knight, 1995). Experiments were performed seven times; the error bars on histograms indicate sd.
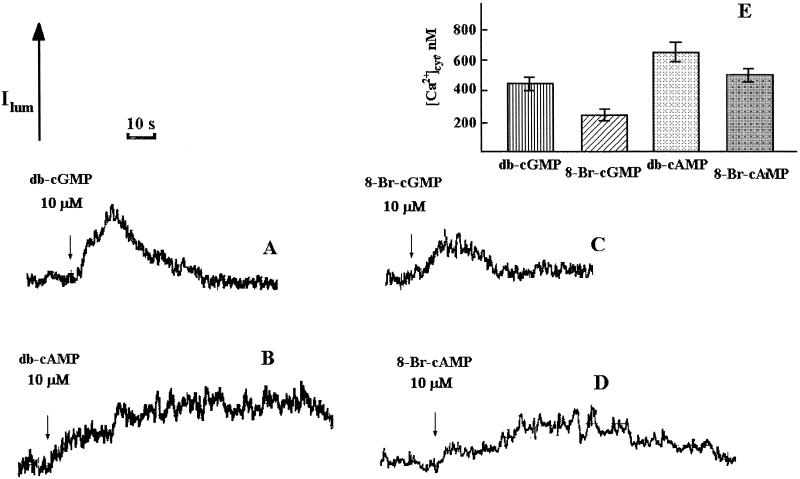
The effects of db-cGMP and db-cAMP on [Ca2+]cyt levels of tobacco protoplasts were tested. As can be seen in Figure 1, A and B, the addition of either of these second messengers at 10 μm provoked large transient increases in [Ca2+]cyt. db-cGMP caused a transient [Ca2+]cyt elevation of around 0.8 μm, lasting for approximately 40 s (Fig. 1, A and C). db-cAMP caused a transient [Ca2+]cyt elevation of around 0.7 μm, lasting for approximately 100 s (Fig. 1C). Figure 2, C and D, demonstrates the effect of 8-Br-cGMP and 8-Br-cAMP. These experiments were performed on protoplasts suspended in Ca2+-deficient medium to compare the results obtained in Figure 1 (in medium containing Ca2+) and hence gauge the involvement of external/internal Ca2+ stores in the [Ca2+]cyt responses to cAMP/cGMP. Both types of cyclic-nucleotide derivatives caused similar transient [Ca2+]cyt elevations, unequivocally indicating that the mononucleotide parts of the molecules were responsible. The transients also lasted about 40 and 100 s, respectively. Both db-cGMP and db-cAMP were able to produce a [Ca2+]cyt elevation in Ca2+-free medium (Fig. 2, A and B), strongly suggesting Ca2+ release from internal stores of ion. There was substantial variability in the calibrate cyclic-nucleotide-mediated [Ca2+]cyt peak values between experiments performed under the same conditions (compare Figs. 2 and 3). Also, the apparent relative potency of the cAMP and cGMP varied (compare Figs. 2 and 3). This is most likely because of physiological differences between protoplasts from different preparations leading to differences in signaling. Practically, this means that the relative quantitative contributions of intracellular/extracellular stores cannot be gauged. Qualitatively, it is clear from our results that both types of Ca2+ stores are involved, although the 8-Br- derivatives were less potent than the db- derivatives at concentrations that mediated a [Ca2+]cyt increase (Fig. 2). This difference might be attributable to nuances in uptake efficiencies of the two derivative forms of cAMP/cGMP.
Figure 2.
Influence of db-cGMP (A), db-cAMP (B), 8-Br-cGMP (C), and 8-Br-cAMP (D) on Ca2+-dependent chemiluminescence of aequorin in protoplasts isolated from transgenic tobacco leaves and suspended in Ca2+-deficient medium (traces from chart recorder). The conditions of the experiments were the same as in Figure 1. E, Peak [Ca2+]cyt values after the addition of cyclic mononucleotides and Ca2+ to protoplasts were calculated after chemiluminescence discharge under the action of 0.1% Triton X-100/100 mm CaCl2 as described previously (Cobbold and Rink, 1987; Knight and Knight, 1995). Experiments were performed seven times; the error bars on histograms indicate sd.
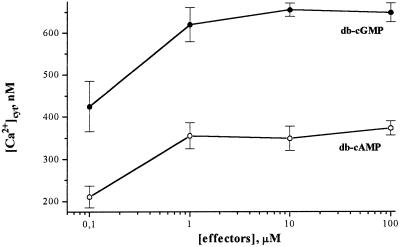
Figure 3.
Dose dependence of [Ca2+]cyt elevation in protoplasts to db-cGMP (•) and db-cAMP (○) concentration without any external Ca2+.
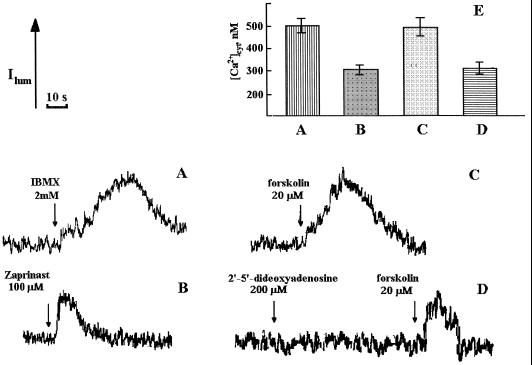
Figure 3 shows a dose-response curve for cyclic-nucleotide concentration plotted against peak [Ca2+]cyt values as a result of intracellular Ca2+ release (this experiment was performed in the absence of external Ca2+). The concentration dependence reached a plateau somewhere between 1 and 10 μm for both cyclic mononucleotides (Fig. 3). The ability of cyclic nucleotides to cause [Ca2+]cyt elevations was further demonstrated by the use of membrane-permeable modulators of anabolic and catabolic pathways of cyclic-mononucleotide metabolism. [Ca2+]cyt elevations were observed with the addition of the cyclic-mononucleotide phosphodiesterase inhibitor IBMX (Fig. 4A), the cGMP phosphodiesterase inhibitor zaprinast (Fig. 4B), and the adenylate cyclase activator forskolin (Fig. 4C). When the adenylate cyclase inhibitor DDOA was added before forskolin, the forskolin-mediated [Ca2+]cyt increase was inhibited (Fig. 4D). It seems that Ca2+-sensitive chemiluminescence of cytosolic aequorin can be induced by the changes in the endogenous cyclic-mononucleotide concentrations, stimulating their formation or inhibiting their hydrolysis.
Figure 4.
Effect of IBMX (A), zaprinast (B), forskolin (C), and DDOA plus forskolin (D) on [Ca2+]cyt in tobacco protoplasts as measured by aequorin chemiluminescence. The compounds were added to the suspension of tobacco protoplasts at the indicated concentrations. The composition of the buffer with no Ca2+ was as described for Figure 1. E, The histograms show the peak [Ca2+]cyt values obtained for each treatment. The error bars on the histograms indicate sd.
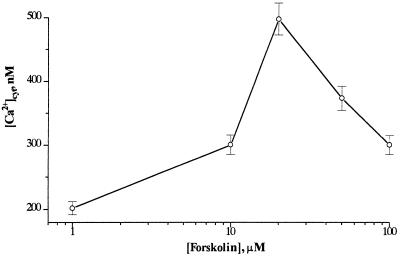
Figure 5 shows the correlation between the concentration of forskolin added and the [Ca2+]cyt responses obtained. The most pronounced effect on [Ca2+]cyt was observed at 20 μm, a concentration that is consistent with the concentration range of optimal activity of forskolin in animal cells (Siamon et al., 1981). Higher concentrations of forskolin seem to have inhibitory effects (Fig. 5), most likely because of cell toxicity at these concentrations.
Figure 5.
Dose dependence of [Ca2+]cyt elevation in tobacco protoplasts to forskolin concentration under the same conditions as described for Figure 1.
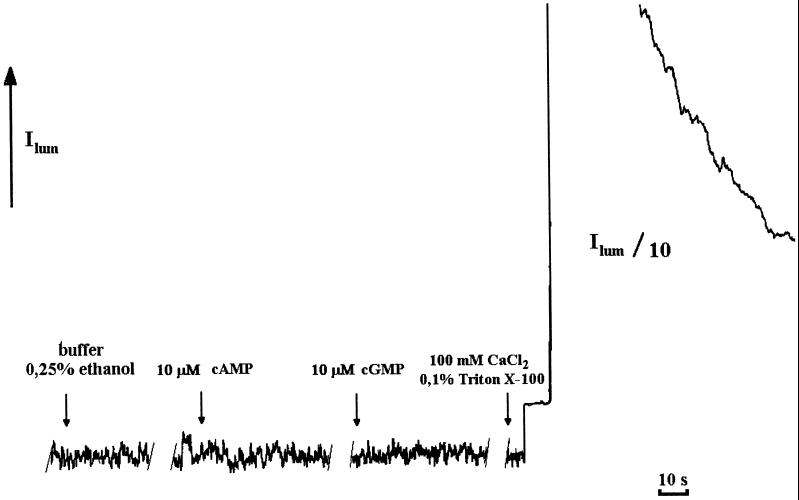
As described previously (Haley et al., 1995), additions to protoplasts in solution may result in the so-called “touch” effect, a consequence of mechanical induction of Ca2+ release from intracellular Ca2+ stores. To minimize the touch effect, Ca2+ solution was added to protoplast suspensions very carefully. This was verified by testing the effect of adding membrane-impermeable cAMP and cGMP to protoplasts as well as by the addition of buffer alone, including ethanol (as a pharmaceutical diluent), as controls (Fig. 6). The addition of cAMP, cGMP, or buffer alone immediately produced very rapid and small transients of cytosolic free Ca2+ attributable to the touch response. As can be seen in Figures 1 and 2, a rapid transient is also seen immediately upon addition of db-cGMP or db-cAMP, which can be attributed to the same phenomenon. This is clearly separate in time and magnitude from the very large and prolonged responses seen with these membrane-permeable second messengers.
Figure 6.
Effects of buffer alone and water-soluble cAMP and cGMP on [Ca2+]cyt levels in tobacco protoplasts. Media for cAMP, cGMP, and buffer plus ethanol (200-μL solutions) were the same as for Figure 1. Peak [Ca2+]cyt values after the addition of cyclic mononucleotides and Ca2+ to protoplasts were calculated after chemiluminescence discharge under the action of 0.1% Triton X-100/100 mm CaCl2 as described previously (Cobbold and Rink, 1987; Knight and Knight, 1995).
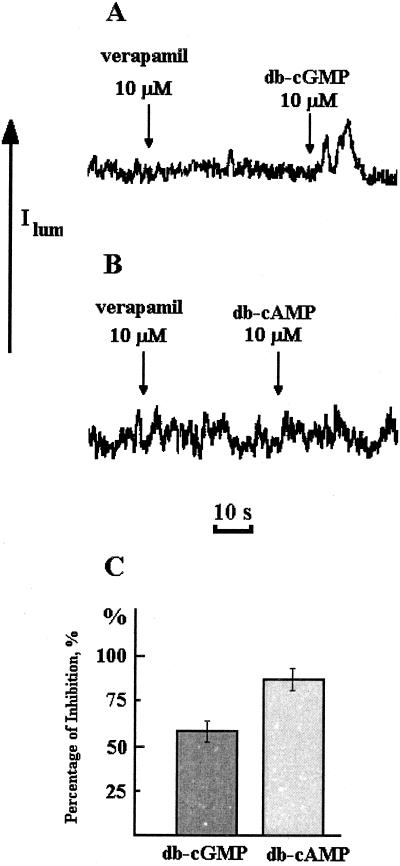
Figure 7 shows the effect of the Ca2+-channel blocker verapamil on cyclic-nucleotide-mediated [Ca2+]cyt increases. Verapamil inhibited the effects of db-cAMP and db-cGMP, producing lower elevations of [Ca2+]cyt. The inhibiting action of verapamil on db-cAMP/db-GMP-induced [Ca2+]cyt elevation was more pronounced for cGMP than for cAMP (Fig. 7C).
Figure 7.
Inhibition of db-cGMP-induced (A) and db-cAMP-induced (B) [Ca2+]cyt elevation by verapamil in Ca2+-deficient medium. C, Percentage of inhibition by verapamil. Experiments were performed three times; the error bars on histograms indicate sd.
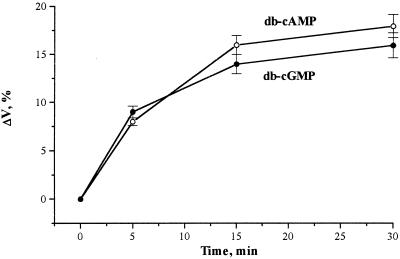
To understand the signaling role of Ca2+ in plants in relation to the involvement of cyclic nucleotides as the messengers, the physiological action of protoplast swelling was investigated. Figure 8 shows that the time course of protoplast swelling was caused by db-cAMP and db-cGMP. According to our results, a 15-min incubation was sufficient for the curves to reach a plateau corresponding to about a 10% and 15% increase in volume for db-cGMP and db-cAMP, respectively.
Figure 8.
Time course of tobacco protoplast swelling after treatment with db-cGMP and db-cAMP in complete darkness at 22°C, expressed as percent volume change (ΔV,%). Protoplasts were suspended in the media described for Figure 1. Cyclic mononucleotides were added at a concentration of 10 μm and incubation times varied as shown. The volume changes were determined as described in Methods. The percent volume change is relative to the control without cyclic mononucleotides. Each point represents the mean of at least three separate experiments; the error bars indicate se.
DISCUSSION
The data presented in this paper show that second messengers such as cGMP and cAMP can provoke [Ca2+]cyt elevations in tobacco protoplasts. In the case of cAMP and cGMP, the targets of action are likely to be intracellular, because even though membrane-permeable analogs at concentrations as low as 0.1 μm provoked a large [Ca2+]cyt response (Fig. 1, A and B), membrane-impermeable cAMP and cGMP did not produce any significant effect (Fig. 6). An intracellular site of action is also supported by the fact that a significant [Ca2+]cyt response was induced by db-cGMP and db-cAMP in the absence of external Ca2+. Protoplasts in EGTA (zero external Ca2+) still showed [Ca2+]cyt responses to these second messengers, implicating the release of Ca2+ from internal stores as a mechanism for this response. Although variability was seen between protoplast preparations, cyclic-nucleotide-mediated [Ca2+]cyt increases were generally higher in the presence of external Ca2+. This suggests that cyclic mononucleotides may also trigger changes in the Ca2+ permeability of the plasma membrane and thus cause a Ca2+ influx. The results obtained with verapamil (Fig. 7) indicate that a significant proportion of the [Ca2+]cyt responses occurs via the activation of verapamil-sensitive Ca2+ channels.
Similar [Ca2+]cyt elevations were also mediated by the addition of 8-Br-cAMP and 8-Br-cGMP, which also penetrate membranes very easily (Fig. 2, C and D). The [Ca2+]cyt responses stimulated by these two types of derivative of membrane-permeable cyclic nucleotides were relatively large, much greater in magnitude, and much more prolonged than the touch [Ca2+]cyt response caused by the mechanical stimulation resulting from the addition of solutions to the protoplasts (Fig. 6).
[Ca2+]cyt elevation in protoplast cytoplasm was also induced by manipulating endogenous cyclic-mononucleotide levels. The addition of various modulators controlling cyclic-nucleotide metabolism to protoplasts produced significant changes in [Ca2+]cyt. As can be seen in Figures 4 and 5, compounds that should increase endogenous cyclic-nucleotide concentration (forskolin, IBMX, and zaprinast) produced increases in [Ca2+]cyt. The interesting result that is consistent with the above explanation was obtained when DDOA was used before forskolin. Forskolin stimulates adenylate cyclase within the cell and, consequently, increases the concentration of endogenous cAMP. It seems likely that DDOA competitively inhibits the adenylate cyclase and prevents its stimulation by forskolin (Fig. 4, C and D).
In addition to [Ca2+]cyt elevation induced by db-cAMP and db-cGMP, the physiological response of protoplast swelling was observed at the same concentrations of cyclic mononucleotides (Fig. 8). These data are consistent with those obtained by other authors (Kim et al., 1986; Bossen et al., 1988; Gilroy and Trewavas, 1994). It has also been shown that protoplast swelling is under the control of red light absorbed by phytochrome (Bossen et al., 1988; Chung et al., 1988; Shacklock et al., 1992). It has been shown that the release of caged Ca2+ can cause protoplast swelling in the absence of light (Shacklock et al., 1992). Therefore, it is possible that cyclic-nucleotide-induced increases in [Ca2+]cyt mediate protoplast swelling. Furthermore, it is possible that the phenomenon described in this paper is relevant to the photophysiology of phytochrome action, i.e. signal transmission from the photoreceptor to target elements within the cell (Barnes et al., 1997), where cyclic mononucleotides have been shown to be downstream signaling molecules (Bowler at al., 1994b).
A key area that needs to be understood concerns the mechanism by which these second messengers bring about Ca2+ release from intracellular Ca2+ stores into cytoplasm. It could be indirect via effects on protein kinases (Stone and Walker, 1995), or possibly by direct action on ion channels (Ward et al., 1995). The first possibility is very important because the activities of many plant cell proteins are modulated by phosphorylation (Ranjeva and Boudet, 1987). The activities of cGMP- and cAMP-dependent protein kinases have been detected in animals (Krebs, 1985). There is also some evidence for cyclic-mononucleotide-regulated kinases in plant cells (Komatsu and Hirano, 1993). However, unlike the situation in mammalian cells, the involvement of cAMP still needs to be unequivocally elucidated in plants (Bolwell, 1995). Generally, the problem is that the low levels of cAMP present in plant cells means that cAMP-dependent processes that are detected experimentally are viewed with suspicion. However, analogs and modulators of cAMP metabolism have been shown to have physiological effects in plant cells (Assmann, 1995). The data presented here suggest the possibility of a role for cAMP in the control of Ca2+ release from intracellular stores in plant cells. The second potential targets for cyclic mononucleotides, at least in animals, are ion channels, which can be directly controlled by cyclic mononucleotides, e.g. in visual and olfactory cells in animals (Fesenko et al., 1985; Delgado et al., 1991). Cyclic mononucleotides in these cases have been shown to be bound directly to specific binding sites located at the components of so-called cGMP- and cAMP-dependent ion channels.
Environmental, chemical, and hormonal stimuli that are capable of inducing changes in [Ca2+]cyt are characterized by individual Ca2+ signatures with different amplitudes, kinetics, and spatial distribution, which, it has been suggested, allow cells to distinguish between stimuli (Gilroy and Trewavas, 1994; Haley et al., 1995; Campbell et al., 1996). Thus, stimulus-induced changes in [Ca2+]cyt may be transient, sustained, or oscillatory (Knight et al., 1991; McAinsh et al., 1995; Campbell et al., 1996). Typically, these [Ca2+]cyt responses can last from a few seconds to several hours. It is generally accepted that changes in [Ca2+]cyt are closely associated with transduction of the biological action of auxin, ABA, cytokinins, gibberellic acid, fungal elicitors, and light (Gilroy and Trevawas, 1994; Bush, 1995). The involvement of second messengers other than Ca2+ in these pathways has not been demonstrated, although cGMP involvement in phytochrome-mediated chs gene expression has been shown (Bowler et al., 1994a, 1994b). cGMP was shown to substitute for the action of red light in the phytochrome-dependent regulatory control of anthocyanin synthesis (Neuhaus et al., 1993; Bowler et al., 1994a). It is clearly possible that cyclic nucleotides (Bolwell, 1995) and metabolites from phosphoinositide cycles are involved in many other plant signal transduction pathways (Alexandre et al., 1991; Allen et al., 1995). The major challenge for the future will be to determine whether cyclic-nucleotide-mediated [Ca2+]cyt increases are actually used in bona fide signal transduction pathways in plants.
ACKNOWLEDGMENTS
We thank Prof. A.A. Milutin (Sakcharov's College, Minsk), who donated coelentrazine, and Dr. V.B. Gavrilov (Institute of Photobiology, Academy of Sciences of Belarus, Minsk) for the use of the chemiluminometer.
Abbreviations:
- 8-Br-cAMP
8-brom-cyclic AMP
- 8-Br-cGMP
8-brom-cyclic GMP
- [Ca2+]cyt
cytosolic Ca2+ concentration
- db-cAMP
2′-O-dibutyryl AMP
- db-cGMP
2′-O-dibutyryl GMP
- DDOA
2′,5′-dideoxyadenosine
- IBMX
3-isobutyl-1-methylxanthine
- IP3
inositol 1,4,5-trisphosphate
LITERATURE CITED
- Alexandre J, Lassalles JP, Kado RT. Opening of Ca2+ channels in isolated red beet root vacuole membrane by inositol 1,4,5-trisphosphate. Nature. 1990;343:567–570. [Google Scholar]
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- Assmann SM. Cyclic AMP as a second messenger in higher plants. Plant Physiol. 1995;108:885–889. doi: 10.1104/pp.108.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, McGrath RB, Chua N-H. Light signal transduction in plants. Trends Cell Biol. 1997;7:21–26. doi: 10.1016/S0962-8924(97)10043-5. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bolwell GP. Cyclic AMP, the reluctant messenger in plants. Trends Biochem Sci. 1995;12:492–495. doi: 10.1016/s0968-0004(00)89114-8. [DOI] [PubMed] [Google Scholar]
- Bossen ME, Dassen HHA, Kendrick RE, Vredenberg WJ. The role of calcium ions in phytochrome-controlled swelling of etiolated wheat (Triticum aestivum L.) protoplasts. Planta. 1988;174:94–100. doi: 10.1007/BF00394879. [DOI] [PubMed] [Google Scholar]
- Bowler C, Neuhaus G, Yamagata H, Chua N-H. Cyclic GMP and calcium mediate phytochrome phototransduction. Cell. 1994a;77:73–81. doi: 10.1016/0092-8674(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Bowler C, Yamagata H, Neuhaus G, Chua N-H. Phytochrome signal transduction pathways are regulated by reciprocal control mechanisms. Genes Dev. 1994b;8:2188–2202. doi: 10.1101/gad.8.18.2188. [DOI] [PubMed] [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signalling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Campbell AK, Trewavas AJ, Knight MR. Calcium imaging shows differential sensitivity to cooling and communication in luminous transgenic plants. Cell Calcium. 1996;19:211–218. doi: 10.1016/s0143-4160(96)90022-6. [DOI] [PubMed] [Google Scholar]
- Chae Q, Park HJ, Hong SD. Loading of quin2 into the oat protoplasts and measurement of cytosolic calcium ion concentration changes by phytochrome action. Biochim Biophys Acta. 1990;1051:115–122. doi: 10.1016/0167-4889(90)90182-d. [DOI] [PubMed] [Google Scholar]
- Chung CH, Lim S-V, Song P-S, Berlin JD, Gooding JR. Swelling of etiolated oat protoplasts induced by phytochrome, cAMP, and gibberellic acid: a kinetic study. Plant Cell Physiol. 1988;29:855–860. [Google Scholar]
- Cobbold PH, Rink TJ. Fluorescence and bioluminescence measurements of cytoplasmic free calcium. Biochem J. 1987;248:313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R, Hidalgo P, Diaz F, Latorre R, Labarca P. A cyclic AMP-activated K+ channel in Drosophila larva muscle is persistently activated in dunce. Proc Natl Acad Sci USA. 1991;88:557–560. doi: 10.1073/pnas.88.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon KM, Shacklock PS, Trewavas AJ. Detection in vivo of very rapid red light-induced calcium-sensitive protein phosphorylation in etiolated wheat (Triticum aestivum) leaf protoplasts. Plant Physiol. 1993;101:1039–1045. doi: 10.1104/pp.101.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H. Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta. 1988;174:495–499. doi: 10.1007/BF00634478. [DOI] [PubMed] [Google Scholar]
- Fesenko EE, Kolesnikov SS, Lyubarsky AL. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985;313:310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Parish RW. Effect of abscisic acid on cytosolic calcium and pH in plant cell. Proc Natl Acad Sci USA. 1990;87:9645–9649. doi: 10.1073/pnas.87.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Read ND, Trewavas AJ. Elevation of cytosolic calcium by caged inositol trisphosphate initiates stomatal closure. Nature. 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Trewavas AJ. A decade of plant signals. BioEssays. 1994;16:677–682. [Google Scholar]
- Haley A, Russel AJ, Wood N, Allan AC, Knight MR, Campbell AK, Trewavas AJ. Effects of mechanical signalling on plant cell cytosolic calcium. Proc Natl Acad Sci USA. 1995;92:4124–4128. doi: 10.1073/pnas.92.10.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-S, Moon DK, Goodin JR, Sdong P-S. Swelling of etiolated protoplasts induced by cAMP and red light. Plant Cell Physiol. 1986;27:193–197. [Google Scholar]
- Knight H, Knight MR. Recombinant aequorin methods for intracellular calcium measurement in plants. Methods Cell Biol. 1995;49:201–216. doi: 10.1016/s0091-679x(08)61455-7. [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Cold calcium signalling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Knight MR, Read ND, Campbell AK, Trewavas AJ. Imaging calcium dynamics in living plants using semi-synthetic recombinant aequorin. J Cell Biol. 1993;121:83–90. doi: 10.1083/jcb.121.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MR, Smith SM, Trewavas AJ. Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci USA. 1992;89:4967–4971. doi: 10.1073/pnas.89.11.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S, Hirano H. Protein kinase activity and phosphorylation in rice (Oryza sativa L.) leaf. Plant Sci. 1993;94:127–137. [Google Scholar]
- Krebs EG. The phosphorylation of proteins: a major mechanism for biological regulation. Biochem Soc Trans. 1985;13:813–820. doi: 10.1042/bst0130813. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Webb AR, Taylor JE, Hetherington AM. Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus G, Bowler C, Kern R, Chua N-H. Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell. 1993;73:937–952. doi: 10.1016/0092-8674(93)90272-r. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984;225:1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Poovaiah BW, Reddy ASN. Turnover of inositol phospholipids and calcium-dependent protein phosphorylation in signal transduction. In: Morré DJ, Boss WF, Loewus FA, editors. Inositol Metabolism in Plants. New York: Wiley-Liss; 1990. pp. 335–349. [Google Scholar]
- Ranjeva R, Boudet AM. Phosphorylation of proteins in plants: regulatory effects and potential involvement in stimulus/response coupling. Annu Rev Plant Physiol. 1987;38:73–93. [Google Scholar]
- Rudenok AN, Konev SV. On the phenomenon of cell protection against heat damage (in Russian) Dokl Acad Nauk SSSR. 1973;208:812–816. [Google Scholar]
- Shacklock PS, Read ND, Trewavas AJ. Cytosolic free calcium mediates red light-induced photomorphogenesis. Nature. 1992;358:753–755. [Google Scholar]
- Shillito R, Potrykus I. Protoplasts: isolation, culture, plant regeneration. Methods Enzymol. 1985;118:549–578. [Google Scholar]
- Siamon KB, Padgett W, Daly DW. Forskolin: unique diterpene activation of adenylate cyclase in membrane and in intact cells. Proc Natl Acad Sci USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Walker JC. Plant protein kinase families and signal transduction. Plant Physiol. 1995;108:451–457. doi: 10.1104/pp.108.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper CG, Jayadev S, Liu B, Bielawska A, Wolff R, Yonehara S, Hannun YA, Seldin MF. Role for ceramide as an endogenous mediator of Fas-induced cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8443–8447. doi: 10.1073/pnas.92.18.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Pei Z-M, Schroeder JI. Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell. 1995;7:833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]