Abstract
Stimulus repetition in identification tasks leads to improved behavioral performance ("repetition priming") but attenuated neural responses ("repetition suppression") throughout task-engaged cortical regions. While it's clear that this pervasive brain-behavior relationship reflects some form of improved processing efficiency, the exact form that it takes remains elusive. In this Discussion Paper, we review four different theoretical proposals that have the potential to link repetition suppression and priming, with a particular focus on a proposal that stimulus repetition affects improved efficiency through enhanced neural synchronization. We argue that despite exciting recent work on the role of neural synchronization in cognitive processes such as attention and perception, similar studies in the domain of learning and memory - and priming, in particular - have been lacking. We emphasize the need for new studies with adequate spatiotemporal resolution, formulate several novel predictions, and discuss our ongoing efforts to disentangle the current proposals.
Keywords: repetition priming, repetition suppression, synchrony, prediction, expectation
When we repeatedly encounter an object in the environment, we become faster and more accurate at identifying it, a phenomenon referred to as "repetition priming" (see Tulving and Schacter, 1990; Schacter & Buckner, 1998, for review). Repetition priming is stimulus-specific, builds up over several stimulus repetitions (e.g. Logan, 1990; Ostergaard, 1998; Wiggs, Martin, & Sunderland, 1997), and while it attenuates over short delays (e.g. McKone, 1995, 1998), it can be extremely long-lasting with significant residual effects lasting days, months, and even years (e.g. Cave, 1997; Mitchell, 2006; van Turennout, Ellmore, & Martin, 2000). It is also relatively automatic in the sense that it often occurs without subjects' awareness (e.g. Cave & Squire, 1992) and is robust to attentional manipulations (e.g. Kellogg, Newcombe, Kammer, & Schmitt, 1996; Szymanski & MacLeod, 1996) and modest alterations of stimulus form (e.g. Biederman & Cooper, 1991, 1992; Cave, Bost, & Cobb, 1996; Srinivas, 1996). Historically, repetition priming has played an important role in our understanding of the organization of human memory due to its neuropsychological dissociation from more explicit forms of memory in amnesic patients (e.g. Warrington & Weiskrantz, 1974; Graf, Squire, & Mandler, 1984). Amnesic patients with damage to the medial temporal lobes, including the hippocampus, can exhibit profound impairments in recall and recognition of recent events while at the same time demonstrating normal or nearly normal repetition priming effects (see Squire, 1992, for review). While caution is warranted in attributing priming entirely to implicit as opposed to explicit memory processes (both would typically be expected to contribute in normal individuals, e.g. Henson & Gagnepain, 2010; Voss & Paller, 2008), the basic dissociation has led researchers to focus primarily on the role of neocortical plasticity mechanisms, with priming potentially serving as a window into the formation of long-term knowledge representations that reside primarily in the neocortex (e.g. McClelland, McNaughton, & O'Reilly, 1995; Stark & McClelland, 2000). Indeed, stimulus repetition paradigms in neuroimaging studies (e.g. fMRI) are routinely used as a tool to infer the nature of neocortical representations in a variety of cognitive domains (e.g. Andresen, Vinberg, & Grill-Spector, 2009; Bedny, McGill, & Thompson-Schill, 2008; Cant, Large, McCall, & Goodale, 2008; Fairhall, Anzellotti, Pajtas, & Caramazza, 2011; Gold, Balota, Kirchhoff, & Buckner, 2005; Gotts, Milleville, Bellgowan, & Martin, 2011; Konen & Kastner, 2008; Mahon, Milleville, Negri, Rumiati, Caramazza, & Martin, 2008; Piazza, Izard, Pinel, Le Bihan, & Dehaene, 2004). Recent work has identified separate contributions of perceptual, conceptual, and decision/response-related processing to both task performance and priming effects (e.g. Dobbins, Schnyer, Verfaellie, & Schacter, 2004; Wig, Grafton, Demos, & Kelly, 2005; Wig, Buckner, & Schacter, 2009; Horner & Henson, 2008, 2011; Race, Shanker, & Wagner, 2009; Race, Badre, & Wagner, 2010).
While behavioral performance improves with stimulus repetition, neural activity in humans (BOLD fMRI) and monkeys (single-cell firing rates) tends to decrease, a phenomenon often referred to as "repetition suppression" (see Desimone, 1996; Henson, 2003; Grill-Spector, Henson, & Martin, 2006, for reviews). Like priming, repetition suppression is stimulus-specific, builds up over several repetitions (e.g. Jiang, Haxby, Martin, Ungerleider, & Parasuraman, 2000; Miller, Gochin, & Gross, 1991), and has both short-lived and long-lasting components (e.g. Li, Miller, & Desimone, 1993; Grill-Spector & Malach, 2001; van Turennout, Bielamowicz, & Martin, 2003). It occurs relatively automatically (e.g. under anesthesia: Miller et al., 1991) and in a wide range of neocortical brain regions. Indeed, the agreement of the empirical properties of repetition priming and repetition suppression was initially met with enthusiasm that the relationship between the two would clarify the mechanisms underlying priming (e.g. Schacter & Buckner, 1998; Wiggs & Martin, 1998). Given the automatic nature and generality of the two phenomena throughout different cognitive domains, tasks, and brain regions, the promise of understanding this link is that it could pay large dividends in understanding the basic relationships between brain and mind.
However, the relationship between repetition priming and repetition suppression also presents a major puzzle: how is it that reductions in neural activity can mediate better behavioral performance? After all, the propagation of neural activity from sensory areas through to decision- and response-related brain regions (ultimately in motor cortex) is what is thought to mediate performance in an identification task. In studies of repetition priming using common objects and other familiar stimuli, there is little evidence of repetition-related increases in neural activity (see Henson, 2003, for review). So where does the behavioral facilitation come from? Just to highlight how puzzling this basic situation is, it's worth remembering that the major "activation-based' theories of priming that existed prior to the first neuroimaging studies of priming in the mid-1990's (e.g. spreading activation, connectionist models) posited repetition-related accumulation or increases in activity in the nodes or units that represented a given stimulus (e.g. Anderson, 1983; Becker, Moscovitch, Behrmann, & Joordens, 1997; Collins & Loftus, 1975; McClelland & Rumelhart, 1985). This issue would also appear to cut across the distinction between implicit versus explicit memory, since both sets of processes are likely to be reflected in some mixture in neural and behavioral repetition effects. One must still explain how less neural activity somehow produces a more effective behavioral response. It is worth noting that in a variety of cognitive domains that do not intrinsically involve stimulus repetition (e.g. attention, visual search, working memory, motion discrimination) better behavioral performance is generally associated with increased rather than decreased activity in cells that prefer a stimulus, location, or response (e.g. Luck, Chelazzi, Hillyard, & Desimone, 1997; Newsome, Britten, & Movshon, 1989; Rainer, Asaad, & Miller, 1998; Schall & Hanes, 1993). Indeed, the basic logic used in mapping visual receptive fields in single-unit studies, in evaluating the results of functional localizers in neuroimaging studies, or in quantitatively comparing neural responses to different experimental conditions implicitly relies on the assumption that greater activity corresponds to greater involvement in processing. The disconnect with this logic that is represented by the joint observation of repetition priming and repetition suppression makes these phenomena even more important and fundamental to understand. Joint repetition priming/suppression appears to reflect some kind of improved efficiency mechanism or set of mechanisms that apply over a wide range of repetition lags. While the exact form of these mechanisms is unclear, the need for such mechanisms is clear, given the high energy cost of neural signaling (see Raichle & Mintun, 2006, for review). It is likely that processes of natural selection discovered solutions that optimize both performance and energy use simultaneously (e.g. Aiello & Wheeler, 1995; Allman, 1990). Below, we review four of the main theoretical proposals about what form these solutions might take (see also Grill-Spector et al., 2006).
Theoretical Models of Repetition Suppression and Priming
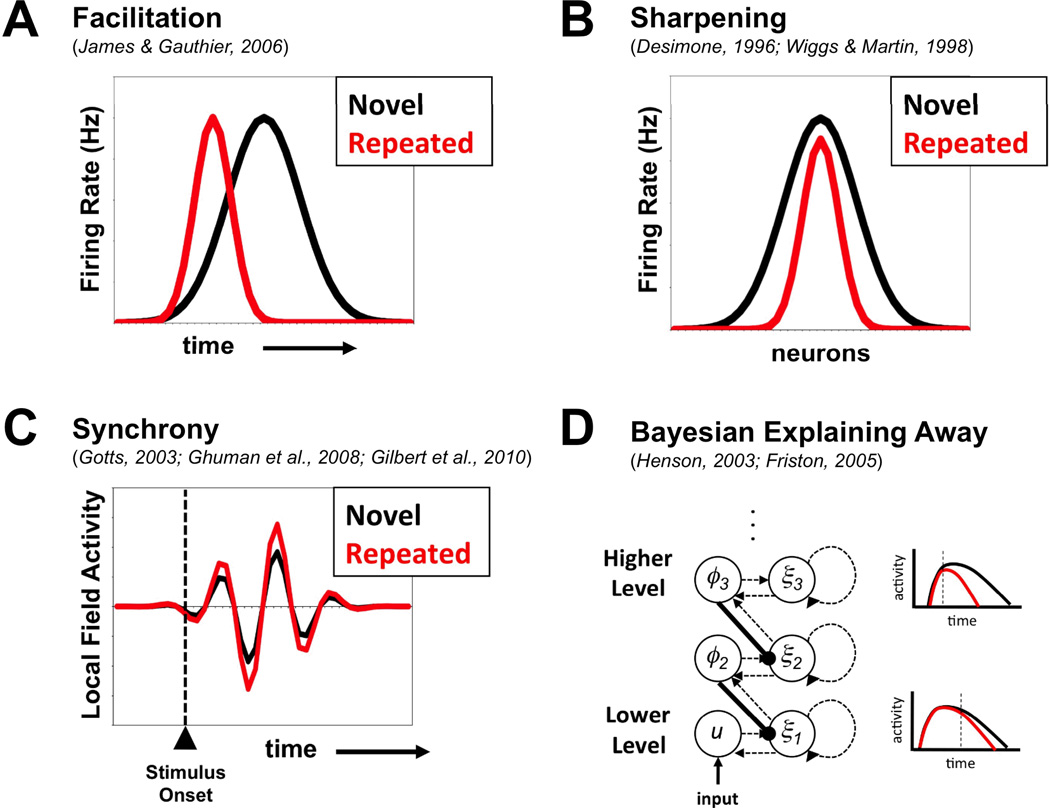
Facilitation
The "Facilitation" model (Henson, 2003; James, Humphrey, Gati, Menon, & Goodale, 2000; James & Gauthier, 2006) is perhaps the most straightforward resolution, positing that with repetition, neural activity is advanced in time with a more rapid overall time course (see Figure 1A). In BOLD fMRI experiments, rapid timing differences such as this would be lost due to the slow time course of the BOLD response. This view has received some support in fMRI experiments that either slowed down the time course of a trial by gradually unmasking the stimuli (e.g. James et al., 2000; although see Eger, Henson, Driver, & Dolan, 2007) or that attempted to measure BOLD latency differences directly (e.g. Gagnepain et al., 2008; Henson, Price, Rugg, Turner, & Friston, 2002). However, direct electrical recordings of single-cell activity in a variety of brain regions in monkeys (e.g. Anderson, Mruczek, Kawasaki, & Sheinberg, 2008; Freedman, Riesenhuber, Poggio, & Miller, 2006; Li et al., 1993; McMahon & Olson, 2007; Rainer & Miller, 2000; Verhoef, Kayaert, Franko, Vangeneugden, & Vogels, 2008) and in human patients undergoing neurosurgery (e.g. Pedreira et al., 2010) have presented strong counter evidence to this idea under typical stimulus presentation conditions. Firing-rate curves to repeated stimuli show no evidence of advancing in time and are subsumed under the firing-rate curves to novel stimuli. A more sophisticated version of this hypothesis (Bayesian Networks and "Explaining Away") is discussed below.
Figure 1. Theories that explain repetition priming in the face of repetition suppression.
Graphical depictions of the theories discussed in the text are shown for (A) Facilitation, (B) Sharpening, (C) Synchrony, and (D) Bayesian Explaining Away. Hypothetical Novel and Repeated conditions are shown with black and red curves, respectively. In D, suppressive feedback from higher levels to lower levels in the network structure is highlighted with the use of thick black lines, and the earlier separation of novel and repeated conditions in higher levels relative to lower levels is indicated with vertical dashed lines in the activity plots to the right.
Sharpening
A second idea, often referred to as "Sharpening" (Desimone, 1996; Wiggs & Martin, 1998), holds that while neural activity is decreasing on the average, the decreases are carried mainly by the cells that are poorly tuned and/or weakly responsive to the repeated stimuli with the "best" cells (i.e. most selective/responsive) instead retaining their activity levels (Figure 1B). If the poorly-responsive cells are dropping out while the best-responsive cells keep their responses, the distribution of cell responses over the population is actually more informative about the identity of the stimulus even though the firing rates have decreased overall. In monkeys, there is certainly some evidence consistent with sharpening in single-cell recordings, particularly after lengthy periods of training with the same set of stimuli (e.g. several months: Baker, Behrmann, & Olson, 2002; Rainer & Miller, 2000; Freedman et al., 2006). However, stimulus repetitions that occur solely within a single experimental session have tended to elicit changes in firing-rate that are more consistent with proportional "scaling", in which the "best" responses exhibit the largest decreases (e.g. Li et al., 1993; McMahon & Olson, 2007; Miller, Li, & Desimone, 1993). It is particularly challenging to understand how priming can occur under these circumstances because the cells that are most responsible for driving downstream responses are the ones that are decreasing the most. fMRI studies in humans that have attempted to evaluate sharpening of visual object representations with experience have similarly generated mixed results. Jiang et al. (2007) trained subjects to discriminate between morphed pictures of cars that were assigned to distinct categories. Using an fMRI-adaptation paradigm (see Grill-Spector & Malach, 2001) they found greater release from adaptation with small changes in visual stimulus form in the right lateral occipital cortex after training relative to pre-training, consistent with "sharper", less-overlapping visual form representations. However, another recent fMRI-adaptation study by Weiner, Sayres, Vinberg, and Grill-Spector (2010), using short- and longer-lag repetitions to measure changes in category selectivity, found proportional changes for preferred and non-preferred categories throughout the lateral aspects of ventral occipitotemporal cortex, consistent with proportional "scaling" (i.e. multiplying by a value between 0 and 1) rather than sharpening. Only the more medial aspects of ventral temporal cortex showed larger repetition suppression effects for non-preferred relative to preferred categories, and only for longer-lag repetitions. Similar attempts to use rapid adaptation paradigms to measure tuning changes in single-cell firing rates in monkeys have failed to yield support for sharpening (e.g. De Baene & Vogels, 2010). Even if sharpening were shown to occur robustly in the experimental contexts in which repetition priming is observed, additional assumptions would need to be articulated in order for sharpening to explain priming. For example, when neural representations are distributed over many cells in a "population code" (e.g. Georgopoulos, Schwartz, & Kettner, 1986), each individual cell - even ones that fire at lower rates - could potentially contribute to activating cells in downstream regions that prefer the current stimulus. What is to guarantee that a large loss of firing rate in the poorly responsive cells will not result in weaker or slower onset of firing in the preferred cells downstream? This point highlights another elusive aspect of the sharpening idea. In order for sharpening of firing rate responses to go through as an explanation of priming, there still seems to be a need for an increase in firing rate at earlier latencies in the cells that most prefer the repeated stimulus somewhere in the brain (akin to the Facilitation model). Perhaps this wouldn't occur until the ultimate or penultimate stage of processing in executing a response, but it would still appear to be necessary. Indeed, most neural network models that exhibit sharpening through the application of a supervised learning algorithm predict a mixture of repetition suppression and enhancement effects (e.g. McClelland & Rumelhart, 1985; Norman & O'Reilly, 2003). To date, we still have little or no evidence of such an enhancement occurring, even in lateral prefrontal sites that may play a more central role in decision/response selection (e.g. Rainer & Miller, 2000).
Enhanced Neural Synchronization
A very different proposal that may help to resolve this puzzle is that as cells are firing at lower overall rates, they are firing more synchronously with one another, leading to more efficient neural processing (Gilbert, Gotts, Carver, & Martin, 2010; Gotts, 2003) (Figure 1C). Neurons are not only sensitive to the average firing rates of their inputs, they are also sensitive to the relative timing of their input spikes due to the passive membrane property of "capacitance" (e.g. Koester & Siegelbaum, 2000). Input spikes only transiently depolarize a receiving cell, after which the membrane voltage decays back toward the resting potential at a rate dictated by the membrane time constant. Small depolarizations that occur simultaneously (i.e. synchronously) in a receiving cell will be much more likely to sum above the voltage threshold needed to evoke an action potential. Biophysical models and in vitro physiology experiments on cortical cells have substantiated this relationship, demonstrating separate contributions of input firing rate and synchrony to a receiving cell's responses (e.g. Reyes, 2003; Salinas & Sejnowski, 2000, 2001). In the extreme, volleys of single spikes could travel along reliably through a processing pathway from sensory to motor, perhaps only requiring a few spikes to generate an appropriate response. Note that this mechanism would also not require elevated firing rates in downstream areas for priming to occur, potentially allowing for decreases in firing rate throughout the entire system. In this view, what increases is the likelihood of generating a single post-synaptic spike when a pre-synaptic spike occurs. It predicts that stimulus-repetition should be accompanied by larger fluctuations in local measures of neural population activity (e.g. local field voltages and magnetic field measurements, multi-neuron firing rate binned over short time windows, etc.; e.g. Gilbert et al., 2010), as well as greater phase-locking/coherence between task-engaged cortical sites (e.g. Ghuman, Bar, Dobbins, & Schnyer, 2008).
In a simplified neocortical circuit model that incorporated biologically proportionate numbers of excitatory and inhibitory cells and short-term plasticity mechanisms, Gotts (2003) showed that it was possible to simultaneously address short-lag repetition suppression and priming effects through enhanced synchronization. The model included synaptic depression, an attenuation of transmitter release following spiking activity (e.g. Abbott, Varela, Sen, & Nelson, 1997; Tsodyks & Markram, 1997), and spike-frequency adaptation, the spike-dependent activation of K+ currents that hyperpolarize the membrane postsynaptically and decrease the membrane resistance (e.g. Constanti & Sim, 1987; Madison & Nicoll, 1984), both parameterized to independent in vitro and in vivo physiological recordings of neocortical cells (e.g. Ahmed, Allison, Douglas, Martin, & Whitteridge, 1998; Varela, Song, Turrigiano, & Nelson, 1999). The model was able to address short-term (i.e. a few seconds) repetition suppression effects quantitatively as well as qualitatively in a variety of monkey single-cell recording and human fMRI experiments (e.g. Grill-Spector & Malach, 2001; Jiang et al., 2000; Miller, Gochin, & Gross, 1991; Miller, Li, & Desimone, 1993), and it naturally produced "scaling" of the firing-rate distributions as observed in several experiments (e.g. Miller et al., 1993; MacMahon & Olson, 2007; Weiner et al., 2010). Importantly, as the model's firing rates decreased with repetition, the synchronization of the spike times simultaneously increased. This enhanced synchronization could be propagated between separate simulated regions in the model, and it was robust to expected synaptic delays and a modest amount of variability in the firing-rate distribution. Simulating reaction time as the amount of time required for a single receiving output cell to reach a threshold number of spikes, the model also produced repetition priming effects as synchronization increased. Repetition priming that occurs through enhanced synchronization - and in the face of firing-rate decreases - constitutes a particular form of neural efficiency mechanism. A model quite similar to the Gotts (2003) model has also been applied to account for repetition-related decreases in firing rate and enhanced spike synchronization in the insect antennal lobe (olfaction) with good success (Bazhenov, Stopfer, Sejnowski, & Laurent, 2005). While the cellular mechanisms in these models would not enhance synchronization over the much longer repetition lags discussed above, good candidates would include longer-term synaptic plasticity mechanisms such as spike-timing dependent long-term potentiation and depression (LTP/LTD) (e.g. Bi & Poo, 1998; Markram, Lubke, Frotscher, & Sakmann, 1997; Sjöstrom, Turrigiano, & Nelson, 2001). With repetition, spike-timing dependent LTP/LTD mechanisms have the potential to improve and coordinate the timing of spikes across cells, permitting enhanced local and long-range synchronization among task-engaged brain regions.
Despite the promise that the Synchrony model holds for resolving the puzzle of repetition priming and repetition suppression, there are relatively few studies that have evaluated it empirically. A burgeoning literature on neural synchronization has developed over the last 10–15 years in domains such as attention and perceptual binding (see Engel et al., 2001; Fries, 2005; Gregoriou, Gotts, Zhou, & Desimone, 2009b, for reviews). However, only a handful of studies involving stimulus repetition in the neocortex have used multi-electrode recording techniques that are capable of measuring spike synchronization directly. For example, von Stein, Chiang, and Konig (2000) recorded simultaneously from Areas 17 and 7 in cat visual cortex while the cats performed a go/no-go task. When they compared trained to novel stimuli, they found greater phase-locking between the two visual areas in the alpha frequency range (8–12 Hz) for trained stimuli. Two recent recording studies by Dragoi and colleagues in monkeys, one in V1 using multi-contact, cross-laminar electrodes (Hansen & Dragoi, 2011) and another in V4 using multiple single electrodes (Wang, Iliescu, Ma, Josić, & Dragoi, 2011), examined local changes in synchronization after brief visual adaptation (duration=300 ms) to oriented sine-wave gratings. In both studies, firing rates were reduced to a test grating presented 100 ms after the adaptor. Spikes elicited by the test grating were simultaneously more synchronized with the local field potential (LFP) (spike-LFP coherence) in the gamma frequency range (30–80 Hz) relative to a control condition in which the adapting grating was replaced with a random dot patch of matched luminance. The increases in gamma synchronization in both studies were associated with improvement in neuronal orientation discrimination performance of the test gratings. For the study in V1, for which laminar information was available, the improvement in neuronal orientation discrimination performance was only associated with increases in gamma synchronization within the superficial cortical layers that serve as output to subsequent visual areas. In models, it is not clear whether synchronization effects should have a different impact at higher versus lower frequencies, since similar benefits can be observed over a range of frequencies (e.g. Salinas & Sejnowski, 2000; 2001). However, given that the brain's activity dynamics are generally weighted towards lower frequencies (e.g. He, Zempel, Snyder, & Raichle, 2010), one might expect changes in lower frequencies to have a larger impact relative to higher frequencies that have weaker overall amplitudes (such as gamma).
A few additional studies using single electrodes have provided relevant data for evaluating the Synchrony model. Anderson et al. (2008) exposed monkeys to novel and familiar images during passive viewing while recording both multi-unit spiking activity and LFPs in inferior temporal cortex. In addition to observing repetition suppression effects in firing rate to the familiar images, they simultaneously observed larger low-frequency fluctuations in the LFPs (~ 5–10 Hz) that were phase-locked to the stimulus onset (i.e. larger evoked responses). In a related study, Peissig, Singer, Kawasaki, and Sheinberg (2007) observed a similar pattern in LFPs that they recorded using transcranial electrodes implanted over occipitotemporal sites. They first trained monkeys to classify a set of bird and object pictures. During testing, the monkeys performed the same classifications on both previously trained and novel pictures. Behaviorally, they observed repetition priming effects for trained relative to novel pictures (faster reaction time and improved accuracy), while they observed larger low-frequency fluctuations in the LFPs that were particularly prominent at 170 ms after stimulus onset. In a different study aimed at evaluating changes in stimulus selectivity to familiar pictures, Freedman, Riesenhuber, Poggio, and Miller (2006) analyzed the firing-rate responses of a large number of single cells (~ 300) in inferior temporal cortex (area TE) to familiar and novel stimuli during passive viewing. They observed increases in stimulus selectivity to familiar pictures (consistent with the Sharpening model), while also observing a hint of periodicity in the firing rate curves to familiar stimuli, with fluctuations at approximately 5–10 Hz (see their Figure 8). Closer inspection of the firing rate curves reported for the 3 monkeys in Anderson et al. (2008, their Figure 4) also reveals a similar tendency for periodicity. Taken together, these studies all provide evidence that supports the basic premise of the Synchrony model, namely that cells should fire in a more synchronous and temporally coordinated manner following stimulus repetition, both locally and in inter-areal interactions among task-engaged cortical sites. It is important to note that such evidence is not limited to monkeys and other mammals. Striking similarities also exist in electrode recordings in insects during stimulus repetition. For example, Stopfer and Laurent (1999) repeatedly presented odor puffs to the antennae of locusts and recorded spikes and LFP responses in the antennal lobe (i.e. the insect equivalent of the olfactory bulb in mammals). Across a series of repetitions presented at a rate of one stimulus per 10 seconds, they found both repetition suppression in firing rates, as well as increased synchrony between the spikes and the LFPs in the 20–30 Hz frequency range. In a separate conditioning experiment in honeybees, Stopfer, Bhagavan, Smith, and Laurent (1997), were able to pharmacologically block odor-selective synchronous firing while leaving odor-selective firing rates intact. Under these conditions, the bees' odor discrimination performance was impaired, demonstrating a causal role of synchrony in their behavior.
Having just reviewed many of the microelectrode recording studies in animals that are relevant for the evaluation of the Synchrony model, what relevant data exist in humans and in repetition priming tasks? In most human studies, measurements of neural activity are restricted to non-invasive neuroimaging methods such as fMRI, MEG, and EEG/ERP. The most extensive literature in humans that employs a method with the appropriate temporal resolution is the EEG/ERP literature on repetition priming (e.g. Bentin & Peled, 1990; Henson et al., 2003; Henson, Rylands, Ross, Vuilleumier, & Rugg, 2004; Kiefer, 2005; Olichney et al., 2000; Paller & Gross, 1998; Rugg, Brovedani, & Doyle, 1992; Rugg, Mark, Gilchrist, & Roberts, 1997; Swick, 1998). While scalp EEG/ERP studies have occasionally found evidence consistent with larger evoked responses to repeated stimuli for select electrode sites (e.g. Schendan & Kutas, 2003; Scott et al., 2006), most studies have failed to find such evidence or have even reported attenuated ERPs with repetition (e.g. Fiebach, Gruber, & Supp, 2005; Gruber & Muller, 2005; Race, Badre, & Wagner, 2010). The discrepancy with the results reviewed above for electrode-recording studies with animals could occur for several reasons: 1) there is a species difference with humans and larger ERPs with repetition are simply not occurring (i.e. the Synchrony model is wrong), 2) the timing of fluctuations in the ERPs, such as those in the alpha to gamma frequency range (~ 8–80 Hz), are somewhat idiosyncratic from subject to subject and group-averaging across subjects (or low-pass filtering the voltage signals below 20 Hz) washes these differences away, or 3) the spatial resolution of scalp EEG signals is too coarse and requires source estimation to see spatially localized effects, particularly for those in deeper sources that may carry the largest effects (e.g. the fusiform gyrus). Two recent source-localized MEG studies of repetition priming in humans suggest that the answer may be one of the latter two reasons (Ghuman et al., 2008; Gilbert et al., 2010). Gilbert et al. (2010) asked subjects to covertly name pictures of common objects by pressing a response button when they knew the correct name, with randomly intermixed novel and repeated trials. They measured evoked power (i.e. phase-locked to the stimulus onset) in source-estimated data using an event-related beamformer approach (event-related synthetic aperture magnetometry or "ER-SAM", Cheyne, Bostan, Gaetz, & Pang, 2007), focusing the analyses on brain regions known to exhibit repetition suppression in fMRI studies (e.g. extrastriate visual cortex, the fusiform gyrus, and the lateral prefrontal cortex). In order to retain phase information in the MEG signals, source-estimated responses in different frequency bands (5–15 Hz: theta/alpha; 15–35 Hz: beta; 35–60 Hz: gamma) were first averaged in the time domain across trials, either novel or repeated. Evoked power estimates were calculated in 100-ms bins around the stimulus onset. Gilbert et al. (2010) observed increases in low-frequency evoked power (5–15 Hz) for repeated stimuli in the right fusiform gyrus and right lateral prefrontal cortex, with the earliest effects occurring between 100 and 200 ms post-stimulus onset in the fusiform gyrus. Similar results were observed in striate/extrastriate visual cortex, albeit in a slightly higher frequency range (beta: 15–35 Hz). Ghuman et al. (2008) measured changes in phase-locking between distant cortical sites in lateral prefrontal and occipitotemporal cortex while subjects made size judgments about novel and repeated objects. They found increases in fronto-temporal phase-locking between 10 and 15 Hz for repeated relative to novel objects. Importantly, the latency of the phase-locking increase predicted the magnitude of repetition priming for individual subjects. Taken together, these studies suggest that stimulus repetition in humans indeed leads to similar changes to those observed in electrode recording studies in animals. Repetition leads to larger local fluctuations in neural activity, as well as increased coupling between distant task-engaged sites, providing support for the Synchrony model.
Bayesian Networks and "Explaining Away"
The final proposal that we'll consider is a more sophisticated variant of the Facilitation model proposed by Friston and Henson (Friston, 2005; Henson, 2003; see Grill-Spector et al., 2006, for further discussion). In this proposal, the cortex is cast as a form of hierarchical generative Bayesian statistical model (see also Dayan, Hinton, Neal, & Zemel, 1995; Lee & Mumford, 2003; Lewicki & Sejnowski, 1996; Mumford, 1992; Rao & Ballard, 1999). Perceptual inference occurs as a progressive interaction between bottom-up sensory input ("evidence") and top-down expectations ("prediction") throughout the cortical hierarchy. A critical aspect of this view is that top-down predictions serve to inhibit or suppress the bottom-up sensory evidence, with residual activity in the lower levels of the cortical hierarchy serving as "prediction error" that is, in turn, relayed back toward the higher levels. The learning mechanism (expectation maximization, or EM algorithm) improves the top-down predictions in service of reducing prediction error, leading to reductions in neural activity in lower levels with stimulus repetition (i.e. Repetition Suppression) (see Figure 1D). This process is commonly referred to in the literature on Bayesian networks as "explaining away" (e.g. Pearl, 1988), since as the appropriate causes of the sensory evidence are learned, the incorrect causes (i.e. prediction error) are reduced and explained away. The proposal bears similarity to the simple Facilitation model in that stimulus repetition leads to progressively earlier termination of activity, potentially supporting earlier and improved behavioral identification/discrimination.
The Bayesian Explaining Away model makes a number of novel predictions in stimulus repetition paradigms. Given that repetition suppression in a certain brain region results from top-down input and that this input can be further propagated to progressively lower levels, the model predicts: 1) repetition suppression effects should tend to occur earlier in higher-level regions than in lower-level regions, 2) repetition should lead to stronger top-down causal interactions as assessed by methods such as Grainger causality and Dynamic Causal Modeling (DCM) (Friston, Harrison, & Penny, 2003), and 3) the nature of those stronger top-down causal interactions should be suppressive/inhibitory (i.e. negative coupling). One relatively novel feature of the Bayesian view is that higher levels of the processing hierarchy can track the likelihood of encountering particular objects, as well as more abstract variables such as the likelihood of object repetition in a stream of stimuli. This feature leads to another prediction: 4) a high likelihood of stimulus repetition in an experimental session (or block of trials) should produce a stronger top-down expectation/prediction from brain regions representing this more abstract contextual information (possibly in prefrontal regions). Hence, larger repetition suppression effects should be observed in brain regions receiving this kind of input (perhaps in object or category selective cortex in the temporal lobes). This last prediction has been evaluated in several recent experiments. Summerfield, Trittschuh, Monti, Mesulam, and Enger (2008) embedded short-term repetitions of face pictures in blocks of trials in which repetitions were either frequent (60% of trials) or infrequent (20% of trials). They found that repetition suppression effects in the fusiform face area (FFA) were stronger when repetitions were expected, with similar recent results reported in EEG/ERP (Summerfield, Wyart, Johnen, & de Gardelle, 2011) and MEG (Todorovic, van Ede, Maris, & de Lange, 2011). In contrast, a study of repetition suppression in monkey TE by Kaliukhovich and Vogels (2010) failed to find evidence of this kind of contextual sensitivity in single-cell firing rates or in LFP gamma band power. Another recent fMRI study in humans, while able to replicate the effect of repetition expectation on repetition suppression magnitude, found that this expectation effect disappeared when subjects had their attention diverted away from the stimuli (Larsson & Smith, 2011). This would appear to rule out the extreme version of the Explaining Away view in which all repetition suppression effects are explained by relatively high-level repetition expectation. However, it is important to keep in mind that this extreme version probably had few adherents to start, since earlier, more perceptual levels in the Bayesian hierarchy would not be expected to be influenced directly by more abstract variables such as the frequency of stimulus repetition. Taken together, these results provide partial support for a role of high-level expectation in modulating short-term repetition suppression effects, at least at particular points along the cortical processing hierarchy. The first three predictions listed above have been evaluated less thoroughly. However, one recent study by Ewbank et al. (2011) has provided some support for the prediction that top-down causal interactions should be stronger following stimulus repetition. They used DCM in fMRI to investigate changes in causal interactions between the fusiform body area (FBA) and the extrastriate body area (EBA) while subjects viewed pictures of human bodies. Pictures were either repetitions of the same body identity or different identities, shown in blocked conditions. They also evaluated the effect of varying picture size and viewpoint on repetition suppression and causal interactions. They found repetition suppression in both EBA and FBA to all viewing conditions (the same identity evoked less activity than different identities). Simultaneously, the DCM analyses revealed increased top-down causal interactions from FBA to EBA for same-identity relative to different-identity blocks in all conditions, with the same size/same view condition also showing greater causal interactions in the bottom-up direction. The fact that repetition suppression and greater top-down causal interactions occurred in the same experimental circumstances is consistent with prediction 2 listed above. However, these authors did not evaluate the more direct association between the strength of top-down coupling from FBA to EBA and the magnitude of repetition suppression in EBA, nor did they focus discussion on the apparently positive sign of the top-down coupling (relevant for prediction 3 listed above; for another study evaluating positive versus negative causal interactions with DCM, see a recent paper by Cardin, Friston, & Zeki, 2011). Positive coupling suggests an excitatory rather than inhibitory top-down influence on the lower-level activity, inconsistent with the Explaining Away account of repetition suppression but potentially consistent with the Synchrony model (e.g. Ghuman et al., 2008). The use of a blocked design also brings with it issues of interpretation, due to potential differences in attentional state and processing strategy (see below for further discussion). Nevertheless, these preliminary results provide some partial support for the Bayesian Explaining Away proposal. Future experiments will need to focus on how proposals such as the Synchrony model and Explaining Away might be further teased apart.
Going Forward
Having reviewed four basic proposals as to how repetition suppression might afford repetition priming, the only view that we consider definitively ruled out by current data is the Facilitation Model, at least in its existing form. Firing-rate recordings in a variety of areas in monkeys and even in humans (e.g. Pedreira et al., 2010) have shown that the onset of neural responses in typical stimulus viewing conditions is not temporally advanced. In many experimental circumstances used to measure priming, particularly those in which stimulus repetitions occur within a single experimental session, tests of the Sharpening model have also yielded a surprising lack of support. At present, we view the Synchrony model as the most promising. Recent experiments in variety of cognitive domains in animals and humans have provided converging support for the role of neural synchronization in behavior. However, the Bayesian Explaining Away model has received experimental support, as well, and neither the Synchrony nor Explaining Away models have been run through a full gauntlet of experimental tests. Below we lay out three basic experimental methods, that if applied, should help to bring about more clarity to the relationship between repetition suppression and priming.
Spike-LFP recordings in animals and human patients
The most direct way to evaluate the Synchrony model would be to measure single-unit and/or multi-unit spiking responses, along with LFPs, in several task engaged cortical regions. For example, monkeys could be trained to perform a discrimination task on visual stimuli with responses indicated through eye movements, taking behavioral measures of response time and accuracy (as in McMahon & Olson, 2007). Responses could then be recorded simultaneously in object/form-selective temporal regions such as TE and areas involved in the execution of eye movements such as the frontal eye fields (FEF). The Synchrony model would predict that spike-LFP coherence, possibly in lower frequencies such as alpha (8–12 Hz) or beta (13–30 Hz), should be greater for repeated stimuli within-area as well as across areas (for an example of this type of experiment in visual attention, see Gregoriou, Gotts, Zhou, & Desimone, 2009a)). Furthermore, this increased coherence should predict the magnitude of repetition priming. Interestingly, the Bayesian Explaining Away model would also predict increased coherence between spikes in higher-level areas such as FEF and LFPs in lower-level areas (e.g TE, in this case, due to suppression by top-down predictions). Both models would expect similar results in other paired locations within the ventral visual pathway that are involved in object form processing (e.g. V1, V2, V4, and TEO). The Explaining Away model would posit a further relationship between coherence increases and the magnitude of repetition suppression in the more bottom-up region of a pair of recording sites (with larger repetition suppression expected for larger coherence). Taking measures of causality in LFP-LFP recordings between two connected regions (e.g. Grainger causality, DCM, etc.), the Explaining Away model clearly predicts that the directionality of the interactions should flow more in the top-down direction for repeated stimuli compared to novel stimuli. Repetition suppression effects should also occur earlier in top-down regions than in bottom-up regions. The quantitative relationship between repetition suppression and increased synchronization, as well as the direction of information flow following repetition, is less constrained in the Synchrony model, potentially allowing for somewhat independent effects and symmetrical top-down/bottom-up causal interactions (see discussion below). However, the Synchrony and Explaining Away proposals differ critically in which cells should show the increased coupling. The Synchrony model posits that task-engaged cells that carry information critical for task-performance are the ones that are synchronizing, activating each other more reliably and effectively with single spikes. The prediction that follows is that cells that prefer a repeated stimulus are the ones that should synchronize (relative to those that are weakly tuned or weakly responsive). In contrast, the Bayesian Explaining Away proposal holds that there are two separate subpopulations of cells, cells that encode the conditional expectation of perceptual causes (φi) and those encoding prediction error (ξi) (see Figure 1D and Friston, 2005, p. 826, for discussion). After learning, the "error" cells are the ones that are suppressed by top-down predictions, and it is the firing of these cells that should carry the effects of the more strongly negative top-down coupling (perhaps exhibiting hyperpolarized voltages following spiking in higher-level areas representing predictions). Occasionally, experiments of this type (i.e. recording spikes and LFPs with microelectrodes) can be conducted in human patients undergoing brain surgery (e.g. Kraskov, Quiroga, Reddy, Fried, & Koch, 2007), and the same sorts of predictions would be expected to hold in these contexts.
Intracranial EEG in humans
While we view recent source-estimated MEG experiments in humans as supporting the Synchrony model (and potentially the Bayesian Explaining Away model), source-estimation procedures are forced to make many assumptions in order to provide an inverse solution, and the algorithms are complex. Direct electrical recordings with good spatial resolution (< 1–2 cm) would be useful for verifying the basic pattern of results observed in these MEG experiments, as well as for testing further predictions of the two models. Such measures are currently available in intracranial EEG studies in human patients who are undergoing surgery for intractable epilepsy, referred to as electrocorticography or ECoG (e.g. Canolty et al., 2006; Puce, Allison, & McCarthy, 1999). While the subdural electrodes used in these studies typically do not allow the recordings of spikes, they permit recordings of field voltages directly from the cortical surface and often provide coverage over a large extent of one cerebral hemisphere, recording signals from up to 100 electrodes simultaneously per patient. We have reported preliminary results of one such study utilizing an object-naming task in two patients with coverage of the lateral surface of the left frontal and temporal lobes (Gotts, Crone, & Martin, 2010, Society for Neuroscience Abstracts, Program 94.11). We found that stimulus repetition led to repetition priming in both patients and increases in low-frequency evoked power (1–15 Hz) for virtually all task-engaged electrodes (i.e. those that exhibited significant evoked responses), replicating the basic pattern ofGilbert et al. (2010). Like Ghuman et al. (2008), we also observed increases in phase-locking (LFP-LFP coherence) between task-engaged frontal and temporal electrodes in the alpha (8–12 Hz) and low beta (12–18 Hz) frequency ranges. With additional patients, we should have the ability to test several of the predictions discussed above for the Spike-LFP experiments, such as the timing and directionality of changes in the top-down and bottom-up directions, as well as the association between coherence changes and the magnitude of repetition priming. While the inability to record spikes in single-cells will necessarily create some ambiguity in interpretation with respect to the exact form that changes in synchronization take (e.g. spike synchrony versus rapid co-modulation of firing rates), the advantage of this method over the spike-LFP recordings is the nearly whole-hemisphere coverage that it provides. To our knowledge, only one other ECoG study to date in humans has examined the effect of stimulus repetition on local field activity (Puce et al., 1999). However, this study examined only short-term repetitions in ventral temporal cortex during passive viewing (as in Miller et al., 1991), and no measures of repetition priming were taken.
Connectivity Methods in fMRI
One large downside in using source-estimated MEG or ECoG to assess changes in neural synchronization is that the analog of repetition suppression in these methods is unclear. Fluctuations in field activity, either magnetic or electrical, may eventually be found to have a reliable correlate in terms of overall neural activity level, but this relationship is currently unknown. The two types of measures could theoretically be unrelated in the same manner that the mean and variance of a random variable can be independent and separate quantities. For example, firing rate that is uniformly distributed in time may have no detectable effect on field fluctuations, resulting in a blindness to certain sorts of changes in activity level when taking field measurements. In order to relate repetition suppression, repetition priming, and changes in synchrony, it would be best to measure these phenomena in the same experiments. While this should be possible for the Spike-LFP recording methods in animals, it might also be possible in coarser methods that are available to more researchers, such as fMRI in humans. First emphasized by Friston and colleagues (Friston et al., 1997; Friston, Harrison, & Penny, 2003), fMRI studies that measure patterns of temporal covariation in the BOLD response across pairs or collections of brain regions have become commonplace following the advent of resting-state functional connectivity methods (see Fox & Raichle, 2007, for review). If cells in two brain regions are engaging in more synchronous interactions with increased coupling while processing repeated compared to novel stimuli, one might expect the magnitudes of the corresponding BOLD responses to co-vary at higher levels, as well. This idea suggests a relatively straightforward fMRI experiment in which it should be possible to evaluate the separate effects of stimulus repetition on the mean BOLD response versus on the magnitude of BOLD covariation between pairs of task-engaged voxels/regions. However, there is at least one main stumbling block to carrying out this experiment. When novel and repeated stimuli are randomly intermixed in a typical rapid event-related design, standard analysis methods do a good job at estimating the mean BOLD response to each condition, even with a great deal of overlap of the slow responses to individual stimuli as long as baseline periods are appropriately interleaved. However, the same is not true of estimating the variation around the mean to each individual stimulus. This is what would be necessary in order to measure a condition-specific change in correlation/coupling cleanly, with correlation/coupling between two brain regions being calculated over the set of individual stimulus responses in each experimental condition (e.g. novel versus repeated). One solution would be to use a blocked design with no temporal overlap of the novel and repeated conditions, although that has well-known downsides, creating problems of interpretation with respect to strategic effects and differences in attentional state (e.g. D'Esposito, Zarahn, & Aguirre, 1999; Hamburger & Slowiaczek, 1998). A better solution would be to space individual stimuli far enough apart so that the peak of the BOLD responses are no longer overlapping (~ 8–10 seconds, closer to a "slow" event-related design, e.g. Bandettini & Cox, 2000), still permitting randomly interleaved conditions. In an experiment such as this (currently underway in our lab), the Synchrony model predicts that while the mean BOLD response is decreased to repeated stimuli (repetition suppression), correlations of the response magnitudes to individual repeated stimuli across task-engaged voxels should increase. Furthermore, beta weights or causal model parameters (e.g. DCM) that assess the strength of inter-regional coupling should be more positive and facilitatory for repeated compared to novel stimuli. The Bayesian Explaining Away model makes at least two novel predictions in this experiment: 1) methods of assessing causality (e.g. Grainger, DCM) should reveal a greater top-down flow of information (see discussion of Ewbank et al., 2011, above), and 2) beta weights or DCM model parameters between two connected brain regions should be negative, rather than positive as in the Synchrony model, between top-down and bottom-up areas for repeated stimuli. The magnitude of this negative coupling should be associated with the magnitude of repetition suppression in the bottom-up areas.
A Final Note on Repetition Suppression and the Synchrony Model
The Synchrony model posits that stimulus repetition should lead to enhanced local and long-range synchronization among task-engaged cortical regions, and this, in turn, should lead to improved accuracy and more rapid response times. What does this model have to say about repetition suppression? In the Gotts (2003) neural network model, short-term repetitions produced repetition suppression and synchronization in a more or less unitary fashion, through short-term plasticity mechanisms of synaptic depression and spike-frequency adaptation. However, these mechanisms recover over tens of seconds and don't apply at the longer lags used to study repetition suppression in many experiments. At longer lags, long-term plasticity mechanisms such as LTP/LTD are likely to be responsible for any observed changes in synchronization, perhaps through spike-timing-dependent plasticity (e.g. Bi & Poo, 1998; Markram et al., 1997; Sjöstrom et al., 2001) that improves the timing relations among cells that prefer the repeated stimulus. It is further possible that LTD dominates the changes such that activities will be reduced overall, producing repetition suppression, but how this would relate to changes in synchrony is quite unclear. We would tentatively suggest that the mechanisms producing changes in synchronization and those resulting in overall activity decreases may be at least partially independent, possibly explaining the lack of relationship between repetition suppression and repetition priming that has occasionally been observed (e.g. McMahon & Olson, 2007; Race et al., 2009; Xu, Turk-Browne, & Chun, 2007). Partial independence would require at least two mechanisms that would tend to be engaged when stimuli are repeatedly encountered in the service of improving neural processing efficiency. With more data in spike-LFP and slow event-related fMRI experiments, the relative importance of repetition suppression and synchronization in explaining priming may be put to the appropriate tests.
Acknowledgements
The authors would like to thank Nathan Crone, David Plaut, Jay McClelland, David McMahon, Carl Olson, and Avniel Ghuman for helpful discussions. The preparation of this paper was supported by the National Institute of Mental Health, NIH, Division of Intramural Research.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Ahmed B, Allison JD, Douglas RJ, Martin KAC, Whitteridge D. Estimates of the net excitatory currents evoked by visual stimulation of identified neurons in cat visual cortex. Cerebral Cortex. 1998;8:462–476. doi: 10.1093/cercor/8.5.462. [DOI] [PubMed] [Google Scholar]
- Aiello LC, Wheeler P. The expensive tissue hypothesis: the brain and digestive system in human and primate evolution. Current Anthropology. 1995;36:199–221. [Google Scholar]
- Allman JM. The origin of the neocortex. Seminars in the Neurosciences. 1990;2:257–262. [Google Scholar]
- Anderson B, Mruczek RE, Kawasaki K, Sheinberg D. Effects of familiarity on neural activity in monkey inferior temporal lobe. Cerebral Cortex. 2008;18:2540–2552. doi: 10.1093/cercor/bhn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR. The architecture of cognition. Cambridge, MA: Harvard University Press; 1983. [Google Scholar]
- Andresen DR, Vinberg J, Grill-Spector K. The representation of object viewpoint in human visual cortex. Neuroimage. 2009;45:522–536. doi: 10.1016/j.neuroimage.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CI, Behrmann M, Olson CR. Impact of learning on representation of parts and wholes in monkey inferotemporal cortex. Nature Neuroscience. 2002;5:1210–1216. doi: 10.1038/nn960. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Cox RW. Event-related fMRI contrast when using constant interstimulus interval: theory and experiment. Magnetic Resonance in Medicine. 2000;43:540–548. doi: 10.1002/(sici)1522-2594(200004)43:4<540::aid-mrm8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Bazhenov M, Stopfer M, Sejnowski TJ, Laurent G. Fast odor learning improves reliability of odor responses in the locust antennal lobe. Neuron. 2005;46:483–492. doi: 10.1016/j.neuron.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Moscovitch M, Behrmann M, Joordens S. Long-term semantic priming: a computational account and empirical evidence. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1997;23:1059–1082. [PubMed] [Google Scholar]
- Bedny M, McGill M, Thompson-Schill SL. Semantic adaptation and competition during word comprehension. Cerebral Cortex. 2008;18:2574–2585. doi: 10.1093/cercor/bhn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Peled BS. The contribution of task-related factors to ERP repetition effects at short and long lags. Memory & Cognition. 1990;18:359–366. doi: 10.3758/bf03197125. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. Journal of Neuroscience. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman I, Cooper EE. Evidence for complete translational and reflectional invariance in visual object recognition. Perception. 1991;20:585–593. doi: 10.1068/p200585. [DOI] [PubMed] [Google Scholar]
- Biederman I, Cooper EE. Size invariance in visual object priming. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:121–133. [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant JS, Large ME, McCall L, Goodale MA. Independent processing of form, colour, and texture in object perception. Perception. 2008;37:57–78. doi: 10.1068/p5727. [DOI] [PubMed] [Google Scholar]
- Cardin V, Friston KJ, Zeki S. Top-down modulations in the visual form pathway revealed with dynamic causal modeling. Cerebral Cortex. 2011;21:550–562. doi: 10.1093/cercor/bhq122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave CB. Very long-lasting priming in picture naming. Psychological Science. 1997;8:322–325. [Google Scholar]
- Cave CB, Bost PR, Cobb RE. Effects of color and pattern on implicit and explicit picture memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:639–653. doi: 10.1037//0278-7393.22.3.639. [DOI] [PubMed] [Google Scholar]
- Cave CB, Squire LR. Intact and long lasting repetition priming in amnesia. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:509–520. doi: 10.1037//0278-7393.18.3.509. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bostan AC, Gaetz W, Pang EW. Event-related beamforming: a robust method for presurgical functional mapping using MEG. Clinical Neurophysiology. 2007;118:1691–1704. doi: 10.1016/j.clinph.2007.05.064. [DOI] [PubMed] [Google Scholar]
- Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychological Review. 1975;82:407–428. [Google Scholar]
- Constanti A, Sim JA. Calcium-dependent potassium conductance in guinea-pig olfactory cortex neurones in vitro. Journal of Physiology (London) 1987;387:173–194. doi: 10.1113/jphysiol.1987.sp016569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Hinton GE, Neal RM, Zemel RS. The Helmholtz machine. Neural Computation. 1995;7:889–904. doi: 10.1162/neco.1995.7.5.889. [DOI] [PubMed] [Google Scholar]
- De Baene W, Vogels R. Effects of adaptation on the stimulus selectivity of macaque inferior temporal spiking activity and local field potentials. Cerebral Cortex. 2010;20:2145–2165. doi: 10.1093/cercor/bhp277. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proceedings of the National Academy of Sciences, USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK. Event-related functional MRI: Implications for cognitive psychology. Psychological Bulletin. 1999;125:155–164. doi: 10.1037/0033-2909.125.1.155. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Eger E, Henson RN, Driver J, Dolan RJ. Mechanisms of top-down facilitation in perception of visual objects studied by fMRI. Cerebral Cortex. 2007;17:2123–2133. doi: 10.1093/cercor/bhl119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nature Reviews Neuroscience. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Lawson RP, Henson RN, Rowe JB, Passamonti L, Calder AJ. Changes in "top-down" connectivity underlie repetition suppression in the ventral visual pathway. Journal of Neuroscience. 2011;31:5635–5642. doi: 10.1523/JNEUROSCI.5013-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall SL, Anzellotti S, Pajtas PE, Caramazza A. Concordance between perceptual and categorical repetition effects in the ventral visual stream. Journal of Neurophysiology. 2011;106:398–408. doi: 10.1152/jn.01138.2010. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Gruber T, Supp GG. Neuronal mechanisms of repetition priming in occipitotemporal cortex: spatiotemporal evidence from functional magnetic resonance imaging and electroencephalography. Journal of Neuroscience. 2005;25:3414–3422. doi: 10.1523/JNEUROSCI.4107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Experience-dependent sharpening of visual shape selectivity in inferior temporal cortex. Cerebral Cortex. 2006;16:1631–1644. doi: 10.1093/cercor/bhj100. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Sciences. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Friston K. A theory of cortical responses. Philosophical Transactions of the Royal Society London B: Biological Sciences. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modeling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gagnepain P, Chételat G, Landeau B, Dayan J, Eustache F, Lebreton K. Spoken word memory traces within the human auditory cortex revealed by repetition priming and functional magnetic resonance imaging. Journal of Neuroscience. 2008;28:5281–5289. doi: 10.1523/JNEUROSCI.0565-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- Ghuman AS, Bar M, Dobbins IG, Schnyer DM. The effects of priming on frontal-temporal communication. Procedures of the National Academy of Science, USA. 2008;105:8405–8409. doi: 10.1073/pnas.0710674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JR, Gotts SJ, Carver FW, Martin A. Object repetition leads to local increases in the temporal coordination of neural responses. Frontiers in Human Neuroscience. 2010;4:30. doi: 10.3389/fnhum.2010.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, Buckner RL. Common and dissociable activation patterns associated with controlled semantic and phonological processing: evidence from FMRI adaptation. Cerebral Cortex. 2005;15:1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- Gotts SJ. Mechanisms Underlying Enhanced Processing Efficiency in Neural Systems. Pittsburgh, PA: Carnegie Mellon University; 2003. [Google Scholar]
- Gotts SJ, Milleville SC, Bellgowan PSF, Martin A. Broad and narrow conceptual tuning in the human frontal lobes. Cerebral Cortex. 2011;21:477–491. doi: 10.1093/cercor/bhq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf P, Squire LR, Mandler G. The information that amnesic patients do not forget. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1984;10:164–178. doi: 10.1037//0278-7393.10.1.164. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009a;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. Long-range neural coupling through synchronization with attention. Progress in Brain Research. 2009b;176:35–45. doi: 10.1016/S0079-6123(09)17603-3. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Science. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychologica. 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Gruber T, Muller MM. Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cerebral Cortex. 2005;15:109–116. doi: 10.1093/cercor/bhh113. [DOI] [PubMed] [Google Scholar]
- Hamburger M, Slowiaczek LM. Repetition priming and experimental context effects. American Journal of Psychology. 1998;111:1–31. [PubMed] [Google Scholar]
- Hansen BJ, Dragoi V. Adaptation-induced synchronization in laminar cortical circuits. Proceedings of the National Academy of Sciences, USA. 2011;108:10720–10725. doi: 10.1073/pnas.1102017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Zempel JM, Snyder AZ, Raichle ME. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010;66:353–369. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RN, Goshen-Gottstein Y, Ganel T, Otten LJ, Quayle A, Rugg MD. Electrophysiological and haemodynamic correlates of face perception, recognition and priming. Cerebral Cortex. 2003;13:793–805. doi: 10.1093/cercor/13.7.793. [DOI] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rylands A, Ross E, Vuilleumier P, Rugg MD. The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. Neuroimage. 2004;21:1674–1689. doi: 10.1016/j.neuroimage.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Horner AJ, Henson RN. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46:1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AJ, Henson RN. Incongruent abstract stimulus-response bindings result in response interference: fMRI and EEG evidence from visual object classification priming. Journal of Cognitive Neuroscience. 2011 Nov 8; doi: 10.1162/jocn_a_00163. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TW, Gauthier I. Repetition-induced changes in BOLD response reflect accumulation of neural activity. Human Brain Mapping. 2006;27:37–45. doi: 10.1002/hbm.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Menon RS, Goodale MA. The effects of visual object priming on brain activation before and after recognition. Current Biology. 2000;10:1017–1024. doi: 10.1016/s0960-9822(00)00655-2. [DOI] [PubMed] [Google Scholar]
- Jiang X, Bradley E, Rini RA, Zeffiro T, Vanmeter J, Riesenhuber M. Categorization training results in shape- and category-selective human neural plasticity. Neuron. 2007;53:891–903. doi: 10.1016/j.neuron.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking items in human working memory. Science. 2000;287:643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- Kaliukhovich DA, Vogels R. Stimulus repetition probability does not affect repetition suppression in macaque inferior temporal cortex. Cerebral Cortex. 2011;21:1547–1558. doi: 10.1093/cercor/bhq207. [DOI] [PubMed] [Google Scholar]
- Kiefer M. Repetition priming modulates category-related effects on event-related potentials: further evidence for multiple cortical semantic systems. Journal of Cognitive Neuroscience. 2005;17:199–211. doi: 10.1162/0898929053124938. [DOI] [PubMed] [Google Scholar]
- Koester J, Siegelbaum SA. Membrane potential. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4 th Ed. New York: McGraw-Hill; 2000. pp. 125–139. [Google Scholar]
- Konen CS, Kastner S. The hierarchically organized neural systems for object information in human visual cortex. Nature Neuroscience. 2008;11:224–231. doi: 10.1038/nn2036. [DOI] [PubMed] [Google Scholar]
- Kraskov A, Quiroga RQ, Reddy L, Fried I, Koch C. Local field potentials and spikes in the human medial temporal lobe are selective to category. Journal of Cognitive Neuroscience. 2007;19:479–492. doi: 10.1162/jocn.2007.19.3.479. [DOI] [PubMed] [Google Scholar]
- Larsson J, Smith AT. fMRI repetition suppression: neuronal adaptation or stimulus expectation? Cerebral Cortex. 2011 Jun 20; doi: 10.1093/cercor/bhr119. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Mumford D. Hierarchical Bayesian inference in the visual cortex. Journal of the Optical Society of America. 2003;20:1434–1448. doi: 10.1364/josaa.20.001434. [DOI] [PubMed] [Google Scholar]
- Lewicki MS, Sejnowski TJ. Bayesian unsupervised learning of higher order structure. Advances in Neural Information Processing Systems. 1996;9:529–535. [Google Scholar]
- Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. Journal of Neurophysiology. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- Logan GD. Repetition priming and automaticity: Common underlying mechanisms? Cognitive Psychology. 1990;22:1–35. [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. Journal of Neurophysiology. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA1 pyramidal neurones in vitro. Journal of Physiology (London) 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Milleville SC, Negri GAL, Rumiati RI, Alfonso C, Martin A. Action-related properties shape object representations in the ventral stream. Neuron. 2007;55:507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rumelhart DE. Distributed memory and the representation of general and specific information. Journal of Experimental Psychology: General. 1985;114:159–188. doi: 10.1037//0096-3445.114.2.159. [DOI] [PubMed] [Google Scholar]
- McKone E. Short-term implicit memory for words and nonwords. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:1108–1126. [Google Scholar]
- McKone E. The decay of short-term implicit memory: Unpacking lag. Memory & Cognition. 1998;26:1173–1186. doi: 10.3758/bf03201193. [DOI] [PubMed] [Google Scholar]
- McMahon DB, Olson CR. Repetition suppression in monkey inferotemporal cortex: relation to behavioral priming. Journal of Neurophysiology. 2007;97:3532–3543. doi: 10.1152/jn.01042.2006. [DOI] [PubMed] [Google Scholar]
- Miller EK, Gochin PM, Gross CG. Habituation-like decrease in the responses of neurons in inferior temporal cortex of the macaque. Visual Neuroscience. 1991;7:357–362. doi: 10.1017/s0952523800004843. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. Journal of Neuroscience. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DB. Nonconscious priming after 17 years: Invulnerable implicit memory? Psychological Science. 2006;17:925–929. doi: 10.1111/j.1467-9280.2006.01805.x. [DOI] [PubMed] [Google Scholar]
- Mumford D. On the computational architecture of the neocortex II. Biological Cybernetics. 1992;66:241–251. doi: 10.1007/BF00198477. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary learning-systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia: electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Ostergaard AL. The effects on priming of word frequency, number of repetitions, and delay depend on the magnitude of priming. Memory & Cognition. 1998;26:40–60. doi: 10.3758/bf03211369. [DOI] [PubMed] [Google Scholar]
- Paller KA, Gross M. Brain potentials associated with perceptual priming vs explicit remembering during the repetition of visual word-form. Neuropsychologia. 1998;36:559–571. doi: 10.1016/s0028-3932(97)00132-2. [DOI] [PubMed] [Google Scholar]
- Pearl J. Probabilistic Reasoning in Intelligent Systems: Networks of Plausible Inference. San Mateo, CA: Morgan Kaufmann; 1988. [Google Scholar]
- Pedreira C, Mormann F, Kraskov A, Cerf M, Fried I, Koch C, Quiroga RQ. Responses of human medial temporal lobe neurons are modulated by stimulus repetition. Journal of Neurophysiology. 2010;103:97–107. doi: 10.1152/jn.91323.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peissig JJ, Singer J, Kawasaki K, Sheinberg DL. Effects of long-term object familiarity on event-related potentials in the monkey. Cerebral Cortex. 2007;17:1323–1334. doi: 10.1093/cercor/bhl043. [DOI] [PubMed] [Google Scholar]
- Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004;44:547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, McCarthy G. Electrophysiological studies of human face perception. III: effects of top-down processing on face-specific potentials. Cerebral Cortex. 1999;9:445–458. doi: 10.1093/cercor/9.5.445. [DOI] [PubMed] [Google Scholar]
- Race EA, Badre D, Wagner AD. Multiple forms of learning yield temporally distinct electrophysiological repetition effects. Cerebral Cortex. 2010;20:1726–1738. doi: 10.1093/cercor/bhp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race EA, Shanker S, Wagner AD. Neural priming in human frontal cortex: multiple forms of learning reduce demands on the prefrontal executive system. Journal of Cognitive Neuroscience. 2009;21:1766–1781. doi: 10.1162/jocn.2009.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annual Review of Neuroscience. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Rainer G, Miller EK. Effects of visual experience on the representation of objects in the prefrontal cortex. Neuron. 2000;27:179–189. doi: 10.1016/s0896-6273(00)00019-2. [DOI] [PubMed] [Google Scholar]
- Rao RP, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nature Neuroscience. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Reyes AD. Synchrony-dependent propagation of firing rate in iteratively constructed networks in vitro. Nature Neuroscience. 2003;6:593–599. doi: 10.1038/nn1056. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Brovedani P, Doyle MC. Modulation of event-related potentials (ERPs) by word repetition in a task with inconsistent mapping between repetition and response. Electroencephalography and Clinical Neurophysiology. 1992;84:521–531. doi: 10.1016/0168-5597(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Mark RE, Gilchrist J, Roberts RC. ERP repetition effects in indirect and direct tasks: effects of age and interitem lag. Psychophysiology. 1997;34:572–586. doi: 10.1111/j.1469-8986.1997.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Impact of correlated synaptic input on output firing rate and variability in simple neuronal models. Journal of Neuroscience. 2000;20:6193–6209. doi: 10.1523/JNEUROSCI.20-16-06193.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nature Reviews Neuroscience. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Kutas M. Time course of processes and representations supporting visual object identification and memory. Journal of Cognitive Neuroscience. 2003;15:111–135. doi: 10.1162/089892903321107864. [DOI] [PubMed] [Google Scholar]
- Scott LS, Tanaka JW, Sheinberg DL, Curran T. A reevaluation of the electrophysiological correlates of expert object processing. Journal of Cognitive Neuroscience. 2006;18:1453–1465. doi: 10.1162/jocn.2006.18.9.1453. [DOI] [PubMed] [Google Scholar]
- Sjöstrom PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Srinivas K. Size and reflection effects in priming: A test of transfer-appropriate processing. Memory & Cognition. 1996;24:441–452. doi: 10.3758/bf03200933. [DOI] [PubMed] [Google Scholar]
- Stark CE, McClelland JL. Repetition priming of words, pseudowords, and nonwords. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:945–972. doi: 10.1037//0278-7393.26.4.945. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Laurent G. Short-term memory in olfactory network dynamics. Nature. 1999;402:664–668. doi: 10.1038/45244. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh EH, Monti JM, Mesulam MM, Egner T. Neural repetition suppression reflects fulfilled perceptual expectations. Nature Neuroscience. 2008;11:1004–1006. doi: 10.1038/nn.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Wyart V, Johnen VM, de Gardelle V. Human scalp electroencephalography reveals that repetition suppression varies with expectation. Frontiers in Human Neuroscience. 2011;5:67. doi: 10.3389/fnhum.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D. Effects of prefrontal lesions on lexical processing and repetition priming: an ERP study. Cognitive Brain Research. 1998;7:143–157. doi: 10.1016/s0926-6410(98)00019-6. [DOI] [PubMed] [Google Scholar]
- Szymanski KF, MacLeod CM. Manipulation of attention at study affects an explicit but not an implicit test of memory. Consciousness & Cognition: An International Journal. 1996;5:165–175. [PubMed] [Google Scholar]
- Todorovic A, van Ede F, Maris E, de Lange FP. Prior expectation mediates neural adaptation to repeated sounds in the auditory cortex: an MEG study. Journal of Neuroscience. 2011;31:9118–9123. doi: 10.1523/JNEUROSCI.1425-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between cortical pyramidal neurons depends on neurotransmitter release probability. Proceedings of the National Academy of Sciences - USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Bielamowicz L, Martin A. Modulation of neural activity during object naming: effects of time and practice. Cerebral Cortex. 2003;13:381–391. doi: 10.1093/cercor/13.4.381. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Ellmore T, Martin A. Long-lasting cortical plasticity in the object naming system. Nature Neuroscience. 2000;3:1329–1334. doi: 10.1038/81873. [DOI] [PubMed] [Google Scholar]
- Varela JA, Song S, Turrigiano GG, Nelson SB. Differential depression at excitatory and inhibitory synapses in visual cortex. Journal of Neuroscience. 1999;19:4293–4304. doi: 10.1523/JNEUROSCI.19-11-04293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef B-E, Kayaert G, Franko E, Vangeneugden J, Vogels R. Stimulus similarity-contingent neural adaptation can be time and cortical area dependent. Journal of Neuroscience. 2008;28:10631–10640. doi: 10.1523/JNEUROSCI.3333-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein A, Chiang C, Konig P. Top-down processing mediated by interareal synchronization. Proceedings of the National Academy of Sciences, USA. 2000;97:14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Iliescu BF, Ma J, Josić K, Dragoi V. Adaptive changes in neuronal synchronization in macaque V4. Journal of Neuroscience. 2011;31:13204–13213. doi: 10.1523/JNEUROSCI.6227-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. The effect of prior learning on subsequent retention in amnesic patients. Neuropsychologia. 1974;12:419–428. doi: 10.1016/0028-3932(74)90072-4. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Sayres R, Vinberg J, Grill-Spector K. fMRI-adaptation and category selectivity in human ventral temporal cortex: regional differences across time scales. Journal of Neurophysiology. 2010;103:3349–3365. doi: 10.1152/jn.01108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. Journal of Neurophysiology. 2009;101:2632–2648. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nature Neuroscience. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A, Sunderland T. Monitoring frequency of occurrence without awareness: evidence from patients with Alzheimer's disease. Journal of Clinical and Experimental Neuropsychology. 1997;19(2):235–244. doi: 10.1080/01688639708403854. [DOI] [PubMed] [Google Scholar]
- Xu Y, Turk-Browne NB, Chun MM. Dissociating task performance from fMRI repetition attenuation in ventral visual cortex. Journal of Neuroscience. 2007;27:5981–5985. doi: 10.1523/JNEUROSCI.5527-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]