Abstract
Purpose
This investigation sought to optimize ultrasmall particles of iron oxide (USPIO) contrast agent dosage for visualizing cerebral microvasculature on an 8.0-Tesla ultra high field magnetic resonance imaging system.
Materials and Methods
USPIO contrast agent was intravenously administered to 3 groups of 4 rats at 1, 2, and 3 mg Fe/kg. Each animal was scanned before and after injection of USPIO using a high resolution T2*-weighted gradient recalled echo sequence with an in-plane resolution of 78 µm. The signal-to-noise ratio (SNR) and the number of microvessels visualized within the cortex and basal ganglia were calculated and compared before and after the administration of USPIO.
Results
As the USPIO dose increased, microvascular conspicuity increased, and SNR decreased. A dosage of 2 mg Fe/kg improved microvascular visualization in both cortex and basal ganglion regions relative to 1 mg Fe/kg without significantly sacrificing SNR as was the case at 3 mg Fe/kg.
Conclusion
Two mg Fe/kg USPIO is an optimal dose when imaging normal rat cerebral microvasculature using GRE T2*-weighted MR imaging at a field strength of 8 T.
Keywords: USPIO, magnetic resonance imaging, brain, microvasculature
Ultrasmall particles of iron oxide (USPIOs) are magnetic resonance (MR) contrast agents, which are cleared by the polymorphonuclear system. Because of their unique clearance characteristics, the plasma half-life of USPIOs is prolonged and has been categorized as a blood-pool MR contrast agent for magnetic resonance angiography (MRA).1–5 In these studies, USPIO produced a strong T1 effect to visualize the vessels at conventional 1.5-Tesla (T) magnetic field strength. In addition, USPIO also has a strong T2* effect, which can be detected by T2*-sensitive sequences, such as gradient recalled echo (GRE).6–8 In the central nervous system, the distinct macrophage-microglia is quiescent in the presence of an intact blood-brain barrier. Under physiological conditions, USPIO only circulates in cerebral blood vessels rather than penetrating the blood-brain barrier.7 This leads to a concentration gradient between cerebral vessels and surrounding brain parenchyma, which provides an opportunity to visualize cerebral microvasculature on steady state T2* GRE sequences.
Ultra high field (UHF) MR systems provide significantly higher signal to noise ratio (SNR) and more pronounced susceptibility effect relative to lower field systems. 9–12 These 2 advantages of UHF MRI combined with the use of USPIO intravascular contrast agents make it possible to obtain high-resolution negative-enhanced images of the normal rodent brain microvasculature.
The purpose of the present study was to determine the optimal dosage of USPIO in normal Wistar rat brain using an 8-T MRI system. This dosage can then be applied toward the in vivo visualization of microvascularity in rodent brain pathologies such as stroke and glioma. Ultimately, it can serve as a reference for establishing dosages for imaging microvascularity in human pathology.
MATERIALS AND METHODS
Animal Preparation
This study was performed according to the guidelines of the National Institutes of Health and the recommendations of the committee on animal research of our institution. The protocol was approved by the Institutional Animal Care and Use Committee (ILACUC). Male adult Wistar rats (n = 12), body weight 250–350 g, were divided randomly in 3 dosage groups which received 1, 2, and 3 mg Fe/kg USPIO (n = 4).
The rats were anesthetized initially by spontaneous inhalation of 5% isoflurane, mixed with O2 at a rate of 1000 mL/min, and followed by 1.5–2% isoflurane with 500 mL/ min O2 through a nose cone. The left femoral vein was then cannulized by a 0.47-mm out-diameter silicone tube filled with 2% heparin. The body temperature of the rats was kept at 37 ± 1°C during the operation by using a preheated isothermal pad (Deltaphase; Braintree Scientific, Braintree, MA).
Contrast Agent Preparation
The USPIO agent used in this study was 0.5 mol/L solution of SHU 555 C (Supravist, Schering AG, Germany). It consists of superparamagnetic iron oxide particles coated with carboxydextran. The mean diameter of the particles is 21 nm. The blood plasma half-life of USPIO in rats is approximately 2–3 hours at a dose of 40 µmol/kg (2.24 mg Fe/kg). Furthermore, the USPIO particles have an R1 relaxivity of approximately 25 seconds−1 nM−1 and an R2 relaxivity of approximately 164 seconds−1 nM−1 at 0.47 T, 37°C in water.7
Dosages were initially derived from preceding MRA, lymph node inflammation, and macrophage activity in brain ischemia studies published previously,13–17 in which the dosage of USPIO varied from 2.5 mg Fe/kg to 16.8 mg Fe/kg. Initial experience at our institution with doses 5 mg/kg or more resulted in poor quality images suspected to be due to signal loss from the susceptibility effects of iron within the particles in the cerebral circulation. This obscured visualization of finer structures such as microvessels but not lymph nodes. As a result, doses used in this study were smaller than those used in studies evaluating lymph node inflammation and macrophage activity. Before administration, the SHU 555 C solution was diluted with 0.9% sodium chloride to 0.1 mmol/ml at room temperature. Either 1, 2, or 3 mg Fe/kg doses were prepared for each rat. A total of 3 groups of 4 rats were used in this study.
8-T MRI Scanning
In vivo MRI was performed on an 8-T/80cm MRI system (Magnex-GE, Abingdon, UK) equipped with a Bruker AVANCE console (Bruker, Billerica, MA) interfaced with Techron (Crown International, Elkhart, IN) gradient amplifiers and Magnex gradients (Magnex Scientific, Abingdon, UK) using a custom-built radiofrequency front end. A custom-made 3-cm diameter birdcage coil was tuned to the head of each rat at 340 MHz while the rat was in a prone position. The rat was anesthetized and its body temperature maintained with the same protocol used for surgery (see the section “Animal Preparation”) and fixed on a custom-made holder. The coil was placed inside the bore of the magnet. A T2*- weighted 2-dimensional multislice GRE sequence (TR/TE 700/16 milliseconds, flip angle 45°, matrix 512 × 512, field of view 4 cm, slice thickness 1 mm, gap 0.1 mm, number of average 2, acquisition time 12 minutes) was used to acquire 16 coronal images with an in-plane resolution of 78 µm. These parameters were derived from previous studies to obtain favorable SNR. After the precontrast imaging, a dose of USPIO was administrated through the silicon tube canalized in the left femoral vein at a rate of 1 mL/min, followed by an equivalent volume of normal saline flushing at the same rate. The postcontrast sequence started within 2 minutes after administration of the USPIO.
After imaging, the rat was removed from the coil and put in a 35°C warm cage with room air for a full recovery from anesthesia. All rats were monitored until they ambulated All rats tolerated MR scanning well and recovered from anesthesia.
Image Analysis
Among the 16 consecutive coronal GRE images for each rat, the slice that best displayed the telencephalon was selected for image SNR analysis and microvascular quantification. This slice covered both cortex and basal ganglia, and showed little susceptibility artifact from the base of the skull.
Signal was measured using Paravision software (version 2.1; Bruker Biospin Corp.; Billerica, MA). Four regions of interest (ROIs) were selected, 2 covering most of the cortex in each hemisphere separately and the basal ganglia in each hemisphere. To minimize variation in slice selection before and after contrast administration, contrast was administered to the rodents while they were in the magnet without moving the rodent. The exact same size and site of the regions of interest were selected before and after USPIO administration. Two narrowed vertical rectangular ROIs were obtained from the top corners of the same image to get the average standard deviation (SD) of the noise. The SNR was calculated as:
Two observers, M.Y. and T.G., independently counted the number of visualized cortical penetrating microvessels and visualized basal ganglion microvessels from both hemispheres on the same slice used for the SNR measurement. This generated a total of 2 basal ganglion regions and 2 cortical regions in each rat. A consensus definition for the microvessels was used. Microvessels were considered to be cortical perforating vessels if they displayed a radial pattern extending from the convexities, whereas basal ganglion branches presented a tortuous pattern extending from the base of the brain.
Statistical analysis on SNR change, microvascular conspicuity after administration of USPIO in each dosage group and interobserver agreement were performed by student t test. A P value less than 0.05 was regarded as significant. In addition, multiple comparisons among 3 dosage groups on overall SNR change and improvement of microvascular conspicuity were performed using Bonferroni correction in which a P value less than 0.0167 was regarded as significant.
RESULTS
SNR Decrease in the Rat Brain After the Administration of USPIO
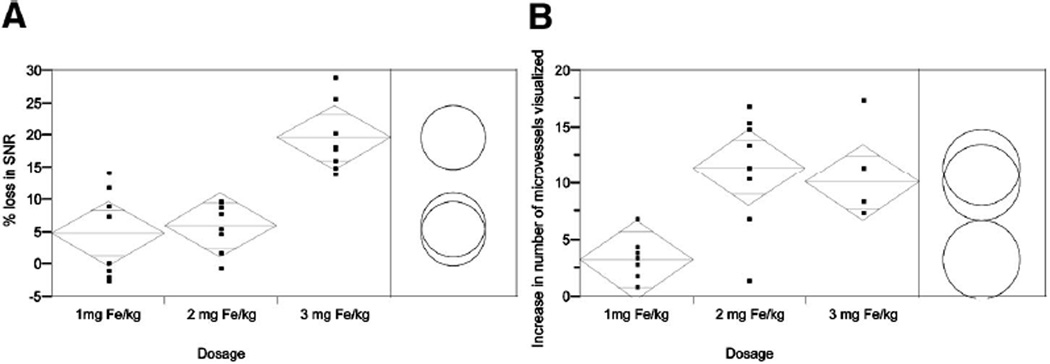
As expected, after the administration of USPIOs, the SNR on T2* GRE imaging of the rodent brain decreased as the dosage increased (Table 1 and Fig. 1). With each successive increment in USPIO administration, SNR decreased by 3.80%, 6.53%, and 17.83%, respectively, in the cortex, and 5.44%, 6.59%, and 22.42% in the basal ganglia. The decrease in SNR in cortex and basal ganglion areas in the 3 mg Fe/kg group was significantly more, relative to the other 2 dosage groups respectively (P < 0.0167), whereas there was no significant difference between the 1- and 2-mg Fe/kg groups (Fig. 1A).
TABLE 1.
Mean SNR and Percent Decrease in SNR in the Cortex and Basal Ganglion Regions in Different Dosage Groups
| SNR in the Cortex ± σ |
SNR in the Basal Ganglia ± σ |
|||||
|---|---|---|---|---|---|---|
| USPIO | Pre-USPIO | Post-USPIO | % Decrease | Pre-USPIO | Post-USPIO | % Decrease |
| 1 mg Fe/kg | 14.5 ± 1.0 | 13.9 ± 1.6 | 3.80 | 15.1 ± 0.9 | 14.3 ± 1.2 | 5.44 |
| 2 mg Fe/kg | 18.7 ± 0.9 | 17.5 ± 0.5 | 6.53 | 17.2 ± 1.4 | 16.0 ± 0.9 | 6.59 |
| 3 mg Fe/kg | 14.5 ± 2.9 | 11.9 ± 2.3 | 17.83* | 14.1 ± 1.2 | 10.9 ± 0.6 | 22.42* |
Mean SNR and percent decrease in the 3-mg Fe/kg group are significantly different from either 1 or 2 mg Fe/kg in both cortical and basal ganglion regions (P < 0.0167, Student t test with Bonferroni correction).
FIGURE 1.
Comparison of mean percentage SNR loss (A) and of mean difference in the number of microvessels visualized (B) among the 3 dosage groups. Standard error bars with upper and lower range limits set to 98.3% are reflected in the diamonds. Circles represent the range in each dosage group using the Student t test with α = 0.0167 for Bonferroni correction. Separation between circles thus reflects a significant difference.
Microvascular Conspicuity
The average number of visualized microvessels within the cortex and basal ganglion regions are quantified and are presented in Table 2. In the precontrast series, the number of vessels identified in all 12 rats ranged between 0 and 12. Among all 12 rats, 4 (33%) displayed recognized branches of cortical penetrating vessels, whereas in the basal ganglia area, 8 rats (67%) displayed microvessels.
TABLE 2.
Microvascular Quantification Pre- and Postadministration of USPIO in Cortex and Basal Ganglia
| Mean Number of Microvessels Identified in the Cortex ± σ |
Mean Number of Microvessels Identified in the Basal Ganglia ± σ |
|||||
|---|---|---|---|---|---|---|
| USPIO | Pre-USPIO | Post-USPIO | P * | Pre-USPIO | Post-USPIO | P * |
| 1 mg Fe/kg | 5.8 ± 3.2 | 9.8 ± 2.2 | 0.0377 | 6.8 ± 1.26 | 8.9 ± 1.3 | 0.0936 |
| 2 mg Fe/kg | 3.5 ± 1.9 | 16.6 ± 3.9 | 0.0009 | 4.6 ± 3.1 | 14.3 ± 3.6 | 0.0229 |
| 3 mg Fe/kg | 4.3 ± 0.9 | 15.5 ± 1.9 | 0.0154 | 3.8 ± 1.7 | 12.8 ± 1.2 | 0.001 |
Student t test P-values derived when comparing mean number of microvessels identified pre- and post-USPIO administration for each dosage group.
Vessel conspicuity and number of vessels identified increased following contrast injection relative to precontrast images. Cortical and basal ganglion microvasculature was visualized in all 12 rats after contrast administration. The overall improvements of microvascular visualization in the 2- and 3-mg Fe/kg groups were significantly higher than that in (P < 0.0167), whereas there was no significant difference between 2- and 3-mg Fe/kg groups (Fig. 1B). No significant interobserver difference was found (P = 0.55). The 2 mg Fe/kg provided an improved microvascularity visualization without significant loss in SNR (Figs. 1 and 2).
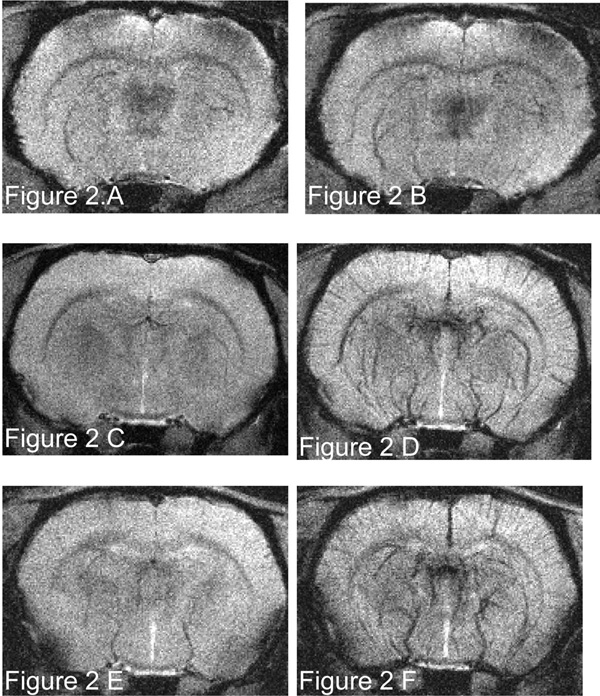
FIGURE 2.
Coronal T2*-weighted GRE image (TR/TE 700/16 milliseconds, flip angle = 45°, matrix 512 × 512, FOV = 4 cm, slice thickness 1 mm, slice gap 0.1 mm, NEX = 2, in-plane resolution 78 µm) before and after USPIO administration of 1 mg Fe/kg (A and B, respectively), 2 mg Fe/kg (C and D, respectively), and 3 mg Fe/kg (E and F, respectively).
DISCUSSION
The present study demonstrates that intravenous USPIO at a dosage of 2 mg Fe/kg produces a favorable negative contrast for the visualization of microvasculature in the Wistar rat brain on the 8-T MRI system. To the best of our knowledge, this is the first study that uses USPIO to visualize the microvessels in the rodent brain. High-resolution, high-field MR imaging of cerebral microvasculature has significant value because it can potentially provide evidence for increased tumoral microvascularity, which can be a critical marker for tumor growth, metastatic potential, and therapeutic potential.11,12,18 From this point of view, identification of new vessel formation on UHF MR systems with the aid of USPIO agents, may predict the prognosis of tumor as well as disease progression.
Eight-Tesla MRI provides a unique platform to study the microvasculature caused by high resolution (78 µm) and high SNR. 9,11,12,19 Moreover, the microsusceptibility changes of the local static magnetic field increase linearly with the field strength, causing a significant decrease in T2* of the local tissue which leads to a reduction of the MR signal in the affected region relative to lower field strengths.10,20 Echo time (TE) plays an important role in T2* weighted imaging. As TE values increase, there is a corresponding decrease in the MR signal. The TE value (16 milliseconds) adapted in the current study was based on preceding 8-T parameter optimization.9,21 As the TE value increases, there is a corresponding decrease of MR signal causing image deterioration due to the strong macro susceptibility artifacts in the regions of the bone tissue contacts and near the cranial cavities.20 As a result selection of imaging parameters balance the enhancement of susceptibility effects, which allow improved visualization of microvessels, to that of image deterioration as a result of susceptibility effects.
Susceptibility effects induced by the iron particles in the blood vessels shorten the transverse T2 and T2* relaxation times and cause a signal loss in T2* sensitive sequences. These effects have been shown to be valuable in the diagnosis of some diseases.8,17,22 As a tissue-specific MR contrast agent, USPIO has been used to detect lesions in organs where macrophages exhibit strong activity. In these studies, a dosage from 2.5 mg Fe/kg to 16.8 mg Fe/kg has been determined to be safe without side effects or adverse effects in experimental clinical and animal studies.13,22–25 USPIO has a prolonged intravascular half-life varying from 2 to 5 hours in animal studies; thus, imaging of the microvessels is possible in the steady state even with prolonged imaging times without significant leakage of USPIO into the surrounding tissues.13,26 Images were acquired immediately after administration of the contrast agent to take advantage of the highest possible blood pool concentration of the agent.
In this study, the precontrast images inconsistently demonstrated microvessels in the cortex and basal ganglia, which is expected to result from a combination of susceptibility effect from paramagnetic properties of deoxyhemoglobin predominantly in the venous system as well as blood flow.9,11,12 In the postcontrast series, magnetic susceptibility generated by USPIO overwhelmed the effect of deoxyhemoglobin in cerebral vessels as reflected in the visualization of a greater number of microvessels. Larger doses of USPIO were observed to decrease the SNR, thus resulting in some loss of vessel and tissue conspicuity. It is expected that a higher dosage of USPIO will reduce image quality further.7,22 Because there is a difference in density and morphology of microvessels located in the basal ganglia versus cortex, the cortex showed more penetrating microvessels and less decrease in SNR than basal ganglia.
An overall comparison of SNR of brain parenchyma with microvascular conspicuity at different doses indicates that as dosage increased, postcontrast decrease in SNR concealed the enhancement effect in conspicuity of microvessels (Fig. 1). As a result, the 2 mg Fe/kg dosage provides good visualization of microvessels in the areas of interest without significantly sacrificing image quality.
Interpretation of T2*-weighted images is influenced by many factors, which include sequence parameters, the subject’s physiological condition, and the strength of the magnetic field.27 Compared with other approaches that attempt to improve microvascular conspicuity, including Gd-DTPA enhanced MRA, USPIO-aided T2*-weighted GRE imaging at UHF strengths takes advantage of high resolution, susceptibility effect, and high SNR to provide direct evidence of microvasacularity. Indeed, the susceptibility effect enhanced by the use of gradient echo techniques allows for imaging vascular structures containing paramagnetic deoxyhemoglobin which are substantially smaller than the in-plane resolution of the acquired image.9–12,28,29 It is intuitive that the use of superparamagnetic agents would also enhance the capability to image microvascular structures beyond the in-plane resolution of the imaging sequence.
Currently, many different kinds of iron containing MR agents are being investigated.5,26,30,31 Because of the differences in pharmacodynamics and pharmacokinetics, their enhancement patterns in microvasculature imaging at UHF MR system deserve to be clarified.
CONCLUSION
An intravenous USPIO dose of 2 mg Fe/kg immediately before imaging using high-resolution GRE imaging on an 8-T UHF MR system with the parameters described in this study was found to be an optimal dose for the enhanced visualization of microvascularity in the Wistar rat brain. Compared with other doses this dose showed clear conspicuity of microvascular structures without significant loss of SNR in the brain parenchyma.
Acknowledgments
Supported in part by NCI Grant R21CA/NS92846-01A1.
REFERENCES
- 1.Chambon C, Clement O, Le Blanche A, et al. Superparamagnetic iron oxides as positive MR contrast agents: in vitro and in vivo evidence. Magn Reson Imaging. 1993;11:509–519. doi: 10.1016/0730-725x(93)90470-x. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz SA, Albrecht T, Wolf KJ. MR angiography with superparamagnetic iron oxide: feasibility study. Radiology. 1999;213:603–607. doi: 10.1148/radiology.213.2.r99oc24603. [DOI] [PubMed] [Google Scholar]
- 3.Stillman AE. New contrast agents for cardiovascular MRI and MRA. Int J Cardiovasc Imaging. 2001;17:471–472. doi: 10.1023/a:1020166005656. [DOI] [PubMed] [Google Scholar]
- 4.Saeed M, Wendland MF, Higgins CB. Blood pool MR contrast agents for cardiovascular imaging. J Magn Reson Imaging. 2000;12:890–898. doi: 10.1002/1522-2586(200012)12:6<890::aid-jmri12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Schnorr J, Wagner S, Abramjuk C, et al. Comparison of the iron oxide-based blood-pool contrast medium VSOP-C184 with gado-pentetate dimeglumine for first-pass magnetic resonance angiography of the aorta and renal arteries in pigs. Invest Radiol. 2004;39:546–553. doi: 10.1097/01.rli.0000133944.30119.cc. [DOI] [PubMed] [Google Scholar]
- 6.Weissleder R, Elizondo G, Wittenberg J, et al. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175:489–493. doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]
- 7.Allkemper T, Bremer C, Matuszewski L, et al. Contrast-enhanced blood-pool MR angiography with optimized iron oxides: effect of size and dose on vascular contrast enhancement in rabbits. Radiology. 2002;223:432–438. doi: 10.1148/radiol.2232010241. [DOI] [PubMed] [Google Scholar]
- 8.Bjerner T, Johansson L, Wikstrom G, et al. In and ex vivo MR evaluation of acute myocardial ischemia in pigs by determining R1 in steady state after the administration of the intravascular contrast agent NC100150 injection. Invest Radiol. 2004;39:479–486. doi: 10.1097/01.rli.0000128658.63611.b3. [DOI] [PubMed] [Google Scholar]
- 9.Christoforidis GA, Bourekas EC, Baujan M, et al. High resolution MRI of the deep brain vascular anatomy at 8 Tesla: susceptibility-based enhancement of the venous structures. J Comput Assist Tomogr. 1999;23:857–866. doi: 10.1097/00004728-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Abduljalil AM, Schmalbrock P, Novak V, Chakeres DW. Enhanced gray and white matter contrast of phase susceptibility-weighted images in ultra-high-field magnetic resonance imaging. J Magn Reson Imaging. 2003;18:284–290. doi: 10.1002/jmri.10362. [DOI] [PubMed] [Google Scholar]
- 11.Christoforidis GA, Grecula JC, Newton HB, et al. Visualization of microvascularity in glioblastoma multiforme with 8-T high-spatialresolution MR imaging. AJNR Am J Neuroradiol. 2002;23:1553–1556. [PMC free article] [PubMed] [Google Scholar]
- 12.Christoforidis GA, Kangarlu A, Abduljalil AM, et al. Susceptibility-based imaging of glioblastoma microvascularity at 8 T: correlation of MR imaging and postmortem pathology. AJNR Am J Neuroradiol. 2004;25:756–760. [PMC free article] [PubMed] [Google Scholar]
- 13.Dousset V, Gomez C, Petry KG, et al. Dose and scanning delay using USPIO for central nervous system macrophage imaging. Magma. 1999;8:185–189. doi: 10.1007/BF02594597. [DOI] [PubMed] [Google Scholar]
- 14.Kaim AH, Wischer T, O’Reilly T, et al. MR imaging with ultrasmall superparamagnetic iron oxide particles in experimental soft-tissue infections in rats. Radiology. 2002;225:808–814. doi: 10.1148/radiol.2253011485. [DOI] [PubMed] [Google Scholar]
- 15.Weissleder R, Elizondo G, Wittenberg J, et al. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology. 1990;175:494–498. doi: 10.1148/radiology.175.2.2326475. [DOI] [PubMed] [Google Scholar]
- 16.Rausch M, Sauter A, Frohlich J, et al. Dynamic patterns of USPIO enhancement can be observed in macrophages after ischemic brain damage. Magn Reson Med. 2001;46:1018–1022. doi: 10.1002/mrm.1290. [DOI] [PubMed] [Google Scholar]
- 17.Bjerner T, Wikstrom G, Johansson L, et al. High in-plane resolution T2-weighted magnetic resonance imaging of acute myocardial ischemia in pigs using the intravascular contrast agent NC100150 injection. Invest Radiol. 2004;39:470–478. doi: 10.1097/01.rli.0000129153.64225.7f. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 19.Abduljalil AM, Kangarlu A, Zhang X, et al. Acquisition of human multislice MR images at 8 Tesla. J Comput Assist Tomogr. 1999;23:335–340. doi: 10.1097/00004728-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Abduljalil AM, Robitaille PM. Macroscopic susceptibility in ultra high field MRI. J Comput Assist Tomogr. 1999;23:832–841. doi: 10.1097/00004728-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Kangarlu A, Abduljalil AM, Robitaille PM. T1- and T2-weighted imaging at 8 Tesla. J Comput Assist Tomogr. 1999;23:875–878. doi: 10.1097/00004728-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Laghi A, Paolantonio P, Panebianco V, et al. Decrease of signal intensity of myometrium and cervical stroma after ultrasmall superparamagnetic iron oxide (USPIO) particles administration: an MR finding with potential benefits in T staging of uterine neoplasms. Invest Radiol. 2004;39:666–670. doi: 10.1097/00004424-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Mack MG, Balzer JO, Straub R, et al. Superparamagnetic iron oxideenhanced MR imaging of head and neck lymph nodes. Radiology. 2002;222:239–244. doi: 10.1148/radiol.2221010225. [DOI] [PubMed] [Google Scholar]
- 24.Ruehm SG, Corot C, Vogt P, et al. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–422. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 25.Saini S, Edelman RR, Sharma P, et al. Blood-pool MR contrast material for detection and characterization of focal hepatic lesions: initial clinical experience with ultrasmall superparamagnetic iron oxide (AMI-227) AJR Am J Roentgenol. 1995;164:1147–1152. doi: 10.2214/ajr.164.5.7717222. [DOI] [PubMed] [Google Scholar]
- 26.Taupitz M, Schnorr J, Abramjuk C, et al. New generation of monomerstabilized very small superparamagnetic iron oxide particles (VSOP) as contrast medium for MR angiography: preclinical results in rats and rabbits. J Magn Reson Imaging. 2000;12:905–911. doi: 10.1002/1522-2586(200012)12:6<905::aid-jmri14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Haacke EM, Xu Y, Cheng YC, et al. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 28.Dashner RA, Kangarlu A, Clark DL, et al. Limits of 8-Tesla magnetic resonance imaging spatial resolution of the deoxygenated cerebral microvasculature. J Magn Reson Imaging. 2004;19:303–307. doi: 10.1002/jmri.20006. [DOI] [PubMed] [Google Scholar]
- 29.Dashner RA, Chakeres DW, Kangarlu A, et al. MR imaging visualization of the cerebral microvasculature: a comparison of live and postmortem studies at 8 T. Am J Neuroradiol. 2003;24:1738–1739. [PMC free article] [PubMed] [Google Scholar]
- 30.Raynal I, Prigent P, Peyramaure S, et al. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004;39:56–63. doi: 10.1097/01.rli.0000101027.57021.28. [DOI] [PubMed] [Google Scholar]
- 31.Taupitz M, Wagner S, Schnorr J, et al. Phase I clinical evaluation of citrate-coated monocrystalline very small superparamagnetic iron oxide particles as a new contrast medium for magnetic resonance imaging. Invest Radiol. 2004;39:394–405. doi: 10.1097/01.rli.0000129472.45832.b0. [DOI] [PubMed] [Google Scholar]