Abstract
Experiential factors shape the neural circuits underlying social and emotional behavior from the prenatal period to the end of life. These factors include both incidental influences such as early adversity as well as intentional influences that can be produced in humans through specific interventions designed to promote prosocial behavior and well-being. Key extant evidence in animal models and humans is reviewed. While the precise mechanisms of plasticity are still not fully understood, moderate to severe stress appears to increase growth of several sectors of the amygdala while effects in the hippocampus and prefrontal cortex tend to be opposite. Structural and functional changes in the brain have been observed with cognitive therapy and certain forms of meditation and lead to the suggestion that well-being and other prosocial characteristics might be enhanced through training.
I. Introduction
Among the influences on brain structure and function that are most powerful in inducing plastic change are social influences. The vertebrate brain appears to be particularly sensitive to social influences and this sensitivity may be especially acute in primates.1
The brain is constantly being shaped, wittingly and unwittingly, by environmental forces that impinge upon organisms. The circuitry implicated in social and emotional behavior is among those circuits that appear importantly shaped by experience, and early experience in these domains likely plays a key role in governing differences among individuals in their vulnerability or resilience to future adversity. Studies in both animal models and humans provide a foundation for understanding how explicit interventions designed to promote prosocial behavior and well-being might induce plasticity-related changes in the brain. There is growing corpus of evidence that suggests that interventions ranging from regular moderate physical exercise2 to cognitive therapy3,4 and to interventions derived from ancient contemplative practices5 induce plasticity-related alterations in the brain and support a range of positive behavioral outcomes.
There are many different mechanisms of plasticity and at the human level, there are methodological constraints that limit the mechanisms that can be directly studied. Most human work has focused on alterations in different indices of brain structure that can be measured with modern magnetic resonance imaging (MRI). Enduring functional alterations can also be assessed using functional MRI (fMRI) and related techniques.
Experience-dependent influences on particular features of cognitive function such as language learning appear to have a robust sensitive period6. Interestingly, however, even a competence as clearly “cognitive” as language acquisition is importantly influenced by social context and social interaction (see 6 for review). The social deprivation of orphanages for abandoned children in Bucharest, Romania has been found to produce profound cognitive impairment that can be partially remediated by early placement in foster care.7 The earlier the age of foster care placement and removal from the orphanage, the less severe was the observed cognitive deficit. The extent of such sensitive periods in the realms of social and emotional behavior is not yet known. Yet there are some hints: e.g., there is recent evidence in a rodent model that amygdale circuits are kept in an immature state in an infant by the presence of the mother and yet can be stimulated to mature by corticosterone to promote maturation to allow aversive learning.8 Once a developmental event has occurred can it be reversed? Research on recovery of vision in adult amblyopic subjects points toward mechanisms that might be used to remove the “brakes” on adult plasticity, including through the use of behavioral interventions.9 Whether similar mechanisms might be present to facilitate adult plasticity of social behavior has not been studied.
We do know that early stressful and nurturing environments have robust effects on the developing brain, some of which persist for the life of the organism. The effects of stress have been most well characterized and key findings at the animal level will be reviewed in the next section.
The research at the human level that has focused on the experience-dependent effects of stressful life events has taken advantage of largely unintended environmental circumstances such as child maltreatment, or exposure to early stress. In addition to this corpus, there is now a growing literature on the impact of interventions explicitly designed to promote positive outcomes such as physical exercise2, cognitive therapy3,4, social service programs for older individuals10 and meditation5,11. There are also a growing number of interventions designed to promote prosocial behavior in children that include social-emotional learning12, and executive function training13. While the evidence for their efficacy is mostly behavioral at this point in time, the mechanisms through which such interventions operate has not been systematically examined though it is likely that some features of neuroplasticity will be key for at least some of the behavioral effects that have been described.
This article will first review some key findings at the animal level that establish experience-induced structural plasticity in response to social influences. While most of the findings have focused on stressful environmental influences, there are some data on specific environmental influences that appear to promote positive social and emotional behavior. The second half of the article will showcase experience-induced plasticity in humans arising from both unintended influences such as early life stress, and from explicit intervention strategies that are designed to promote more effective coping with stress and salubrious social and emotional behavior. Some of these interventions are derived from ancient contemplative practices while others emerge from the modern research context. One critical upshot of this work is the notion that just as we as a society are learning to take more responsibility for our physical health by engaging in the regular practice of physical exercise, so too can we take more responsibility for our minds and brains by engaging in the regular practice of certain mental exercises that can induce plastic changes in the brain and that potentially have enduring beneficial consequences for social and emotional behavior. It also invites the perspective that qualities such as well-being ought to be viewed, at least in part, as a product of trainable skills and that interventions explicitly designed to promote well-being may have beneficial behavioral and biological effects. While well-being and other similar constructs exhibit moderate stability in the absence of either unwitting or intentional influences, in the presence of such factors the evidence suggests that change can occur.
II. Basic Research at the animal level
Evidence that the healthy mature animal brain is capable of structural plasticity can be traced to the so-called “enriched environment” studies of Bennett and coworkers14 based on the findings of D O Hebb for enhanced problem solving behavior in rats living as pets in a complex environment15. Rats that lived for weeks in an environment filled with toys that were changed daily in a larger and more complex living space showed increased thickness of cerebral cortical areas. This was also true of aging rats16. Subsequent studies showed that cortical neurons increased dendritic branching and complexity in such an environment compared to normal laboratory cages along with increased numbers of glial cells and increased blood supply17.
More recent investigations have shown that both acute and chronic stress alter spine density and dendritic length and branching in brain regions such as hippocampus, prefrontal cortex and amygdala18. Measured by conventional neuroanatomical methods, the time course of these changes were found to occur over days and are largely reversible, at least in young adult animals18,19. Yet, a recent study using transcranial two-photon microscopy to track the formation and elimination of dendritic spines in vivo after treatment with glucocorticoids in developing and adult mice revealed spine turnover within several hours that was higher in the developing barrel cortex but still very much present in the adult, and similar changes occurred in multiple cortical areas, suggesting a generalized effect that may occur in many brain regions20. Mechanisms for such dendritic and synaptic remodeling involve not only glucocorticoids but also excitatory amino acids and other cellular mediators18,21.
Sex hormones also promote structural plasticity in hippocampus, cerebral cortex and hypothalamus and other brain regions22,23. For example, ovarian hormones promote cyclic changes in spine density in the hippocampus as well as in the primary sensory-motor cortex and prefrontal cortex of rodents and monkeys24,25. Mechanisms for these changes involve not only estradiol and progesterone but also excitatory amino acids and other cellular mediators22.
A major breakthrough in brain plasticity came with the rediscovery of neurogenesis in the adult dentate gyrus26 based on pioneering work of Kaplan27 and Altman28 and the studies of songbirds by Nottebohm and colleagues29. Dentate gyrus neurogenesis is stimulated by physical activity and environmental enrichment30 and inhibited by chronic physical and social stressors18. Regular physical activity also increases human hippocampal volume, possibly via stimulating neurogenesis2.
Structural plasticity in the adult brain involving not only neurogenesis but also dendritic and synaptic turnover can be related to social interactions in the visible burrow system for rats31 and in the tree shrew. In the tree shrew, a resident-intruder paradigm shows the powerful effect upon the intruder in terms of reduced neurogenesis and dendritic shrinkage in the hippocampus32,33.
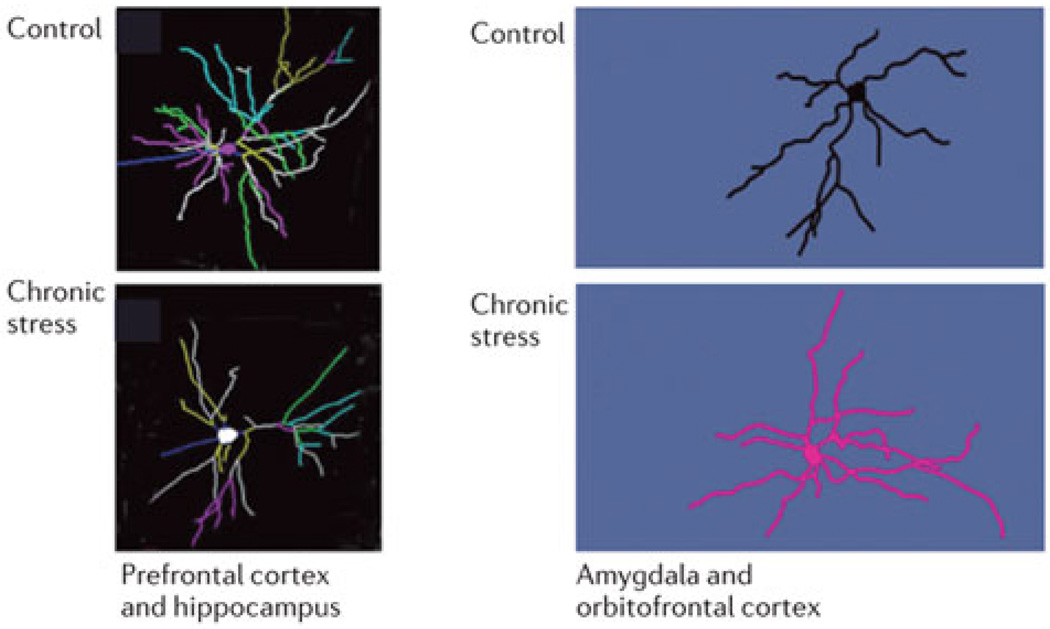
While the hippocampus shows impaired neurogenesis and atrophy of dendritic trees after chronic stress, the same stressor causes dendritic growth in the basolateral amygdala along with increased anxiety (see Fig. 1) and aggression while neurons in the medial prefrontal cortex shrink and those in the orbitofrontal cortex grow18,34,35. These are largely reversible changes at least in young adult animals, although aging compromises reversibility of neuronal atrophy in the medial prefrontal cortex19.
Figure 1.
Chronic stress causes neurons to shrink or grow but not necessarily to die. Representation of the chronic stress effects detected in animal models on growth or retraction of dendrites in the basolateral amygdala and orbitofrontal cortex (growth) and in the CA3 hippocampus, dentate gyrus and medial prefrontal cortex (shrinkage), as described in the text. These effects are largely reversible in young adult animals, although aging appears to compromise resilience and therefore at least in medial prefrontal cortex recovery.21
Stress-induced changes in the circuitry of these brain regions alters the balance between different neural systems that are activated by experiences18,36. For example, low self esteem in humans has been associated with a smaller hippocampus and impulsiveness and poor executive function, with an defective prefrontal cortex; and aggression and anxiety, with an overactive amygdala36.
Early life experiences are potent in this regard37. Thus in both animal models and humans, experiences - good and bad - shape these circuits and their connectivity and experiences can trigger adaptive or maladaptive responses depending on the health and balance of those interconnections. In animals, early life events related to maternal care, as well as parental care in humans, play a powerful role in later mental and physical health, as demonstrated by the adverse childhood experiences (ACE) study38. Prenatal stress impairs hippocampal development in rats, as does stress in adolescence39. Abusive maternal care in rodents and the surprising attachment shown by infant rats to their abusive mothers appears to involve an immature amygdala40, activation of which by glucocorticoids causes aversive conditioning response to emerge. Maternal anxiety in the variable foraging demand model in rhesus monkeys leads to chronic anxiety in the offspring as well as signs of metabolic syndrome41,42.
There is also structural plasticity in the mesolimbic reward system that is affected by social defeat and leads animals to increased drug self-administration. Medium spiny neurons in the nucleus accumbens show altered dendritic spine formation as a result.43 Social defeat, along with maternal separation in infancy, increases the vulnerability to substance self-administration.44 Drugs of abuse alter morphology of many brain regions45 which may or may not drive addictive behavior or reflect compensatory changes.46 Interestingly, there is cross-sensitization of appetitive stimuli in that induction of need-free salt appetite leads to altered dendritic morphology in the shell of the nucleus accumbens and sensitizes the animal to amphetamine self-administration.47
In addition to findings that underscore the deleterious impact of early life stress on later development, there are also some animal findings that suggest protective effects of nurturing environments, as well as resilience-enhancing effects of exposure to mild stress early in life. Starting with the “neonatal handling” studies of Levine and Denenberg48 and the work of Meaney, Syzf and colleagues49, animal models have contributed enormously to our understanding of how the brain and body are affected, Epigenetic, transgenerational effects transmitted by maternal care are central to these findings. Besides the amount of maternal care, the consistency over time of that care and the exposure to novelty are also very important not only in rodents50,51 but also in monkey models52. In a recent study van Hasselt and colleagues53 demonstrated that the rat pups who received high levels of licking and grooming during the first week ofpost natal life showed as young adults, higher levels of glucocorticoid mRNA expression in the hippocampus and enhanced induction of synaptic plasticity in the dentate gyrus in vitro.
In a series of studies in squirrel monkeys, Parker and her colleagues have observed beneficial effects of early exposure to mild stress. After exposure to mild stress from postnatal weeks 17 to 27, as young adults the mildly stressed animals displayed decreased anxiety as measured by decreased maternal clinging, enhanced exploratory behavior and increased food consumption. Moreover, animals exposed to early mild stress had lower basal plasma ACTH and cortisol and lower cortisol following stress exposure.54 In a follow-up study, this group also showed that animals exposed to early mild stress exhibited enhanced prefrontally-dependent response inhibition as young adults suggesting that the early exposure to mild stress enhances prefrontal regulatory mechanisms that facilitate stress inoculation.55 In this same squirrel monkey model, Lyons and his colleagues have demonstrated that mild stress exposure early in life results in increases ventromedial prefrontal cortex (vmPFC) volumes during the peripubertal period.56 The increased vmPFC volume reflects surface area expansion of this PFC zone rather than an increase in cortical thickness. Moreover, these same investigators found increased white matter myelination in this region detected with diffusion tensor imaging.56
One of the longest held notions of brain plasticity is that certain critical periods or windows exist in development during which circuitry is laid down that lasts for the lifetime. Yet, a more recent set of findings suggests that developmentally-induced plasticity, at least of certain kinds, can be reversed by re-opening those windows. For example, ocular dominance imbalance from early monocular deprivation can be reversed by patterned light exposure in adulthood that can be facilitated by fluoxetine, on the one hand57 and food restriction, on the other hand58, in which reducing inhibitory neuronal activity appears to play a key role59. Investigations of underlying mechanisms for the re-establishment of a new window of plasticity are focusing on the balance between excitatory and inhibitory transmission and removing molecules that put the “brakes” on such plasticity9.
Depression is more prevalent in individuals who have had adverse early life experiences38. Neurotrophic factors such as BDNF may be a key feature of the depressive state and elevation of such factors by diverse treatments ranging from antidepressant drugs, such as fluoxetine, to regular physical activity may be a key feature of treatment60. Yet, there are other potential applications, such as the recently reported ability of fluoxetine to enhance recovery from stroke61. However, a key aspect of this new view62 is that the drug is opening a “window of opportunity” that may be capitalized by a positive behavioral intervention, e.g., behavioral therapy in the case of depression or the intensive physiotherapy to promote neuroplasticity to counteract the effects of a stroke.
III. Plasticity in human social brain
A. Experience-induced effects of adversity and stress
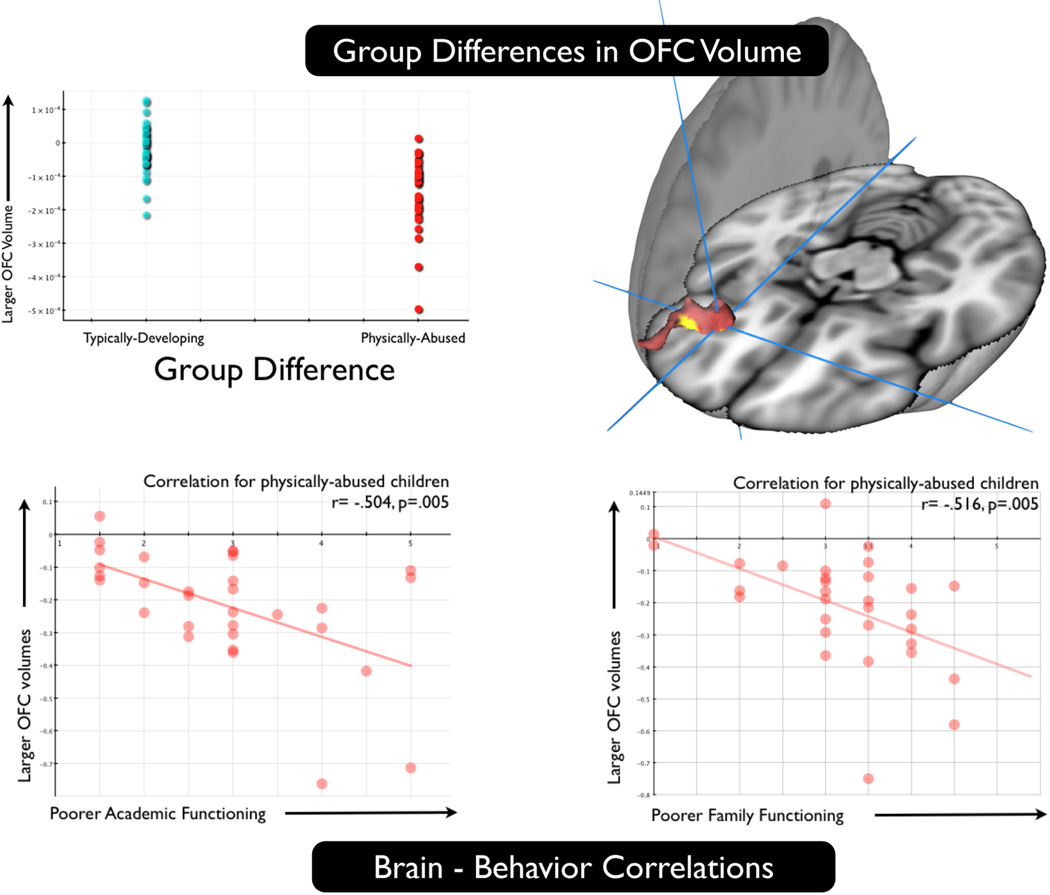
The social and emotional circuitry of the brain is continuously being shaped by forces that impinge upon the nervous system during prenatal development and continuing throughout life. The fact that experience-induced plasticity has been documented in the social brain in a variety of animal models provides the foundation for examining similar effects in humans. There is now a substantial body of evidence on the impact of stressful environments on the developing human brain and associated behavior.62–67 For example, in a sample of 31 physically abused and 41 typically developing teenage children who underwent structural MRI scanning using diffeomorphic image normalization and tensor-based morphometry, Pollak, Davidson and their colleagues found that the abused children had smaller orbitofrontal (OFC) volumes and furthermore, the smaller the OFC volume in the abused sample, the more severe the social stress as reported by children and parents on a structured interview (see Fig. 2).68
Figure 2.
Physically abused children show alterations in orbitofrontal volume compared with typically developing children and volume shrinkage in this region is related to measures of family stress. Top: Physically abused children show reductions in orbitofrontal cortex compared with typically developing controls; Bottom: Among physically abused children, those showing poorer academic function and poorer family functioning (greater family stress) exhibit less volume in orbitofrontal cortex.68 Note that because the voxel-wise analysis was a between groups comparison and the correlational analysis was conducted with the abused children only, this does not suffer from the “double-dipping” problem.
Early life stress modulates the hypothalamic pituitary adrenal axis, especially cortisol as an output measure of this system, though the effects on this system are complex and depend upon the chronicity and timing of the stress.69 Evidence that child abuse is associated with alterations in the epigenetic regulation of the glucocorticoid receptor was obtained in a study of postmortem tissue extracted from the hippocampus of suicide victims with a history of child abuse and those with no abuse history along with controls.70 In hippocampus, McGowan et al. reported decreased levels of glucocorticoid receptor mRNA, as well as mRNA transcripts bearing the glucocorticoid receptor 1F splice variant and increased cytosine methylation of an NR3C1 promoter.70
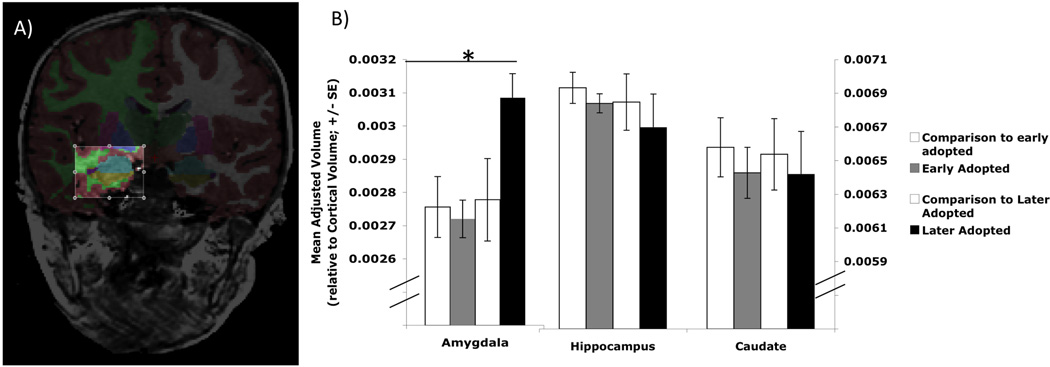
Capitalizing on unfortunate circumstances, Tottenham and colleagues studied 38 post-institutionalized (PI) children who were raised in impoverished orphanages in either Eastern Europe or Asia and 40 non-institutionalized children.71 At the time of testing children were 8.5–9.5 years and were institutionalized on average at age 2.5 months. Using an automated segmentation algorithm, the authors specifically looked at volumetric measures of the amygdala, hippocampus and caudate. When the PI sample was compared with controls, no overall differences between groups were found for any of the three structures examined. However, they also divided the PI sample into those who were adopted early vs. adopted late (<15 months vs. >15 months at age of adoption respectively). When participants were divided in this way, the later adopted PI children were found to have significantly large amygdale compared with the early adopted and control counterparts. There were no significant differences among any of the groups in the volumes of the hippocampus or caudate (see Fig. 3). When examined continuously, the authors found that age at adoption was positively correlated with amygdala volume such that those adopted at a later age had larger amygdala volumes. Higher parental ratings of internalizing behavior and anxiety were also correlated with larger amygdala volume. A similar pattern of results was obtained from a sample of 10 year old children, some of whom were continuously exposed to maternal depressive symptoms since birth and others had no exposure to maternal depressive symptoms.72 At age 10 years, children who had been continuously exposed to maternal depressive symptoms since birth had significantly larger left and right amygdalae compared with children having no such exposure. There were no significant differences in hippocampal volume between these groups. The mean depression score of the mother computed over 7 years predicted amygdala volume of her child at age 10 such mothers with higher levels of depressive symptoms had children with larger amygdala volume.
Figure 3.
Anatomically segmented amygdala volumes are larger in later-adopted post-institutionalized children. a. Anatomical segmentation of the amygdala; b. later-adopted post-institutionalized children show larger amygdala volume compared with both early adopted children and with typically developing controls. No differences among groups were found in hippocampus or caudate.71 Asterisk indicates that the later adopted group exhibits significantly larger amygdala volume compared with each of the comparison groups.
These findings are consistent with the idea that early life stress induces structural changes in the developing brain. The two most prominent structural findings from the human literature suggest that amygdala volume is increased while sectors of the prefrontal cortex are decreased. Some caution regarding the findings with the amygdala are warranted because of methodological complications with automated segmentation algorithms with subcortical structures such as the amygdala.73 Moreover, the precise ages at which these effects occur needs to be carefully studied since particularly for the amygdala, early hypertrophy and enlargement may occur in response to adversity, but then, perhaps in part due to excitotoxic processes, premature volume reduction may be produced74. Such a developmental pattern in the amygdala has been suggested to occur in the autistic brain.75,76 The amygdala and prefrontal cortex and their interconnections have been strongly implicated in emotion regulation77–79 and well-being 80 and dysfunctions and/or structural abnormalities in their interconnections have been implicated in psychopathology81–83
B. Prosocial interventions and training
A key question replete with both theoretical and practical significance is whether explicit interventions or training designed to foster prosocial behavior and well-being, or more naturally-occurring forms of positive social interaction and social support, can induce neuroplastic changes in the brain. In a study examining the impact of holding the hand of one’s spouse, Coan, Schaefer, and Davidson and his colleagues found a significant attenuation of the neural response to the threat of shock in several threat-sensitive brain regions including the anterior insula and ventral anterior cingulate cortex, in women when they were holding their spouse’s hand compared with controls that included holding a stranger’s hand, and an alone condition.84 Since this and other similar studies examine the impact of an acute manipulation, the effects are likely to be phasic and short-lived but they raise the question of whether cumulative exposure to social support would induce beneficial plastic changes.85 Other forms of social support, such as maternal care, appear to modulate the impact of prenatal risk on hippocampal volume, at least in women.86
There is a growing literature documenting functional and structural changes in the brain with specific interventions and training regimes. The behavioral evidence in support of such interventions and training provides a reasonable foundation for the exploration of neural changes that support these behavioral outcomes. For example, interventions designed to promote prosocial behavior such as effective emotion regulation have been developed for incorporation in school curricula to support the development of more positive social and emotional trajectories in K-12 school children. In a recent meta-analysis of 213 programs involving more than 270,000 school children, Durlak and colleagues reported that compared with controls, participants in social emotional learning programs demonstrated significant gains in social and emotional skills and they performed on average 11 percent better on standardized measures of academic achievement.12 Other evidence suggests the efficacy of cognitive therapy for depression4 as well as well-being therapy87 to specifically help to improve positive affect.
In an important review, DeRubeis and colleagues present evidence consistent with the view that cognitive therapy enhances prefrontal function and via this enhanced prefrontal activation, amygdala activation is inhibited.88 de Lange and his colleagues examined the impact of cognitive therapy for patients with chronic fatigue syndrome in a short-term longitudinal study. At baseline these patients showed decreased gray matter volume compared with healthy controls. Patients then underwent 16 one-hour sessions of cognitive therapy and were rescanned following treatment. Increases in lateral prefrontal volume were found in the patients following treatment that were correlated with improvements in digit symbol substitution and in a choice reaction time task.89 Unfortunately changes in mood or social behavior were not reported in this study.
The impact of secular training derived from meditation traditions that emphasize the cultivation of positive affect such as compassion and kindness has received increased empirical attention. A recent review concludes that such exercises, which are oriented toward enhancing the positive emotions compassion and kindness, do indeed increase positive affect and decrease negative affect.90 And Singer and her colleagues have recently found that one day of compassion meditation training increases prosocial behavior in a novel virtual game compared with a one-day memory training control condition.88 Collectively these findings raise the possibility that such interventions and training programs designed to explicitly decrease stress and enhance certain forms of positive emotion may produce specific plasticity-related alterations in brain function and structure.
Davidson and his colleagues have studied functional brain alterations with compassion meditation in expert practitioners who have been meditating for more than 10,000 hours over the course of their lifetime, compared with novices who were just learning to meditate. During a mental practice explicitly designed to enhance compassion, Lutz et al. reported that the practitioners showed enhanced gamma oscillations and gamma synchrony compared with controls91 and enhanced BOLD signal detected with functional MRI in response to emotional sounds in brain regions including the insula and temporoparietal junction that have been implicated in previous studies of empathy.92 The increase in gamma oscillations and gamma synchrony might reflect its role in synaptic plasticity93 and suggest a general enhancement of synaptic plasticity through this form of mental practice.
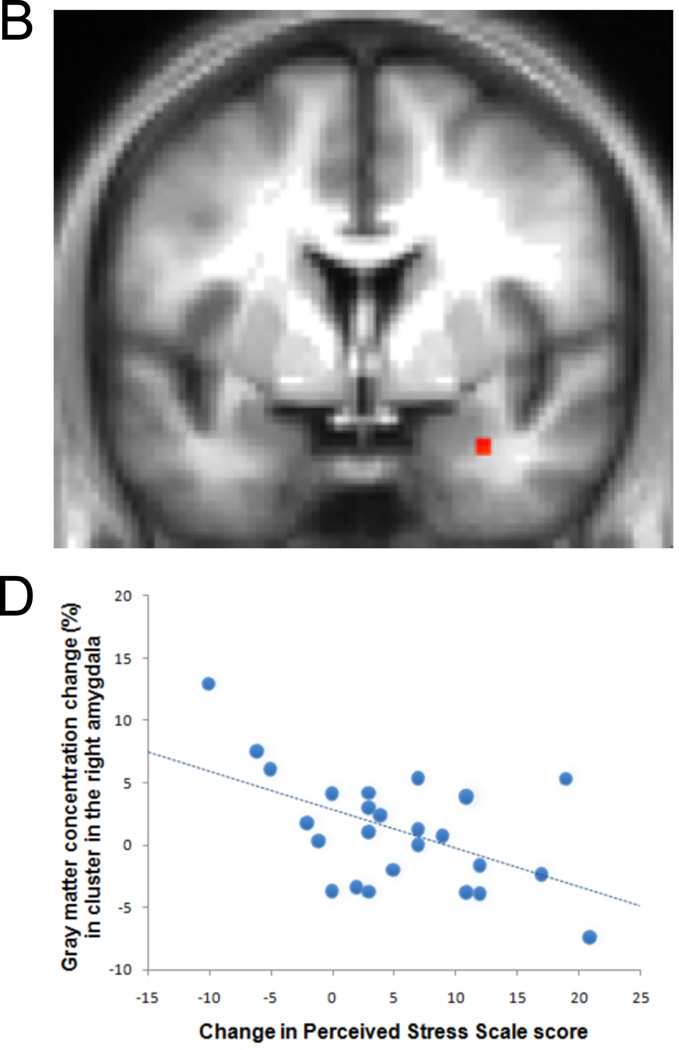
Other research suggests that mindfulness meditation may operate via a distinct neural mode of self-referencing such that favors momentary non-judgmental present-moment experience over narrative self-focused mentation. This form of mental training has been found to decrease anxiety and increase positive affect.94 Farb and his colleagues tested this idea by comparing novices and participants who attended an 8-week course in mindfulness meditation (Mindfulness-based Stress Reduction; MBSR). Functional MRI was measured in response to a task that contrasted an “experiential focus” to a narrative self-focused condition in response to trait adjectives. The MBSR participants exhibited reductions in medial prefrontal activation and increased activation of the insula and lateral prefrontal cortices during the experiential vs. narrative conditions.95 Consistent findings using a different methodological strategy were obtained in a recent study comparing experienced mindfulness meditation practitioners to novices. The experienced practitioners showed decreased medial prefrontal activity in the baseline default BOLD signal compared with the novices.96 Other findings indicate that activation of the medial prefrontal cortex at baseline is associated with mind-wandering97 and Killingworth and Gilbert98 reported that mind wandering is associated with unhappiness. A major limitation of all of the studies described above on the impact of meditation is that they relied upon between group comparisons of a meditation group compared with a control group. To more firmly establish that differences are due to meditation training per se and not to self-selection and other factors that might confound between group comparisons, longitudinal investigations of changes over the course of meditation training are needed. Such a design was used to examine whether certain forms of meditation may operate via effects that are opposite to those produced by stress. As we noted in the section above, early life stress increases amygdala volume. In a longitudinal study of 26 participants undergoing an eight-week training in MBSR, MRI scans were obtained before and after the eight weeks of training. Reductions in perceived stress following MBSR were correlated with reductions in gray matter volume in the right basolateral amygdala that were obtained from MRI scans performed before and after the eight weeks of training (see Fig. 4).11 These findings suggest that plasticity-related alterations in brain regions implicated in stress can occur with as little as eight weeks of mindfulness meditation training.
Figure 4.
Change in gray matter volume in the right basolateral amygdala from pre to post eight weeks of mindfulness-based stress reduction (MBSR) was associated with decreases in perceived stress over this same time period. Individuals undergoing MBSR who showed the largest decreases in perceived stress also showed the largest decreases in basolateral amygdala gray matter volume.11
Summary, conclusions and implications
It has been known for more than a century that social and emotional behavior is importantly modified by experience. Abundant evidence exists demonstrating that stress and adversity, particularly early in life, can produce enduring alterations in behavior. And it has also been claimed for thousands of years that specific forms of mental training can produce robust beneficial and enduring effects on behavior. The rigorous investigation of such effects and the neural mechanisms responsible for producing them has only recently become a serious focus of neuroscientific study. The findings we review underscore the structural plasticity of emotional circuitry in response to both acute and chronic stress, particularly alterations of spine density, and dendritic length and branching in hippocampus, amygdala and prefrontal cortex. Evidence at the animal level has identified several different mechanisms of plasticity including dendritic and synaptic turnover and neurogenesis. The animal and human evidence is consistent in demonstrating that many forms of stress promote excessive growth in sectors of the amygdala while effects in hippocampus tend to be opposite. Whether critical or sensitive periods exist for plasticity in response to social influences has not been thoroughly addressed and more systematic developmental studies are required. Moreover, the reversibility of structural changes following alterations in social and emotional conditions has not been systematically examined.
At the human level, research is beginning to document the impact of explicit interventions designed to decrease stress and promote prosocial behavior and well-being on brain structure and function. These studies are consistent with the basic research in demonstrating increases in specific sectors of prefrontal activation and decreases in amygdala activation. These functional alterations are accompanied by structural changes that show increases in prefrontal and decreases in amygdala volume. The precise differences among the various interventions that have been developed for this general purpose have not been systematically studied, nor has the relation between functional and structural changes been carefully documented. Moreover, it is apparent that both structural and functional connectivity between prefrontal regions and subcortical structures is extremely important for emotion regulation and these connections represent important targets for plasticity-induced changes. This is likely to be an important focus of future studies.
Finally, the studies on interventions explicitly designed to promote positive emotional qualities such as kindness and mindfulness implies that such qualities might best be regarded as the product of skills that can be enhanced through training, just as practice will improve musical performance and produce correlated regionally-specific anatomical changes. Whether these interventions simply modulate the adverse effects of stress, or whether they result in a profile of neurobehavioral functioning that is “better than normal” will require considerably more evidence, though the available evidence points toward this latter possibility. This perspective can lead to the view that social and emotional characteristics can be educated in ways that are not dissimilar from certain forms of cognitive learning. Many forms of meditation and cognitive therapy can enhance self-control or self-regulation.99 Such improvements in self-control are particularly apparent in social and interpersonal contexts. It is in these contexts that attentionally demanding stimuli typically occur and where self-regulation is especially important. In a recent study of a cohort of 1000 participants assessed from birth to age 32 years, Moffitt and her colleagues found that childhood measures of self-control predicted physical health, substance dependence, personal finances and criminal offending outcomes at age 32 years.100 Moffitt and colleagues defined self-control as a family of processes that include delay of gratification, impulse and attentional control, executive function and will power. They suggest that early interventions that enhance self-control might reduce a panoply of societal costs, save tax-payers money and promote prosperity. The mental training at the core of the techniques described above might constitute ideal interventions to promote early self-control and improve later adult prosocial outcomes. For example, mindfulness meditation has been found to strengthen selective and other aspects of attention and executive function.5 Whether such interventions can produce changes that have lasting consequences is a possibility that requires extensive empirical investigation.
Acknowledgments
RJD’s contribution was supported by grants from the National Institute of Mental Health (R01-MH43454 and P50-MH084051), the National Center for Complementary and Alternative Medicine (P01-AT004952), the Fetzer Institute, the John Templeton Foundation and gifts from Bryant Wangard and Ralph Robinson, Ann Down, Keith and Arlene Bronstein and the John W. Kluge Foundation. BSM’s contribution was supported by National Institutes of Health (NIH) grants R01 MH41256 and 5P01 MH58911.
Contributor Information
Richard J. Davidson, Waisman Laboratory for Brain Imaging and Behavior and Center for Investigating Healthy Minds, University of Wisconsin-Madison, 1500 Highland Avenue, Madison, WI 53705, rjdavids@wisc.edu
Bruce S. McEwen, Harold and Margaret Milliken Hatch Laboratory of Neuroendocrinology, The Rockefeller University, New York, New York 10065, mcewen@mail.rockefeller.edu
References
- 1.Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65:752–767. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disner SG, Beevers CG, Haigh EP, Beck AT. Neural mechanisms of the cognitive model of depression. Nature reviews. Neuroscience. 2011;12 doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 4.Clark D, Beck AT. Cognitive theory and therapy of anxiety and depression: convergence with neurobiological findings. Trends in cognitive sciences. 2010;14:418–424. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Lutz A, Slagter H, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in cognitive sciences. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67:713–727. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson C, et al. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science (New York NY) 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neuroscience and biobehavioral reviews. 2010;34:835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. The Journal of neuroscience. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson MC, et al. Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64:1275–1282. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hölzel BK, et al. Stress reduction correlates with structural changes in the amygdala. Social cognitive and affective neuroscience. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durlak J, Weissberg RP, Dymnicki AB, Taylor RD, Schellinger KB. The impact of enhancing students’ social and emotional learning: a meta-analysis of school-based universal interventions. Child development. 2011;82:405–432. doi: 10.1111/j.1467-8624.2010.01564.x. [DOI] [PubMed] [Google Scholar]
- 13.Diamond, Lee K. Interventions Shown to Aid Executive Function Development in Children 4 to 12 Years Old. Science. 2011;333:959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity of the brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- 15.Hebb DO. The organization of behavior a neuropsychological theory. New York, NY: Wiley; 1949. [Google Scholar]
- 16.Diamond MC. The Aging Brain: Some Enlightening and Optimistic Results. American Scientist. 1978;66:66–71. [PubMed] [Google Scholar]
- 17.Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biology. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 19.Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. The Journal of Neuroscience. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liston C, Gan W-B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature reviews. Neuroscience. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Research Reviews. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine Reviews. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 24.Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Annals of the New York Academy of Sciences. 2010;1204:104–112. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J-R, et al. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cerebral Cortex. 2009;19:2719–2727. doi: 10.1093/cercor/bhp048. [DOI] [PubMed] [Google Scholar]
- 26.Cameron HA, Gould E. The Control of Neuronal Birth and Survival. Receptor Dynamics in Neural Development. 1996:141–157. [Google Scholar]
- 27.Kaplan MS. Environment complexity stimulates visual cortex neurogenesis: Death of a dogma and a research career. Trends in Neurosciences. 2001;24:617–620. doi: 10.1016/s0166-2236(00)01967-6. [DOI] [PubMed] [Google Scholar]
- 28.Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. The Journal of Comparative Neurology. 1990;301:325–342. doi: 10.1002/cne.903010302. [DOI] [PubMed] [Google Scholar]
- 29.Nottebohm F. From bird song to neurogenesis. Scientific American. 1989;260:74–79. doi: 10.1038/scientificamerican0289-74. [DOI] [PubMed] [Google Scholar]
- 30.Brown J, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. European Journal of Neuroscience. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 31.Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. The Journal of Neuroscience. 2004;24:6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. The Journal of Neuroscience. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. The Journal of Neuroscience. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. The Journal of Neuroscience. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual review of medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 38.Anda RF, Butchart A, Felitti VJ, Brown DW. Building a framework for global surveillance of the public health implications of adverse childhood experiences. American Journal of Preventive Medicine. 2010;39:93–98. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- 40.Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman D, et al. Early appearance of the metabolic syndrome in socially reared bonnet macaques. The Journal of Clinical Endocrinology and Metabolism. 2005;90:404–408. doi: 10.1210/jc.2004-0452. [DOI] [PubMed] [Google Scholar]
- 42.Coplan JD, et al. Variable foraging demand rearing: Sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biological Psychiatry. 2001;50:200–204. doi: 10.1016/s0006-3223(01)01175-1. [DOI] [PubMed] [Google Scholar]
- 43.Christoffel DJ, et al. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miczek K, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacology & therapeutics. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends in neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roitman MF, Na E, Anderson G, Jones T, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:RC225. doi: 10.1523/JNEUROSCI.22-11-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine S, Haltmeyer GC, Karas GG, Denenberg VH. Physiological and behavioral effects of infantile stimulation. Physiology & Behavior. 1967;2:55–59. [Google Scholar]
- 49.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akers KG, et al. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PloS one. 2008;3:e2840. doi: 10.1371/journal.pone.0002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neuroscience and Biobehavioral Reviews. 2011;35:1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Hasselt FN, et al. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus. 2011 doi: 10.1002/hipo.20892. [DOI] [PubMed] [Google Scholar]
- 54.Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Archives of General Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- 55.Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biological Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 56.Katz M, et al. Prefrontal plasticity and stress inoculation-induced resilience. Developmental Neuroscience. 2009;31:293–299. doi: 10.1159/000216540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vetencourt JFM, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 58.Spolidoro M, et al. Food restriction enhances visual cortex plasticity in adulthood. Nature communications. 2011;2:320. doi: 10.1038/ncomms1323. [DOI] [PubMed] [Google Scholar]
- 59.Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Chollet F, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet neurology. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 62.Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Developmental neurobiology. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 63.Shonkoff JP, Garner AS. The Lifelong Effects of Early Childhood Adversity and Toxic Stress. Pediatrics. 2011 doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 64.Shonkoff JP. Protecting brains, not simply stimulating minds. Science (New York, N.Y.) 2011;333:982–983. doi: 10.1126/science.1206014. [DOI] [PubMed] [Google Scholar]
- 65.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Developmental psychobiology. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 66.Gould F, et al. The effects of child abuse and neglect on cognitive functioning in adulthood. Journal of psychiatric research. 2012:1–7. doi: 10.1016/j.jpsychires.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanson JL, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. The Journal of neuroscience. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience and biobehavioral reviews. 2010;34:867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tottenham N, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lupien SJ, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America. 2011;i:1–6. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morey R, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nacewicz BM, et al. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Archives of general psychiatry. 2006;63:1417–1428. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mosconi MW, et al. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Archives of general psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 78.Wager TD, Davidson ML, Hughes BL, Lindquist M, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Urry HL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davidson RJ. Well-being and affective style: neural substrates and biobehavioural correlates. Philosophical Transactions of the Royal Society of London. 2004;359:1395–1411. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the Neural Circuitry of Emotion Regulation — A Possible Prelude to Violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 82.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coan JA, Schaefer HS, Davidson RJ. Lending a hand: social regulation of the neural response to threat. Psychological science. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 85.Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychological bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- 86.Buss C, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fava Ga, Rafanelli C, Cazzaro M, Conti S, Grandi S. Well-being therapy. A novel psychotherapeutic approach for residual symptoms of affective disorders. Psychological medicine. 1998;28:475–480. doi: 10.1017/s0033291797006363. [DOI] [PubMed] [Google Scholar]
- 88.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nature reviews. Neuroscience. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Lange FP, et al. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain: a journal of neurology. 2008;131:2172–2180. doi: 10.1093/brain/awn140. [DOI] [PubMed] [Google Scholar]
- 90.Hofmann SG, Grossman P, Hinton DE. Loving-kindness and compassion meditation: Potential for psychological interventions. Clinical psychology review. 2011;31:1126–1132. doi: 10.1016/j.cpr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditateors self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16369. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PloS one. 2008;3:e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends in cognitive sciences. 2010;14:72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Kemeny ME, et al. Contemplative/emotion training reduces negative emotional behavior and promotes prosocial responses. Emotion (Washington D.C.) 2011 doi: 10.1037/a0026118. [DOI] [PubMed] [Google Scholar]
- 95.Farb NS, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social cognitive and affective neuroscience. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brewer J, et al. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1–6. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 99.Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clinical Psychology Review. 2009;29:560–572. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Moffitt TE, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]