The Food and Drug Adminstration recently approved a diphtheria-conjugated pneumococcal polysaccharide vaccine for adults, although its long-term immunogenicity is unknown. We report that, in patients with moderate to severe chronic obstructive pulmonary disease, conjugate vaccination elicits a superior immune response to free-polysaccharide vaccine that persists for >2 years.
Abstract
Background. Although the 23-valent pneumococcal polysaccharide vaccine (PPSV23) protects against invasive disease in young healthy persons, randomized controlled trials in chronic obstructive pulmonary disease (COPD) have demonstrated no benefit in the intention-to-treat population. We previously reported that the 7-valent diphtheria-conjugated pneumococcal polysaccharide vaccine (PCV7) is safe and induced greater serotype-specific immunoglobulin G (IgG) and functional antibody than did PPSV23 1 month after vaccination. We hypothesized that these advantages would persist at 1 and 2 years.
Methods. One hundred eighty-one patients with moderate to severe COPD were randomized to receive PPSV23 (n = 90) or PCV7 (1.0 mL; n = 91). We measured IgG by enzyme-linked immunosorbent assay and assessed functional antibody activity by a standardized opsonophagocytosis assay, reported as a killing index (OPK). We determined differences in IgG and OPK between vaccine groups at 1 and 2 years.
Results. Relative to PPSV23, PCV7 induced greater OPK at both 1 and 2 years for 6 of 7 serotypes (not 19F). This response was statistically greater for 5 of 7 serotypes at 1 year and 4 of 7 at 2 years. Comparable differences in IgG were observed but were less often statistically significant. Despite meeting Centers for Disease Control and Prevention criteria for PPSV23 administration, almost 50% of individuals had never been vaccinated. No differences in the frequency of acute exacerbations, pneumonia, or hospitalization were observed.
Conclusions. PCV7 induces a greater functional antibody response than PPSV23 in patients with COPD that persists for 2 years after vaccination. This superior functional response supports testing of conjugate vaccination in studies examining clinical end points.
Clinical Trials Registration: NCT00457977.
Streptococcus pneumoniae is a major cause of pneumonia, hospitalization, and mortality and disproportionately affects those with comorbid illnesses, such as chronic obstructive pulmonary disease (COPD) [1]. Until recently, the only pneumococcal vaccine approved for use in adults in the United States and Europe was the 23-valent pneumococcal polysaccharide vaccine (PPSV23) [2]. Although PPSV23 provides partial protection against invasive pneumococcal disease in young healthy patients, it appears to have limited impact on this risk among older patients and those with underlying medical problems [3]. In addition, the benefit of PPSV23 on the incidence of nonbacteremic pneumonia has not been clearly demonstrated [2, 4], and no randomized, controlled trial of the vaccine in patients with COPD has shown a reduction in clinical outcomes in the intention-to-treat population [5–9]. Although observational studies suggest that PPSV23 offers some protection against pneumococcal infection in patients with lung disease [10], it is well accepted that more effective vaccines are needed [2, 11].
Pneumococcal protein conjugate vaccines link the polysaccharide antigen to a nontoxic protein carrier, thereby increasing its immunogenicity [11]. Conjugate vaccines were developed for young children who mount inadequate antibody responses to unconjugated polysaccharide antigens. The 7-valent diphtheria-protein conjugated pneumococcal vaccine (PCV7) induces a potent immune response in children, and randomized trials revealed reductions in otitis and invasive disease in this population [12]. Since the introduction of PCV7 in the United States in 2000, rates of invasive pneumococcal disease have decreased markedly [13]. In 2011, the US Food and Drug Administration (FDA) approved an extended serotype 13-valent pneumococcal protein conjugate vaccine (PCV13) for use in children [2] and more recently matched the European Commission by extending this approval to adults aged >50 years on the basis of studies suggesting that the immune response to conjugate vaccines may be superior to the response to PPSV23 [11, 14, 15].
In the first 120 patients with COPD who were recruited to this trial, we previously reported that PCV7 (at 1.0 mL, twice the pediatric dose of 0.5 mL) is as safe as PPSV23 and induces a superior immune response 1 month after vaccination, as assessed by serotype-specific IgG and by functional antibody opsonophagocytosis activity (OPK) [16]. Although the short-term superiority of pneumococcal conjugate vaccines has not been a consistent finding in studies in adults [11], our data suggest that there may be advantages in patients with COPD.
Definitive data about the relative efficacy of pneumococcal vaccines would come from randomized trials using clinical end points. However, such studies are difficult to perform because of problems with the accurate detection of pneumococcal infection and ethical issues with the inclusion of a placebo control arm, together necessitating very large sample sizes and major expense [10]. Serotype-specific IgG and OPK serve as the best available surrogate markers of vaccine effectiveness in adults [17, 18]. There are limited data about the long-term immunogenicity of PPSV23 and PCV7 in healthy adults [14, 15], and none are available on patients with COPD. Here, we report the 1-year and 2-year comparative immunogenicity data for the entire study population of patients with moderate to severe COPD (n = 181) who were randomized to receive PCV7 (1.0 mL) or PPSV23.
Methods
Additional Detail Is Available in the Online Supplement
Study Design, Randomization, and Masking
We performed a randomized, open-label trial comparing the safety and immunogenicity of PCV7 (1.0 mL) with those of PPSV23 in 181 patients with COPD. The study was conducted by the 10 centers participating in the National Heart, Lung, and Blood Institute's COPD Clinical Research Network (CCRN). The study was approved by the participating center's Institutional Review Boards and the FDA under an Investigational New Drug approval. The study was registered online (NCT00457977) and completed in May 2011.
Study Population
Detailed inclusion and exclusion criteria are published elsewhere [16]. Participants were men and women >40 years of age with ≥10 pack-year cigarette smoking and a clinical diagnosis of moderate to very severe COPD (as defined by post-bronchodilator–forced expiratory volume [FEV1]/forced vital capacity [FVC] <70% and FEV1<70% predicted). Persons were eligible if they had never received PPSV23 or if it was administered >5 years before randomization. Whenever possible, the receipt and the date of prior PPSV23 administration were confirmed with medical records. Exclusion criteria included a diagnosis of asthma, use of immunosuppressive medications other than systemic and inhaled corticosteroids, presence of conditions known to impair pneumococcal vaccine response, and any illness within the month prior to enrollment that required antibiotics and/or systemic steroids. Participant enrollment and disposition are shown in Figure 1.
Figure 1.
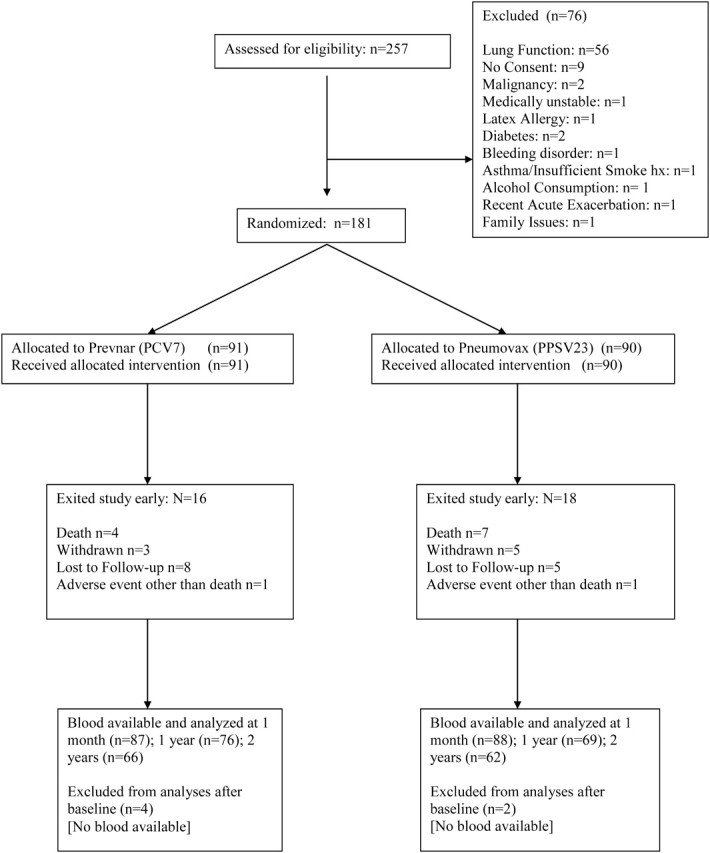
CONSORT diagram of participant recruitment and disposition. Abbreviations: PCV7, 7-valent diphtheria-60 protein conjugated pneumococcal vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Vaccines and Administration
Each 1.0 mL dose of PCV7 contained 4 μg of the capsular polysaccharide from serotypes 4, 9V, 14, 18C, 19F, and 23F and 8 μg of serotype 6B covalently linked to a total of 40 μg of CRM197, a nontoxic diphtheria protein. The 1.0-mL dose of PCV7 also contained 0.250 mg of aluminum phosphate. PPSV23 was injected in the approved volume of 0.5 mL and contained 25 μg of the capsular polysaccharide from each of the 23 included serotypes and 0.25% phenol. Vaccinations were given as a single intramuscular deltoid injection with a 1-inch needle.
Serologic Testing
Blood specimens were obtained immediately before and 1 month, 1 year, and 2 years after vaccination and were shipped to the University of Alabama at Birmingham for serologic testing. The capacity of each serum sample to opsonize S. pneumoniae for ingestion and killing by phagocytes was determined by incubating bacteria in serum and exposing them in vitro to HL-60 cells and complement [19]. Results are reported as OPK, representing the reciprocal of the interpolated serum dilution that led to 50% uptake and killing of pneumococci. Total IgG concentrations to the 7 PCV7 serotypes were also measured using a World Health Organization–recommended enzyme-linked immunosorbent assay protocol (www.vaccine.uab.edu).
Statistics
IgG and OPK were transformed using natural logarithms to account for their skewed distributions and reported as geometric means. Unpaired t tests were used for between-group comparisons of baseline and postvaccination IgG and OPK. Data describing PCV7 and PPSV23 responses in healthy older patients were used to determine a sample size of 180 participants, providing >90% power to detect a statistically significant difference in OPK at 1 year between the 2 study groups for each serotype (primary end point) [20]. The proportions of participants reporting pneumonia, acute exacerbation, or hospitalization or who died during follow-up were compared using Fisher's exact test. Time to first exacerbation was also compared between the groups with use of a Cox proportional hazard model. P values <.05 were considered to be statistically significant. No adjustments were made for multiple comparisons.
RESULTS
From 9 April 2007 through 7 May 2009, we randomized 181 participants. An additional 76 participants signed informed consent and were screened but were excluded before vaccine administration, primarily because they did not meet spirometric criteria (Figure 1). Baseline characteristics of the entire study population were similar to those of the first 120 participants recruited [16] and were well matched between groups (Table 1). Participants had significant COPD, as evidenced by lung function (mean FEV1 45% predicted), episodes of exacerbation and hospitalization during the previous year, frequent use of inhaled steroids (65%), and proportion on supplemental oxygen (28%).
Table 1.
Patient Characteristics
| Characteristic | PCV7 (N = 91) | PPSV23 (N = 90) | P value |
|---|---|---|---|
| Age, years | 63 ± 9 | 64 ± 10 | .56 |
| Male, n, (%) | 59 (65) | 52 (58) | .36 |
| Caucasian, (%) | 70 (77) | 71 (79) | .86 |
| FEV1, (L) | 1.33 ± 0.53 | 1.27 ± 0.50 | .37 |
| FEV1, (% predicted) | 44.9 ± 15 | 44.8 ± 15 | .96 |
| FVC | 2.91 ± 0.85 | 2.86 ± 1.0 | .73 |
| FEV1/FVC | 0.46 ± 0.13 | 0.45 ± 0.12 | .66 |
| Oxygen use, n (%) | 21 (23) | 29 (32) | .19 |
| Inhaled corticosteroid use, n (%) | 60 (66) | 58 (64) | .88 |
| Pack-Years smoking | 52 ± 28 | 55 ± 27 | .48 |
| Current Smoker, n (%) | 33 (36) | 32 (36) | 1.0 |
| Co-Morbid Illness, n (%) | |||
| Coronary Artery Disease | 22 (24) | 19 (21) | .72 |
| Congestive Heart Failure | 3 (3) | 5 (6) | .50 |
| Stroke | 3 (3) | 7 (8) | .21 |
| Diabetes Mellitus | 8 (9) | 7 (8) | 1.0 |
| Prior Malignancy | 9 (10) | 12 (13) | .50 |
| Anemia | 12 (13) | 5 (6) | .12 |
| Previous Pneumonia, n (%) | 44 (48) | 45 (50) | .88 |
| Exacerbation History (Year Prior to Enrollment) | |||
| Hospitalized or unscheduled emergency visit, n (%) | 18 (20) | 11 (12) | .22 |
| Number of hospitalizations or unscheduled ED visits in past year for those with any. | 1.2 ± 0.9 | 1.2 ± 0.6 | .90 |
| Received systemic steroids and/or antibiotics, n (%) | 38 (42) | 34 (38) | .65 |
| Number of courses of systemic steroids and/or antibiotics in past year for those with any courses. | 1.9 ± 1.6 | 1.6 ± 1.0 | .28 |
| Vaccine naive, n (%) | 45 (49) | 42 (47) | .77 |
| Years since last vaccination | 7.6 ± 2.7 | 8.4 ± 3.5 | .19 |
Results are mean ± standard deviation, unless indicated.
Abbreviations: ED, emergency department; FEV1, forced expiratory volume; FVC, forced vital capacity; L, liters; PCV7, 7-valent diphtheria-conjugated pneumococcal polysaccharide vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Almost 50% of participants reported a history of pneumonia, and a similar proportion was vaccine naive despite meeting at least 1 (COPD) and ≤3 (age, >65 years; history of pneumonia) Centers for Disease Control and Prevention (CDC) criteria for PPSV23. Participants who had been previously vaccinated with PPSV23 were older than those who had never received the vaccine (67 vs 60 years; P < .001). In addition, they were more often white (88% vs 67%; P < .001), had more severe airflow limitation (FEV1 41% vs 48% predicted; P = .002), more frequently used oxygen (43% vs 12%; P < .001), and more often reported a history of pneumonia (60% vs 38%; P = .005).
Results of baseline testing showed that IgG levels were generally similar in previously vaccinated and vaccine-naive groups (Table 2), although the former had significantly higher titers against serotype 18C and 23F. By contrast, revaccination participants had higher baseline OPK for all serotypes (statistically greater for 4, 18C, 19F, and 23F).
Table 2.
Baseline Serotype-Specific Antibody (Immunoglobulin G) and Opsonophagocytosis Killing Index in Vaccine-Naive and Previously Vaccinated Participants
| IgG |
OPK |
|||||
|---|---|---|---|---|---|---|
| Serotype | Naive (N = 86) | Previously Vaccinated (N = 93) | P value | Naive (N = 86) | Previously Vaccinated (N = 93) | P value |
| 4 | 0.25 (0.19–0.32) | 0.33 (0.26–0.43) | .11 | 7.14 (4.95–10.31) | 18.53 (12.17–28.21) | <.001 |
| 6B | 0.94 (0.75–1.17) | 0.86 (0.70–1.06) | .60 | 33.47 (19.88–56.34) | 49.04 (32.38–74.27) | .25 |
| 9V | 0.70 (0.54–0.92) | 0.91 (0.72–1.16) | .15 | 25.5 (15.53–41.87) | 44.7 (27.77–71.96) | .11 |
| 14 | 2.05 (1.46–2.88) | 3.14 (2.25–4.38) | .078 | 98.11 (51.95–185.27) | 161.78 (91.15–287.13) | .25 |
| 18C | 0.84 (0.64–1.10) | 1.44 (1.06–1.96) | .009 | 28.26 (17.67–45.20) | 62.17 (37.01–104.44) | .027 |
| 19F | 3.22 (2.62–3.96) | 2.99 (2.45–3.67) | .61 | 8.68 (5.59–13.48) | 25.63 (15.42–42.57) | .002 |
| 23F | 0.51 (0.40–0.66) | 0.83 (0.63–1.09) | .010 | 7.94 (5.10–12.35) | 26.21 (16.69–41.14) | <.001 |
Values are shown as geometric mean and 95% confidence interval. Significant differences between groups are shown in bold.
Abbreviations: IgG, immunoglobulin G; OPK, opsonophagocytosis killing index.
Follow-up visits with collection of serum samples and questionnaires were accomplished in 175 (97%), 145 (81%), and 128 (71%) participants at 1 month, 1 year, and 2 years, respectively. Consistent with our previously reported data [16], OPK values were higher at 1 month after vaccine than at baseline in both groups for all serotypes (P < .001), whereas PCV7 induced a greater response than PPSV23 for 6 of 7 serotypes (statistically greater in 5; Figure 2). Similar results were observed when the ratios between 1 month and baseline OPK were compared.
Figure 2.
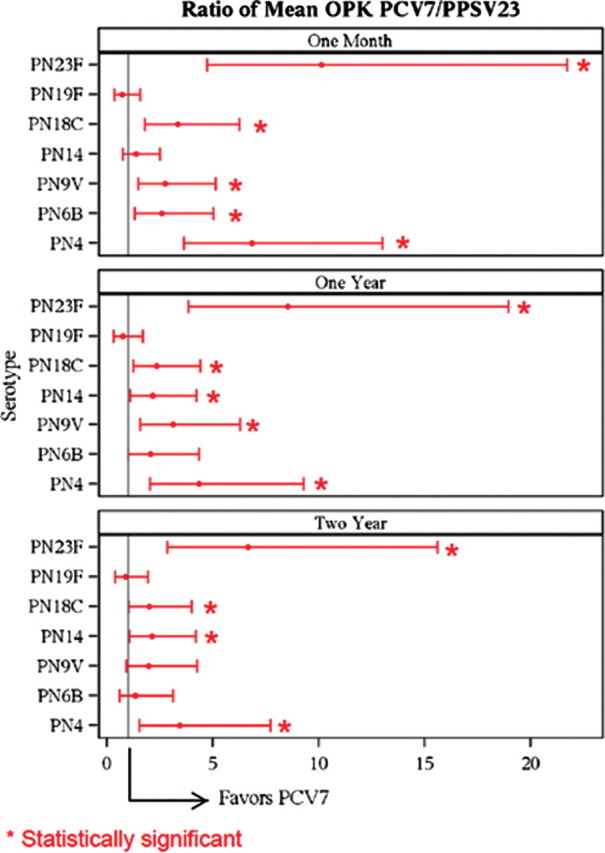
Ratios of the mean opsonophagocytosis killing index (OPK) between the 7-valent diphtheria-60 protein conjugated pneumococcal vaccine (PCV7) and 23-valent pneumococcal polysaccharide vaccine (PPSV23) groups for each serotype at 1 month, 1 year, and 2 years. PCV7 elicited a greater OPK response in 6 of 7 serotypes at all 3 time points that was statistically greater (*) in 5 serotypes at 1 month and 12 months and 4 serotypes at 24 months. Error bars denote 95% confidence interval. At year 1, the statistically greater OPK for PCV7 was observed for serotype 4: 268 vs 61.8, P = .0002; serotype 9V: 444 vs 142, P = .0016; serotype 14: 1801 vs 839, P = .0279; serotype 18C: 665 vs 284, P = .0091; and serotype 23F: 407 vs 47.8, P ≤ .0001. At 2 years, statistically greater OPK was observed for serotype 4: 237 vs 69.1, P = .0032; serotype 14: 1255 vs 594, P = .0319; serotype 18C: 540 vs 268, P = .0446; and serotype 23F: 266 vs 40, P ≤ 0001]. Abbreviations: OPK, opsonophagocytosis killing index; PCV7, 7-valent diphtheria-60 protein conjugated pneumococcal vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
OPK decreased over the next 23 months in both vaccine groups but remained significantly greater than baseline for all serotypes. Multivariate modeling revealed that the rate of OPK decrease was not affected by age, race, FEV1, smoking status, or prior vaccination status in either vaccine group (see Online Supplement). OPK was greater after PCV7 than after PPSV23 at both 1 and 2 years for 6 of 7 serotypes (all but 19F). This difference was statistically significant for 5 of 7 serotypes at 1 year (4, 9V, 14, 18C, and 23F) and numerically higher for serotype 6B (476 vs 229; P = .0526). OPK to serotype 19F was greater after PPSV23, although the difference was not statistically significant (116 vs 86.8; P = .47). PCV7 elicited a statistically superior OPK response for 4 of 7 serotypes at 2 years (4, 14, 18C, and 23F), and this was numerically higher for serotype 9V (433 vs 219; P = .08). Serotype-specific OPK for vaccine-naive and previously vaccinated participants are presented in the Online Supplement.
Both vaccines resulted in significant increases (P < .001) in postvaccination IgG levels for all serotypes (Table 3). Similar to the OPK results, serotype-specific IgG levels at 1 month were greater after PCV7 for all serotypes and were statistically greater in 4 (4, 9V, 18C, and 23F). At 1 year, this superior response persisted in all serotypes and was statistically greater in 3 (4, 18C, and 23F). IgG levels remained higher than baseline levels at 2 years in both groups, and the PCV7 response remained higher than that for PPSV23 for 6 of 7 serotypes, although was statistically greater only in one (23F). Serotype-specific IgG levels for vaccine-naive and previously vaccinated participants are presented in the Online Supplement.
Table 3.
Baseline and Postvaccination Geometric Mean Serotype-Specific Antibody Levels (Immunoglobulin G)
| Baseline (95% CI) |
1 Mo (95% CI) |
1 Y (95% CI) |
2 Y (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Serotype | PCV7 (N = 91) | PPSV23 (N = 88) | PCV7 (N = 87) | PPSV23 (N = 88) | PCV7 (N = 76) | PPSV23 (N = 69) | PCV7 (N = 66) | PPSV23 (N = 62) |
| 4 | 0.28 (.21–.37) | 0.30 (.23–.38) | 1.95 (1.39–2.73) | 0.74 (.54–1.01) | 0.70 (.50–.97) | 0.43 (.31–.60) | 0.53 (.38–.75) | 0.45 (.32–.65) |
| 6B | 0.90 (.72–1.13) | 0.89 (.73–1.09) | 3.19 (2.35–4.34) | 2.26 (1.65–3.09) | 1.77 (1.29–2.42) | 1.32 (1.02–1.72) | 1.33 (.95–1.85) | 1.27 (.95–1.69) |
| 9V | 0.78 (.60–1.01) | 0.83 (.64–1.07) | 4.87 (3.64–6.50) | 2.72 (1.98–3.72) | 2.15 (1.59–2.91) | 1.53 (1.11–2.09) | 1.65 (1.22–2.24) | 1.37 (.98–1.90) |
| 14 | 2.53 (1.77–3.61) | 2.59 (1.87–3.57) | 15.73 (11.25–22.00) | 10.80 (7.47–15.61) | 7.47 (5.15–10.84) | 5.76 (3.97–8.35) | 5.68 (3.75–8.58) | 4.82 (3.38–6.88) |
| 18C | 1.07 (.78–1.46) | 1.16 (.88–1.53) | 9.65 (7.22–12.89) | 4.25 (3.11–5.82) | 3.74 (2.78–5.03) | 2.25 (1.59–3.19) | 2.77 (2.05–3.74) | 1.94 (1.38–2.72) |
| 19F | 3.01 (2.45–3.70) | 3.20 (2.62–3.91) | 7.54 (5.76–9.85) | 6.79 (5.27–8.76) | 4.32 (3.37–5.52) | 4.25 (3.26–5.53) | 3.68 (2.74–4.94) | 3.85 (2.90–5.11) |
| 23F | 0.74 (.56–.98) | 0.58 (.45–.75) | 7.76 (5.49–10.97) | 1.88 (1.29–2.73) | 2.73 (1.94–3.84) | 1.12 (.80–1.55) | 1.99 (1.38–2.86) | 1.00 (.72–1.39) |
Abbreviations: CI, confidence interval; PCV7, 7-valent diphtheria-60 protein conjugated pneumococcal vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Values are shown as geometric mean and 95% confidence interval. Statistically significant differences between 7-valent diphtheria-60 protein conjugated pneumococcal vaccine and 23-valent pneumococcal polysaccharide vaccine groups are shown in bold.
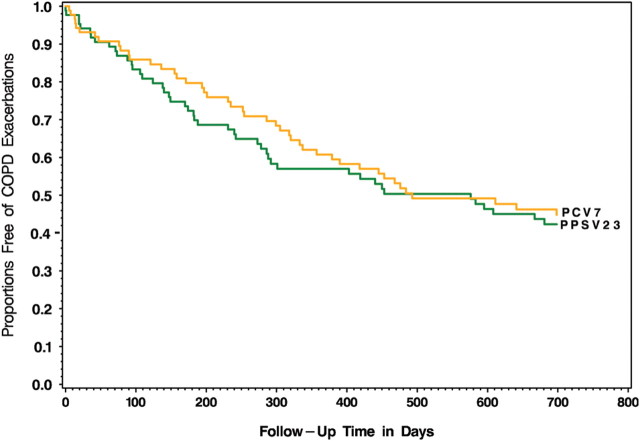
The study was not powered to detect differences between groups in clinical outcomes, and none were observed in the frequency of respiratory infection, hospitalization, or death (Table 4), nor was there any difference (hazard ratio, 0.91; P = .66) in the time to first exacerbation of COPD (Figure 3).
Table 4.
Frequency of Major Respiratory and Cardiac Events, Hospitalization, and Death
| Event | PCV7 (N = 91) | PPSV23 (N = 90) |
|---|---|---|
| Acute exacerbations | ||
| 0 | 46 | 45 |
| 1 | 16 | 21 |
| 2 | 15 | 12 |
| 3 | 4 | 4 |
| ≥4 | 7 | 6 |
| Total number of exacerbations | 93 | 93 |
| Participants with an exacerbation | 42 | 43 |
| Pneumonia | ||
| 0 | 81 | 80 |
| 1 | 9 | 9 |
| 2 | 1 | 0 |
| 3 | 0 | 1 |
| Total number of pneumonias | 11 | 12 |
| Participants with pneumonia | 10 | 10 |
| Hospitalizations—any cause | ||
| 0 | 65 | 66 |
| 1 | 11 | 16 |
| 2 | 12 | 4 |
| 3 | 1 | 2 |
| 4 | 1 | 0 |
| 5 | 1 | 2 |
| Total number of hospitalizations | 47 | 40 |
| Total participants hospitalized | 26 | 24 |
| Deaths | 4 | 7 |
Abbreviations: PCV7, 7-valent diphtheria-60 protein conjugated pneumococcal vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Figure 3.
Cox proportional hazard model adjusted for baseline age, sex, forced expiratory volume 1% predicted, and baseline smoking status, showing the time to first acute exacerbation in the 2 vaccine groups. No difference between 7-valent diphtheria-60 protein conjugated pneumococcal vaccine and 23-valent pneumococcal polysaccharide vaccine was observed (hazard ratio, 0.91; P = .66). Abbreviations: COPD, chronic obstructive pulmonary disease; PCV7, 7-valent diphtheria-60 protein conjugated pneumococcal vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
DISCUSSION
We found that, in patients with moderate to severe COPD, a population at high risk for pneumococcal infection, conjugate vaccination (PCV7) elicits a superior immune response to free-polysaccharide vaccine (PPSV23) that persists for >2 years. To our knowledge, the present study was the first study to examine the long-term (>1 year) comparative immunogenicity of pneumococcal vaccines in adults and specifically in those with underlying COPD. The current data suggest that conjugate vaccination may offer advantages in these patients and motivate further studies examining clinical end points.
An important feature of the study is the use of the 2 best surrogates of pneumococcal immunity: serotype-specific IgG and OPK. No published study has adequately compared long-term immune responses after conjugate or PPSV23 vaccination in adults with use of these measures, and the limited existing data about short-term responses have shown mixed results [11]. Despite consensus that OPK is the best laboratory surrogate for vaccine protection from pneumonia and bacteremia [10, 17, 18], no published report has included this measurement beyond 6 months after vaccination, although preliminary 1-year data on healthy adults are available in abstract form [14, 15]. Our results are consistent with and extend those of Musher et al, who reported that, in patients who had recovered from pneumonia, both PCV7 and PPSV23 elicited comparable IgG levels and OPK at 4–8 weeks but only remained greater than baseline at 6 months in those receiving PCV7 [21]. Our data contrast with those of Ridda et al, who reported no difference in IgG levels at 6 months after PCV7 or PPSV23 in older patients [22], and Goldblatt et al, who found that PCV7 was superior to PPSV23 for only 1 serotype (23F) at 1 year in healthy adults aged 50–80 years [23]. This disparity could be explained in part by PCV7 dose, because each of these studies used the usual pediatric dose (0.5 mL). However, Jackson et al found that IgG responses 1 year after a 1.0-mL dose of PCV7 were superior to those after PPSV23 for only 2 of 7 serotypes [20]. Similarly, although we found numerically greater IgG levels at 1 and 2 years in those receiving PCV7 (1.0 mL), these were statistically superior for only 3 and 1 serotype at each time. Despite this marginal advantage in IgG, the sustained superiority in OPK in those vaccinated with PCV7 argues that conjugate vaccination offers greater protection in patients with COPD for the 7 included serotypes [10, 17, 18].
This study provides novel information about the important policy question of revaccination. We found that baseline OPK were generally higher in those patients who had been previously vaccinated (a mean of 8 years earlier) than in those who were vaccine naive. A similar pattern was observed for baseline IgG, although the differences were less pronounced, reflecting that OPK is both a better correlate of protection, in part because of detection of functional IgM [24], and a more sensitive end point for immunogenicity studies. In addition, participants receiving either vaccine maintained IgG and OPK higher than those at baseline at 1 and 2 years. These results extend prior reports suggesting that, although levels decrease after vaccination, older adults can maintain IgG responses greater than those at baseline for up to 10 years and OPK responses up to 5 years [23, 25, 26]. Despite this immune persistence and some evidence that older persons can respond adequately to repeated PPSV23 vaccination, we previously reported that older age and prior vaccination were associated with a blunted 1-month vaccine response [16]. Our analysis showing that neither of these factors (nor lung function, race, or smoking status) impacts the rate of decrease in OPK shows the importance of the initial peak as the primary driver of long-term immune persistence. Because COPD is a disease of aging and most developed nations are experiencing expansion of their older population, current CDC guidelines recommend PPSV23 for those with disease and for all smokers, and functional antibody levels after vaccination are often short lived, the issue of revaccination is of major importance. A preventive strategy using repeated conjugate vaccination might offer advantages based on the greater immune response and emerging evidence that these vaccines are less likely to induce immune hyporesponsiveness [11].
Several studies including ours [20, 27] have demonstrated that PCV7 offers no advantage over PPSV23 for serotype 19F, emphasizing that protein conjugation of polysaccharide antigens does not ensure superior immunogenicity. Comparative responses to the additional serotypes included in expanded conjugate vaccines must therefore be individually examined. Of note, antibodies against serotype 19F do not cross-react against serotype 19A, which is frequently antibiotic resistant and has emerged as a significant cause of invasive disease since the introduction of PCV7 [28]. PCV13 will contain this replacement serotype and may offer some protection [2], but the long-term efficacy of any serotype-specific vaccine may ultimately be limited by the emergence of infections caused by serotypes that it does not contain.
Our population provides new insight into the usefulness of PPSV23 in the United States. All patients in the trial met current CDC criteria for vaccination with PPSV23 on the basis of their diagnosis of COPD and, in some cases, their age (>65 years) or history of pneumonia [1, 2]. Nevertheless, almost half of our participants reported never having received the vaccine. This finding is particularly telling, because we recruited from university medical centers and their affiliated Veterans Administration hospitals and, thus, sampled a population with access to medical care that is likely to be superior to the national norm. Absence of previous vaccination was more common in those with less advanced lung disease and among African-American persons. These data are compatible with prior reports suggesting the significant underuse of pneumococcal vaccination in healthy African-American individuals and of underuse of influenza vaccination in those with COPD [29, 30]. This phenomenon is poorly understood but is unlikely to be solely attributable to disparities in socioeconomics and access to care [29].
Our study has a number of limitations. First, our primary end point was a 1-year difference in OPK between the 2 study groups for each serotype. Accordingly, although we found no difference in the rates of acute exacerbations, pneumonia, hospitalizations, or mortality, our study was not powered to detect such differences. Second, the protective thresholds for IgG and OPK against respiratory infections are not known in adults, limiting our ability to definitively conclude that the greater immunogenicity observed after PCV7 will translate into superior clinical efficacy and meaningful reductions in these events. This caution applies particularly for the prevention of acute exacerbations, because pneumococcus is not the most frequent bacterial cause and higher IgG and OPK do not appear to result in clearance of pneumococcal carriage [31]. Lastly, we did rely in part on self-reported vaccination, and thus, it is possible that some participants were misclassified as vaccine naive or previously vaccinated or were enrolled <5 years after previous PPSV23.
No study has compared the effectiveness of PCV7 and PPSV23 in adults, and such a trial would necessarily be very large. A placebo-controlled trial of PCV13 enrolling >80 000 patients to detect a difference in rates of pneumonia is under way in Europe but will not resolve the debate regarding the comparative effectiveness of conjugate vaccines and PPSV23 [32]. The reduced serotype coverage offered by PCV13 and its major cost-disadvantage (estimates are that PCV13 will be 5-fold more expensive than will PPSV23, although a recent study suggests that the conjugate vaccine may be cost-effective [33]) raise questions about its widespread introduction [2, 11]. Serum immune end points proved to be powerful surrogates of clinical efficacy in children [12]; however, such correlative data do not exist in adults, and comparative studies using clinical end points are needed despite their complexity and expense. The superior immune response to PCV7 that we observed supports the pursuit of those trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/our_journal/cid). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Pneumo Study Credit Roster and Funding Sources. The COPD Clinical Research Network is supported by a Cooperative Agreement from the Division of Lung Diseases of the National Heart, Lung, and Blood Institute.
Brigham and Women's Hospital: J. J. Reilly, Jr, G. Washko (PIs), S. Peterson (Coordinator). Grant HL074428, GCRC Grant RR02635.
Denver Health Medical Center: R. K. Albert (PI), B. Make (Co-PI), M. Schwarz, C. Welsh (Investigators), C. Verano, J. Binford, J. Herrell (Coordinators). Grant HL074409, GCRC Grant RR00051.
Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center: R. Casaburi, (PI), G. Mason, J. Porszasz, (Investigators), K. Norulak, R.Kiledjian, L. Diaz, R. Love, P. Walker (Coordinators). Grant HL074407, GCRC Grant RR00425.
Minnesota Veterans Research Institute, Minneapolis (Affiliated Sites: HealthPartners Research Foundation, Mayo Clinic): D. E. Niewoehner (PI), C. McEvoy, K. R. Rice, P. D. Scanlon (Co-PIs). C. Andrist, J. Hart, K. Timm (Coordinators).Grant 1U10-HL074416.
Temple University: G. J. Criner (PI), W. Chatila, N. Marchetti, V. Kim, G. D'Alonzo, S. Krachman, F. Cordova, K. Brennan, N. Patel, J. Mamary (Investigators), C. Grabianowski, G. Jones, N. Krayger, A. M. Kuzma, H. Smith (Coordinators). Grant HL074408.
University of Alabama at Birmingham: M. T. Dransfield (PI), J. A. D. Cooper (Co-PI), W. C. Bailey, L. B. Gerald, P. O'Reilly (Investigators), S. Tidwell (Coordinator). Grant HL074418.
University of California, San Francisco: S. C. Lazarus (PI), H. A. Boushey, J. V. Fahy, P. G. Woodruff (Investigators), K. Schardein, C. Nguyen, R.Sakurai, M. Dyjak (Coordinators). 1U10-HL074431.
University of Maryland, Baltimore: S. M. Scharf (PI), M. Alattar, P. Amelung, M. Cowan, J. Hanson, J. Hasday, A. Iacono, C. Shanholtz, N. Todd, A. Verceles (Investigators), W. Bell-Farrell, T. Fitzgerald, P. Wood(Coordinators). Grant HL074441, GCRC Grant RR16500.
University of Michigan, Ann Arbor: F. J. Martinez (PI), J. L. Curtis, M.K. Han, S. E. Gay, T. H. Sisson, P. J. Christensen, M. Mendez (Investigators), D. Thompson (Project Manager), C. Flaherty, T. Felt, S. Smith, L. Husselman, D. White (Coordinators at UM), C. Getty, C. Jett, L. McCloskey, M. Christensen (Coordinators at VA). Grant HL074422.
University of Pittsburgh: F. Sciurba (PI), L. Kniolek, L. Lane, M. Pitaro (Coordinators). Grant HL074439, GCRC Grant RR00056.
University of Minnesota (Data Coordinating Center): J. E. Connett (PI), N. R. Anthonisen (Steering Committee Chair), C.Wendt (Co-PI), S. Harnden, W. Patrek, H. Voelker (Coordinators). Grant 1U10-HL074424.
Data and Safety Monitoring Board: B. B. Bender, C. A. Champlin, S. F. Kelsey, J. R. Landis, B.Phillips, G.M. Turino, R. Veatch, A.L. Waldo, A. Wanner.
Protocol Review Committee: H. W. Kelly, J. Maurer A. J. McSweeny, R. M. Senior, E. A. Thom, P. D. Wagner, R.L. ZuWallack.
NHLBI: G. Weinmann (Deputy Director, Division of Lung Diseases), T. Croxton (Director, Airway Biology & Disease Program), A. Punturieri (Program Officer), M.P. Stylianou (Biostatistician).
Potential conflicts of interest. M. T. D. has served as an advisory board member for GlaxoSmithKline and Boehringer Ingelheim and has received industry contracts for clinical trials from GlaxoSmithKline, Boehringer Ingelheim, Otsuka, Boston Scientific and Centocor. R. C. has served in the speakers’ bureau for Pfizer Pharmaceuticals. M. K. H. has participated in advisory boards for Boehringer Ingelheim GmbH, Pfizer, GlaxoSmithKline, Genentech, Novartis, and Medimmune; has participated on speaker's bureaus for Boehringer Ingelheim GmbH, Pfizer, GlaxoSmithKline, the National Association for Continuing Education, and WebMD; has consulted for Novartis and Nycomed; and has received royalties from UpToDate and ePocrates. B. M. has served on an advisory boards for Merck and Pfizer and has been the local principal investigator for multicenter trials sponsored by Pfizer. F. J. M. has participated in Advisory Boards in COPD development for Actelion, Astra Zeneca, Bayer, BoomComm, fbCommunications, Forest/Almirall, GSK, Ikaria, MedImmune, Merck, Novartis, Nycomed, Pearl, Pfizer, Roche, Schering, and Talecris; has been a member of Steering Committee for COPD studies sponsored by Actelion, GSK, Forest, MPex, and Nycomed; has participated in FDA Mock panels for Boehringer Ingelheim and Forest; has served on speaker's bureaus or in CME activities sponsored by American Lung Association, Almirall, Altana/Nycomed, Astra Zeneca, Boehringer Ingelheim, CME Incite, ePocrates, Forest, France Foundation, GSK, MedEd, NACE, Pfizer, Potomac, Prescott, Sanofi Aventis, Vox Medic, WebMD, and UpToDate; and has received royalties from Associates in Medical Marketing, Castle Connolly. D. E. N has received advisory fees from Merck and Pfizer. P. D, S. has participated in clinical trials funded by Pfizer and Boehringer Ingelheim, and his wife works for Merck Research Laboratories. P. G. W. has had a recent research grant with Genentech, recent consulting for Medimmune, and is co-inventor on a patent pending for asthma diagnostics. The University of Alabama at Birmingham owns the intellectual property rights to some of the reagents described in this work. M. H. N., R. L. B., W. C. B., J. A. D. C. and M. T. D. are employees of the University of Alabama at Birmingham, and M. H. N. has been a consultant to Merck regarding the diagnosis of pneumonia. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Center for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 1997;46:1–24. [Google Scholar]
- 2.Metersky ML, Dransfield MT, Jackson LA. Determining the optimal pneumococcal vaccination strategy for adults: is there a role for the pneumococcal conjugate vaccine? Chest. 2010;138:486–90. doi: 10.1378/chest.10-0738. [DOI] [PubMed] [Google Scholar]
- 3.Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2008;47:1328–38. doi: 10.1086/592691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfageme I, Vazquez R, Reyes N, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax. 2006;61:189–95. doi: 10.1136/thx.2005.043323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis AL, Aranda CP, Schiffman G, Christianson LC. Pneumococcal infection and immunologic response to pneumococcal vaccine in chronic obstructive pulmonary disease. A pilot study. Chest. 1987;92:204–12. doi: 10.1378/chest.92.2.204. [DOI] [PubMed] [Google Scholar]
- 7.Leech JA, Gervais A, Ruben FL. Efficacy of pneumococcal vaccine in severe chronic obstructive pulmonary disease. CMAJ: Canadian Medical Association Journal = Journal de l'Association Medicale Canadienne. 1987;136:361–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Steentoft J, Konradsen HB, Hilskov J, Gislason G, Andersen JR. Response to pneumococcal vaccine in chronic obstructive lung disease–the effect of ongoing, systemic steroid treatment. Vaccine. 2006;24:1408–12. doi: 10.1016/j.vaccine.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Walters JA, Smith S, Poole P, Granger RH, Wood-Baker R. Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010:CD001390. doi: 10.1002/14651858.CD001390.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Schenkein JG, Nahm MH, Dransfield MT. Pneumococcal vaccination for patients with COPD: current practice and future directions. Chest. 2008;133:767–74. doi: 10.1378/chest.07-0996. [DOI] [PubMed] [Google Scholar]
- 11.Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2011;52:633–40. doi: 10.1093/cid/ciq207. [DOI] [PubMed] [Google Scholar]
- 12.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. The Pediatric Infectious Disease Journal. 2000;19:187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA: The Journal of the American Medical Association. 2006;295:1668–74. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 14.LA J. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. doi: 10.1016/j.vaccine.2013.05.010. European Congress of Clinical Microbioogy and Infectious Diseases (ECCMID). (Vienna, Austria).Q425. [DOI] [PubMed] [Google Scholar]
- 15.LA J. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in pneumococcal vaccine naive adults, 50–64 years of age. In: European Conference of Clinical Microbiology and Infectious Diseases (ECCMID). (Vienna, Austria).Q426. [Google Scholar]
- 16.Dransfield MT, Nahm MH, Han MK, et al. Superior immune response to protein-conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2009;180:499–505. doi: 10.1164/rccm.200903-0488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson SE, Rubin L, Romero-Steiner S, et al. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. The Journal of Infectious Diseases. 1999;180:133–40. doi: 10.1086/314845. [DOI] [PubMed] [Google Scholar]
- 18.Musher DM, Phan HM, Watson DA, Baughn RE. Antibody to capsular polysaccharide of Streptococcus pneumoniae at the time of hospital admission for Pneumococcal pneumonia. The Journal of Infectious Diseases. 2000;182:158–67. doi: 10.1086/315697. [DOI] [PubMed] [Google Scholar]
- 19.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clinical and Vaccine Immunology: CVI. 2006;13:1004–9. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson LA, Neuzil KM, Nahm MH, et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2007;25:4029–37. doi: 10.1016/j.vaccine.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 21.Musher DM, Rueda AM, Nahm MH, Graviss EA, Rodriguez-Barradas MC. Initial and subsequent response to pneumococcal polysaccharide and protein-conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. The Journal of Infectious Diseases. 2008;198:1019–27. doi: 10.1086/591629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridda I, Macintyre CR, Lindley R, et al. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009;27:1628–36. doi: 10.1016/j.vaccine.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 23.Goldblatt D, Southern J, Andrews N, et al. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50–80 years. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2009;49:1318–25. doi: 10.1086/606046. [DOI] [PubMed] [Google Scholar]
- 24.Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infection and Immunity. 2011;79:314–20. doi: 10.1128/IAI.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musher DM, Manoff SB, McFetridge RD, et al. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Human Vaccines. 2011;7:919–28. doi: 10.4161/hv.7.9.15996. [DOI] [PubMed] [Google Scholar]
- 26.Manoff SB, Liss C, Caulfield MJ, et al. Revaccination with a 23-valent pneumococcal polysaccharide vaccine induces elevated and persistent functional antibody responses in adults aged 65 > or = years. The Journal of Infectious Diseases. 2010;201:525–33. doi: 10.1086/651131. [DOI] [PubMed] [Google Scholar]
- 27.de Roux A, Schmole-Thoma B, Siber GR, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2008;46:1015–23. doi: 10.1086/529142. [DOI] [PubMed] [Google Scholar]
- 28.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. The Journal of Infectious Diseases. 2007;196:1346–54. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 29.Egede LE, Zheng D. Racial/ethnic differences in influenza vaccination coverage in high-risk adults. American Journal of Public Health. 2003;93:2074–8. doi: 10.2105/ajph.93.12.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Mukamel DB. Racial disparities in receipt of influenza and pneumococcus vaccinations among US nursing-home residents. American Journal of Public Health. 2010;100(Suppl 1):S256–62. doi: 10.2105/AJPH.2009.173468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malley R, Lipsitch M, Bogaert D, et al. Serum antipneumococcal antibodies and pneumococcal colonization in adults with chronic obstructive pulmonary disease. The Journal of Infectious Diseases. 2007;196:928–35. doi: 10.1086/520937. [DOI] [PubMed] [Google Scholar]
- 32.Hak E, Grobbee DE, Sanders EA, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. The Netherlands Journal of Medicine. 2008;66:378–83. [PubMed] [Google Scholar]
- 33.Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA: The Journal of the American Medical Association. 2012;307:804–12. doi: 10.1001/jama.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.