ACTG A5199 compared the neuropsychological effects of 3 antiretroviral regimens in 860 human immunodeficiency virus–positive participants from Brazil, India, Malawi, Peru, South Africa, Thailand, and Zimbabwe. Treatment with either of the World Health Organization–recommended first-line regimens improved neurological and neuropsychological functioning.
Abstract
Background. AIDS Clinical Trials Group (ACTG) A5199 compared the neurological and neuropsychological (NP) effects of 3 antiretroviral regimens in participants infected with human immunodeficiency virus type 1 (HIV-1) in resource-limited settings.
Methods. Participants from Brazil, India, Malawi, Peru, South Africa, Thailand, and Zimbabwe were randomized to 3 antiretroviral treatment arms: A (lamivudine-zidovudine plus efavirenz, n = 289), B (atazanavir, emtricitabine, and didanosine-EC, n = 293), and C (emtricitabine-tenofovir-disoproxil fumarate plus efavirenz, n = 278) as part of the ACTG PEARLS study (A5175). Standardized neurological and neuropsychological (NP) screening examinations (grooved pegboard, timed gait, semantic verbal fluency, and finger tapping) were administered every 24 weeks from February 2006 to May 2010. Associations with neurological and neuropsychological function were estimated from linear and logistic regression models using generalized estimating equations.
Results. The median weeks on study was 168 (Q1 = 96, Q3 = 192) for the 860 participants. NP test scores improved (P < .05) with the exception of semantic verbal fluency. No differences in neurological and neuropsychological functioning between treatment regimens were detected (P > .10). Significant country effects were noted on all NP tests and neurological outcomes (P < .01).
Conclusions. The study detected no significant differences in neuropsychological and neurological outcomes between randomized ART regimens. Significant improvement occurred in neurocognitive and neurological functioning over time after initiation of ARTs. The etiology of these improvements is likely multifactorial, reflecting reduced central nervous system HIV infection, better general health, and practice effects. This study suggests that treatment with either of the World Health Organization –recommended first-line antiretroviral regimens in resource-limited settings will improve neuropsychological functioning and reduce neurological dysfunction.
Clinical trials registration. NCT00096824.
Currently, the greatest burden of the human immunodeficiency virus type 1 (HIV-1) epidemic is in resource-poor, developing parts of the world where the majority of new infections occur [1]. An important component of this epidemic includes its effects on the central and peripheral nervous systems (CNS and PNS), through opportunistic infections and by more direct effects of HIV-1. Considerable evidence exists to suggest the local effects of viral and immune factors in the CNS and PNS underlie the damage done [2].
The spectrum of HIV-related CNS diseases is grouped under HIV-associated neurological disorders (HAND) [3] and includes the more severe form of HIV-associated dementia (HAD), the less severe but more prevalent HIV-associated minor neurocognitive disorder (MND), and asymptomatic neurocognitive impairment (ANI) [4–8]. In the era prior to highly active antiretroviral therapy (HAART), as many as 80% of people who died from AIDS in the United States had autopsy evidence of CNS injury attributable to HIV-1 regardless of whether there had been clear manifestations of HAD during life [9].
Because little is known about the impact of antiretroviral treatment on neuropsychological functioning and neurological dysfunction in HIV-1–infected people in resource-limited settings, this study examined the neurological and neuropsychological effects of 3 randomly assigned antiretroviral regimens, including a direct comparison of 2 regimens recommended by the World Health Organization (WHO) for first-line treatments of HIV-1 in resource-limited setting (efavirenz with either coformulated lamivudine-zidovudine or emtricitabine-tenofovir-DF) [10].
METHODS
Sites
A5199, the International Neurological Study (Clinicaltrials.gov, NCT00096824) enrolled solely from ACTG A5175, a randomized treatment trial of antiretroviral efficacy and safety. The international ACTG sites that participated in A5199 were located in Rio de Janeiro, Brazil; Porto Alegre, Brazil; Chennai, India; Pune, India; Blantyre, Malawi; Lilongwe, Malawi; Lima, Peru; Johannesburg, South Africa; Durban, South Africa; Chiang Mai, Thailand; and Harare, Zimbabwe.
Procedures
Human subjects study reviews and approvals by local and country specific review boards were obtained at each site prior to study initiation, and informed consent was obtained prior to study participation. The National Institutes for Health (NIH), National Institute for Allergy and Infectious Diseases (NIAID), Division of AIDS (DAIDS), and Multinational Data Safety and Monitoring Board monitored the study at intervals to ensure safe and appropriate conduct. Standardized training on administration of the neurological and neuropsychological screening examinations was conducted. Rigorous data monitoring at data entry through computerized range checks, with follow-up data cleaning through multiple queries and replies, was conducted throughout the study. Implausible values were queried, and confirmed or corrected at intervals.
Participants
Participants were a subset of ACTG A5175, a randomized antiretroviral treatment trial (ClinicalTrials.gov, NCT00084136). Eligible subjects for A5175 were men and women 18 years or older who had documented HIV-1 infection, CD4+ lymphocytes less than 300 cells/mm3, Karnofsky performance score greater than or equal to 70, and no more than 7 days of cumulative prior antiretroviral therapy prior to study entry. Participants were excluded from participation in the study if they had any active severe psychiatric illness, active drug or alcohol abuse or dependence, serious illness and/or hospitalization within 14 days of study entry, or any other condition that, in the opinion of the site investigator, would compromise the person's ability to participate in the study, adhere to study requirements, or confound the analysis or interpretation of the results of the study.
Treatment Arms
The parent study, A5175, was a phase IV, randomized, open-label, 3-arm, antiviral efficacy trial designed to evaluate 3 antiretroviral regimens for treatment-naive HIV-infected participants: 1 regimen of 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus an HIV-1 protease inhibitor (PI) and 2 regimens of 2 NRTIs plus a nonnucleoside reverse transcriptase inhibitor (NNRTI). Arm A consisted of coformulated lamivudine (3TC)/zidovudine (ZDV) 150 mg/300 mg PO BIDefavirenz (EFV) 600 mg PO QHS, arm B was emtricitabine (FTC) 200 mg PO QD plus atazanavir (ATV) 400 mg PO QD plus didanosine enteric-coated (ddI-EC) 400 mg PO QD for participants who weighed ≥60 kg or ddI-EC 250 mg PO QD for participants who weighed <60 kg, and arm C was coformulated FTC/tenofovir (TDF) 200 mg/300 mg PO QHS plus EFV 600 mg PO QHS. If a participant had treatment failure, defined as a viral load >1000 copies/mL on study, they entered step 2: those on arm A with treatment failure went on to arm 2A, which consisted of 2 NRTIs plus PI(s); while those on arm B who had treatment failure went on to arm 2B which consisted of 2 NRTIs plus EFV; and those on arm C who had treatment failure went on to arm 2C, which consisted of 2 NRTIs plus PI(s).
The Data and Safety Monitoring Board (DSMB) review of 6 May 2008 concluded that arm B FTC plus ATV plus ddI was inferior to 3TC-ZDV plus EFV and should not be recommended for initial therapy in antiretroviral-naive individuals; arm B was discontinued, and participants offered the option to change regimens in Step 3 to track changes in antiretroviral regimens.
Standardized neurological and neuropsychological (NP) screening examinations (grooved pegboard, timed gait, semantic verbal fluency, and finger tapping) were administered every 24 weeks from February 2006 to May 2010. The neuropsychological tests were chosen on the basis of prior experience in clinical trial and cohort studies in the United States, with particular care taken to keep the battery short and minimizing language- and culture-specific items [11, 12]. Although neuropsychological tests are used for assigning impairment ratings or for diagnoses of HAND, appropriate normative data do not exist to make these assessments in this study.
The neurological examination included a neurological history and symptom review and cognitive, motor, sensory and reflex assessments, and was conducted primarily by physicians, less often by midlevel clinicians such as nurse practitioners. A study-specific diagnosis form was completed for each participant and included HAD, MND, and peripheral neuropathy, in addition to CNS opportunistic infections. The diagnosis of diffuse CNS-disease related to HIV was categorized as MND if impairment was rated as subclinical or equivocal, and as HAD if it was categorized mild, moderate, or severe, on the basis of the severity levels in the ADC Staging criteria as follows. Subclinical or equivocal was defined as either minimal or equivocal symptoms or motor dysfunction characteristic of ADC, or mild signs (snout response, slowed extremity movements) but without impairment of work or capacity to perform activities of daily living (ADL). Mild was defined as unequivocal evidence of functional intellectual or motor impairment but able to perform all but the more demanding aspects of work or ADL. Moderate was defined as cannot work or maintain the more demanding aspects of daily life but able to perform basic activities of self-care. Severe was defined as major intellectual incapacity (cannot follow news or personal events, cannot sustain complex conversation, considerable slowing of all output) or motor disability.
Statistical Analyses
Linear and logistic regression models using generalized estimating equation (GEE) with an autoregressive correlation structure (for within-patient correlation) were constructed to assess the treatment effects as well as the associations of other covariates with neuropsychological test scores and neurological outcomes which included overall neurological examination abnormality, PNS, focal or diffuse CNS abnormality. The covariates included in the model were country, randomized treatment, baseline HIV-1 RNA stratum (<100 000 vs ≥100 000 copies/mL), screening CD4 stratum (<50, 50–99, 100–199, 200–249, and 250–299 cells/mm3) , baseline neurocognitive test scores, age, sex, and years of education. Although CD4+ cell nadir was not available, but because these participants were treatment naive, current CD4+ cell count likely approximates the nadir. A second model used the same covariates as above plus current HIV-1 RNA, and current CD4 to estimate the associations of current CD4 and current HIV-1 RNA on neurological and neuropsychological functions. Parameters were interpreted for a 10-year change in age, a 50-unit change in CD4 count, a 4-year change in education, and a 1 log change in entry plasma HIV-1 RNA. CD4 count and entry plasma HIV-1 RNA were used as continuous variables in modeling. HIV-1 RNA value at study follow-up was dichotomized into detectable vs undetectable using the lower assay detection limit of 400 copies/mL, below which viral load levels were censored. In each case, 95% confidence intervals (CIs) are used to estimate the covariate effects. Letendre has developed a ranking system to assess CNS penetration-effectiveness (CPE) of antiretrovirals based on chemical properties, CSF to plasma concentrations, and effectiveness in clinical studies. Highly penetrating drugs receive a rank of 4, high intermediate drugs a 3, low intermediate drugs a 2, and low penetrating drugs a 1. For the total CPE score in a regimen, the rankings of each individual agent are summed [13]. Treatment arm A had CPE of 9; treatment arms B and C had CPE scores of 7.
Forest plots were generated to summarize associations. Longitudinal plots were used to display temporal trends. All significance testing was performed at the .05 level, a trend for significance was defined as P > .05 and P < .15 level, and no adjustments for multiple testing or multiple comparisons were used. All reported P values are 2-sided.
RESULTS
Demographics
The baseline results for A5199 have been reported elsewhere [14], but a brief summary of the demographics for the cohort is presented here (see Table 1). The total enrollment was 860 participants: 452 (53%) females and 408 (47%) males. The median age was 34 years, and the median educational level was 10 years (Q1-7, Q3-12). By country, there were 161 participants in Brazil, 184 in India, 133 in Malawi, 62 in Peru, 167 in South Africa, 73 in Thailand, and 80 in Zimbabwe. The parent study enrolled in both US and international sites, with a total of 1261 enrolled into A5175 at the A5199 participating sites, and 401 not participating in A5199. There were no significant differences in sex, race, ethnicity, age, intravenous drug use, CD4 count, and plasma HIV RNA between demographics between the A5175 and A5199 participants.
Table 1.
Screening Demographics by Country
| Country |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (N = 860) | Brazil (N = 161) | India (N = 184) | Malawi (N = 133) | Peru (N = 62) | South Africa (N = 167) | Thailand (N = 73) | Zimbabwe (N = 80) | ||
| CD4 count, cells/mm3 | Median | 172.5 | 183 | 198 | 178 | 167 | 157 | 125 | 170.5 |
| Q1, Q3 | 97.5, 232.0 | 75, 257 | 136, 236 | 122, 232 | 88, 234 | 100, 216 | 37, 177 | 98.5, 217.5 | |
| Log10 plasma HIV-1 RNA, copies/mL | Median | 5.0 | 5.2 | 5.1 | 4.8 | 4.8 | 5.2 | 5.0 | 5.2 |
| Q1, Q3 | 4.5, 5.5 | 4.7, 5.6 | 4.5, 5.5 | 4.4, 5.2 | 4.2, 5.1 | 4.6, 5.7 | 4.6, 5.3 | 4.6, 5.6 | |
| Sex | Male | 408 | 102 | 102 | 39 | 37 | 60 | 37 | 31 |
| Female | 452 | 59 | 82 | 94 | 25 | 107 | 36 | 49 | |
| Age | Median | 34 | 36 | 33 | 31 | 33 | 34 | 33 | 36 |
| Education level | Median | 10 | 9 | 9 | 8 | 12.5 | 11 | 10 | 11 |
| Q1, Q3 | 7, 12 | 6, 11 | 6, 11 | 4, 11 | 11, 14 | 9, 12 | 6, 15 | 8.5, 12.5 | |
Treatment Arm Comparisons
No significant differences existed between the 3 randomized treatment arms in neuropsychological or neurological outcomes over time. Therefore, there were no observed differences related to neuropsychological or neurological functioning over time between CPE score for these regimens. Treatment arm A (CPE = 9) vs arm C (CPE = 7) and treatment arm A vs arm B (CPE = 7) were not different from one another in neurological and neuropsychological outcome.
Longitudinal Follow-up
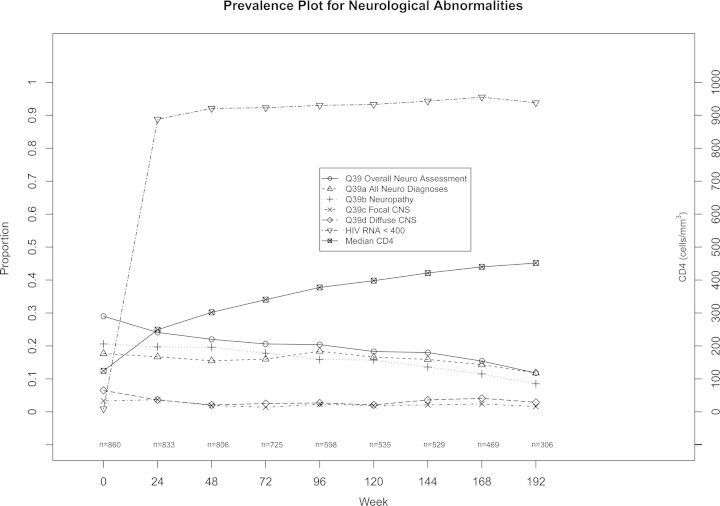
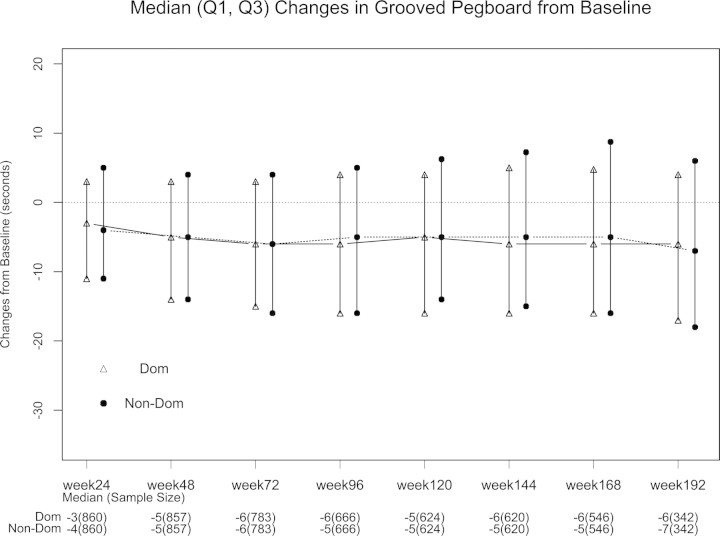
Median follow-up was 168 weeks on study (Q1 = 96, Q3 = 192, see Figure 1). As expected, there were significant increases in CD4+ lymphocyte counts and significant decreases in plasma HIV-1 RNA over the course of the study. Overall neurological abnormality and neuropathy improved over time with initiation of antiretroviral treatment (see Figure 2). For follow-up on study, at week 24 there were 860 participants, 857 at week 48, 783 at week 72, 666 at week 96, 624 at week 120, 620 at week 144, 546 at week 168, 342 at week 192, and 62 at week 216, whose data were included when neurocognitive functions were assessed. Significant improvements were seen in all the neuropsychological test scores over weeks on study after antiretroviral treatment initiation (P < .05) with exception of semantic verbal fluency. These improvements were sustained over 3 years of follow-up (see Figure 3 for example and online supplemental figures).
Figure 1.
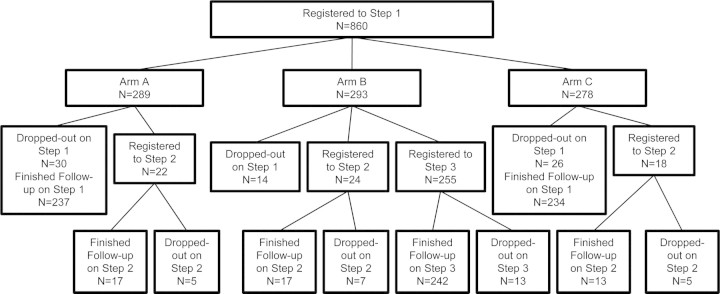
Consort diagram of participants in the study.
Figure 2.
Improvements in neurological disease, human immunodeficiency virus RNA, and CD4 cell counts over time. *Test scores improved at each follow-up visit compared to baseline (each P value < .01 by Wilcoxon signed-rank test). Abbreviations: CNS, central nervous system; HIV, human immunodeficiency virus.
Figure 3.
Median (Q1, Q3) changes in grooved pegboard from baseline. *Test scores improved at each follow-up visit compared to baseline (each P value < .01 by Wilcoxon signed-rank test). Abbreviations: Dom, dominant; Non-Dom, non-dominant.
Country
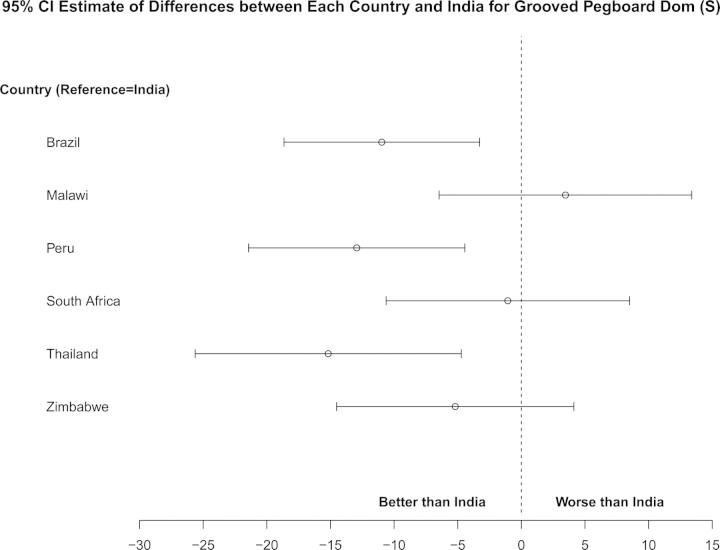
As expected, there was significant variation in neuropsychological performance across countries (see Figure 4 as an example and online supplemental figures). India was chosen as the reference for the following comparisons controlling for covariates of age, sex, education, baseline functioning, screening RNA stratum, screening CD4 count, time, and treatment arm. The South American countries and Thailand performed better on grooved pegboard over time. Malawi and South Africa performed poorer, whereas South America and Zimbabwe performed better in semantic verbal fluency over time. Peru, South Africa, Thailand, and Zimbabwe had better performance on timed gait over time. Malawi and Peru performed worse, whereas Zimbabwe had better performance on finger tapping over time. Substantial country variation was also seen with respect to overall neurological abnormality. South Africa was the reference in the comparisons. There was higher overall abnormality rate in Thailand and Zimbabwe and lower rate in India and Peru over time.
Figure 4.
Country differences in grooved pegboard dominant hand. *P-values for each comparison with India: Brazil (P = .01), Malawi (P = .49), Peru (P < .01), South Africa (P = .83), Thailand (P < .01), and Zimbabwe (P = .27). India was chosen as the reference country due to having the largest sample size. Abbreviations: CI, confidence interval; Dom, dominant.
Correlations With Neurological and Neuropsychological Performance
Higher baseline neuropsychological scores were associated with better future neuropsychological performance on all tests. Increasing education was associated with better performance on finger tapping and semantic verbal fluency, and trends existed for grooved pegboard and timed gait (P < .15). Increasing age was significantly associated with decreased performance on grooved pegboard dominant/nondominant and timed gait but not for finger tapping and semantic verbal fluency. Similar to the neuropsychological findings, older age was associated with higher odds of overall neurologic abnormality for each 10-year increment (odds ratio [OR], 1.38 [95% CI: 1.18–1.62], P < .001), whereas higher education was associated with lower odds of overall neurological abnormality for each 4-year increment (OR, .82 [95% CI: .69–.97], P < .05).
Lower screening RNA stratum was related to better performance over time in grooved pegboard dominance with similar trends for grooved pegboard nondominance, verbal fluency, and timed gait (P < .15). A higher screening CD4 count was related to better performance over time in timed gait, and trends were seen for better performance over time in grooved pegboard and finger tapping (P < .15). Compared to participants without HAD and MND, those given a diagnosis of HAD or MND on the basis of the neurological exam had trends for poorer performance on the neuropsychological tests over time (Table 2).
Table 2.
Association of Current HIV-Associated Dementia/Minor Neurocognitive Disorder With Each Neuropsychological Test
| Test | Estimate | 95% CI | P |
|---|---|---|---|
| Grooved pegboard dominant, s | 10.12 | (−.069 to 20.30) | .05 |
| Grooved pegboard nondominant, s | 6.45 | (−1.16 to 14.07) | .10 |
| Semantic verbal fluency, no. of words | − 0.62 | (−1.41 to − .17) | .13 |
| Timed gait, s | 0.92 | (.19–1.65) | .01 |
| Finger tap dominant, no. of taps | − 2.02 | (−4.24 to − .19) | .07 |
| Finger tap nondominant, no. of taps | −1.77 | (−3.26 to −.27) | .02 |
Association of neuropsychological test scores in subjects without and with minor neurocognitive disorder/HIV-associated dementia on study. Abbreviations: CI, confidence interval; s, seconds to complete.
DISCUSSION
This is the first clinical trial to our knowledge of the impact of randomized antiretroviral regimens on neuropsychological and neurological function in HIV-positive participants across diverse international resource-limited settings.
No significant differences were seen in neurocognitive or neurological functioning between the randomized treatment arms. Importantly, this provides a comparison of WHO first-line recommended treatment regimens for resource-limited settings (3TC/ZDV + EFV vs FTC/TDF + EFV) [10]. The amount of the neuropsychological and neurological improvements did not differ between these 2 arms, consistent with the virological and immunological improvements in the primary study. In addition, this provided evidence that the higher CPE value in arm A (3TC/ZDV + EFV) vs arm C (FTC/TDF + EFV) did not provide additional neurological or neurocognitive benefit.
Sustained antiretroviral therapy improved neuropsychological functioning and reduced overall neurological abnormality in treatment-naive HIV-positive participants. This improvement was sustained over more than 3 years of follow-up. This is an important finding with substantial clinical relevance for individuals living with HIV, especially because the overwhelming majority of the disease burden is in resource-limited settings. The choice of regimen, whether an NNRTI or a PI on a 2 NRTI backbone, or whether that regimen is dosed once or twice daily appeared less important for improving neuropsychological functioning than initiating and maintaining successful antiretroviral treatment.
In the developed world, several studies have shown that ART was associated with improvement in cognitive and peripheral nervous system function [15–18]. Prior to the current study, we did not know whether these neurological benefits would be seen in HIV-infected patients in resource-limited settings. As has been seen in studies from the United States and Europe, we found that neuropsychological function improved and neurological abnormalities were less common after antiretroviral treatment.
There was substantial variation in neurological and neuropsychological outcomes by country in this study. This country variation was not unexpected, and a primary reason the study was conducted in such diverse settings was to assess the degree of such variability. Prior studies have found country differences in neurological and neuropsychological outcomes including resource-limited settings [19–23]. One important factor in our study was differences in education across sites. In addition, the differences between sites could be due to variation in the administration of the neuropsychological tests and neurological examination. We minimized this possibility by conducting on-site and centralized training sessions. Different HIV-1 subtypes likely predominated in the individual countries in which this study was conducted, and those differences may have contributed to the differences in neurological or neuropsychological performance that we observed. For example, Sacktor et al [20] documented that among a group of HIV-infected individuals in Uganda, those infected with subtype D had a greater prevalence of HIV-associated neurological disease than those with subtype A.
This study found a low level of HAD or MND over time, likely because the participants were fairly healthy. A Karnofsky score of 70 or better was an inclusion criterion, thus selecting for individuals who were less likely to have neurological abnormalities. In addition, sites were encouraged to recruit participants who they thought would be compliant and adhere to study procedures, potentially leading to a bias toward enrolling a healthier study population with less neurological impairment.
Improvement in neuropsychological test performance was seen across the study. In part, this could be due to practice or learning effects, but several arguments can be made that the improvements were not solely or substantially due to practice effect. We included timed gait, a test that consists of walking. Walking is an overlearned task that is resistant to practice or learning effects. Timed gait improved over the study, consistent with ART-associated improvement. In addition, practice and learning effects likely had little effect on neurological examination findings, and these improved over the course of the study. However, clinicians were not blinded to the antiretroviral treatment arms, ratings, which could have biased the neurological assessments. Although there were no direct correlations between neuropsychological or neurological outcomes and higher CD4+ cell counts or lower plasma HIV RNA, these systemic disease markers improved over the study as did neurological and neuropsychological functioning.
HIV-associated cognitive impairment has substantial impact on even the simplest activities of daily living. The burden of neurological disease on families and communities is substantial, with loss of productivity and income for the diagnosed, but also for those who take the primary responsibility as caretakers. In resource-limited settings with high rates of HIV infection, the toll has been devastating [24–26]. This study suggests that successful antiretroviral therapy will improve neuropsychological functioning and reduce overall neurological abnormality in naive HIV-positive participants and is an important reason for increasing and sustaining access to therapy in resource-limited settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The content is solely the responsibility of the above authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID), the National Institutes of Mental Health (NIMH), the National Institutes of Health (NIH), or the institutions with which the authors are affiliated.
Financial support. The project described was supported by the National Institute of Mental Health, and the AIDS Clinical Trials Group (ACTG) funded by The NIAID Award number U01AI068636, General Clinical Research Center (GCRC) funded by the National Center for Research Resources, and Statistical and Data Analysis Center (SDAC) grant number AI-068634. C. M., R. A., K. S., A. L. R., B. B., B. S., C. M., C. H., R. W. P., S. T., J. H., and I. S. received NIH grant support from ACTG U01AI068636.
Dianne Rausch, PhD, and Pim Brouwers, PhD, Center for Mental Health Research on AIDS, National Institute of Mental Health.
Thomas Campbell, MD, grant support from NIAID ACTU AI069450.
Deise Vieira, MD, and Marcus Tulius T. Silva MD–PhD-IPEC- FIOCRUZ (Site 12101) CTU grant number AI69476.
Umesh Lalloo, MD, FRCP, and Rosie Mngqibisa, MB ChB—Durban Adult HIV CRS (Site 11201) CTU grant 5U01AI069426-03.
Nagalingeshwaran Kumarasamy, MBBS, PhD, and Jabin Sharma—YRGCARE Medical Centre (Site 11701) CTU grant number AI069432.
Virginia M. Kayoyo and Franklin D. Kilembe, MPh—Franklin Kilembe University of North Carolina Project, Kamuzu Central Hospital, Lilongwe (Site 12001) CTU grant number AI069518.
Mauleen Waison and Rachel Mahachi—Parirenyatwa CRS (Site 30313) CTU grant number BRS-ACURE-Q-08-00173- TOOI-OOO.
Cynthia Firnhaber, MD, Sharla Faesen, and Daphne S. Radebe, BA—Wits HIV Clinical Research Site (Helen Joseph Hosp) (Site 11101) CTU grant AI069463; BRS-ACURE-Q-07-00143 T006.
Thira Sirisanthana, MD, and Daralak Tavornprasit—Research Institute for Health Sciences-Chiang Mai University (Site 11501) CTU grant numbers AI069399 and AACTG.27.5199.06.
Maria Siliprandi, MD, and Renata Londero, MD—Hospital Nossa Senhora da Conceicao CRS (Site 12201) CTU grant number 5 U01 AI069401.
Anjali A. Joglekar, MBBS, and Srikanth Prasad Tripathy, MD, MBBS—NARI Pune CRS (Site 11601) CTU grant number 5U01AI069417-03.
Ben Kalonga and Henry Chamba—College of Medicine-Johns Hopkins Project (Site 30301) CTU grant number U01A1069518.
Carlos Mosquera, MD, and Rosa Infante, MD-—INMENSA-Lince CRS (Site 11302) CTU grant numbers 5U01 AI069438-03 and BRS- ACURE-Q-07-00141-T001-001.
Jorge Sanchez, MD, MPH, and Juan Carlos Hurtado, MD—Asociación Civil Impacta Salud y Educación (Site 11301) CTU grant numbers AI069438 and BRS-ACURE-Q-08-00007-T-002.
Manisha V. Ghate, MBBS, DCH, and Madhura Nene, MBBS—NARI-NIV Clinic (Site 11603) CTU grant number 5U01AI069417-03.
Dr Raman Gnagakhedkar and Usha Katti, MBBS—Dr Kotnis Dispensary, NARI (Site 11602) CTU grant number 5U01AI069417-03.
Dr Scott R Evans and Hongyu Jiang were funded in part by the Statistical and Data Management Center of the Adult AIDS Clinical Trials Group grant 1 U01 068634.
Potential conflicts of interest. K. R. R. has been a consultant for GSK, Abbott, and Tibotec. R. W. P. is involved in an investigator-initiated grant funding for Merck and honorarium for a lecture for Abbott Laboratories. R. M. is a consultant for Gilead. Silvia Montano is an employee of the US government. This work was prepared as part of her official duties. Title 17 U.S.C. $105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 USC $101 defines a US government work as a work prepared by a military service member or employee of the US government as part of that person's official duties. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global summary of the HIV/AIDS epidemic, December 2003. Geneva Switzerland: World Health Organization; December 2003. [Google Scholar]
- 2.Price RW, Spudich SJ. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis. 2008;197(Supp 3):S294–306. doi: 10.1086/533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Neurology. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a working group of the American academy of neurology AIDS task force. Neurology. 1991;41:778–85. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- 5.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–24. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 6.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: neuropathology. Ann Neurol. 1986;19:525–35. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 7.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort study. Neurology. 1993;43:2245–52. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 8.Robertson KR, Hall CD. HIV related mild cognitive impairment and AIDS dementia complex. Semin Neurol. 1992;12:18–27. doi: 10.1055/s-2008-1041153. [DOI] [PubMed] [Google Scholar]
- 9.Elder GA, Sever JL. AIDS and neurological disorders: an overview. Ann Neurol. 1988;23:S4–S6. doi: 10.1002/ana.410230705. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents, recommendations for a public health approach: 2010 revision. 2010 [PubMed] [Google Scholar]
- 11.Price RW, Sidtis JJ. Evaluation of the AIDS dementia complex in clinical trials. J AIDS. 1990;3(Supp 2):S51–60. [PubMed] [Google Scholar]
- 12.Sidtis JJ, Gatsonis C, Price RW, et al. Zidovudine treatment of the AIDS dementia complex: results of a placebo controlled trial. AIDS Clinical Trials Group. Ann Neurol. 1993;33:343–9. doi: 10.1002/ana.410330403. [DOI] [PubMed] [Google Scholar]
- 13.Letendre S 17th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA. Revised CNS penetration-effectiveness ranks. 2010 [Google Scholar]
- 14.Robertson K, Kumwenda J, Supparatpinyo K, et al. he AIDS Clinical Trials Group. A multinational study of neurological performance in antiretroviral therapy-naive HIV-1-infected persons in diverse resource-constrained settings. J Neurovirol. 2011 doi: 10.1007/s13365-011-0044-3. Jul 23. [Epub ahead of print] PMID:21786076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gendleman H, Zheng J, Coulter C, et al. Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in HIV-associated dementia. J Infect Dis. 1998;178:1000–7. doi: 10.1086/515693. [DOI] [PubMed] [Google Scholar]
- 16.McArthur JC, Haughey N, Gartner S, et al. HIV associated dementia: a evolving disease. J Neurovirol. 2003;9:205–21. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 17.Robertson K, Robertson W, Ford S, Watson D, Fiscus S, Harp A, Hall C. Highly active antiretroviral therapy improves neurocognitive function. J AIDS. 2004;36:562–66. doi: 10.1097/00126334-200405010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Sacktor N, McDermott MP, Marder K, et al. HIV-1 associated cognitive impairment before and after the advent of combination therapy. J. Neurovirol. 2002;8:136–42. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 19.Maj M, Satz P, Janssen R, et al. WHO Neuropsychiatric AIDS study, cross-sectional phase II. Neuropsychological and neurological findings. Arch Gen Psyc. 1994;51:51–61. doi: 10.1001/archpsyc.1994.03950010051007. [DOI] [PubMed] [Google Scholar]
- 20.Sacktor N, Nakasujja N, Skolasky RL, et al. HIV subtype D is associated with dementia, compared with subtype A, in immunosuppressed individuals at risk of cognitive impairment in Kampala, Uganda. Clin Infect Dis. 2009;49:780–6. doi: 10.1086/605284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jowi JO, Mativo PM, Musoke SS. Clinical and laboratory characteristics of hospitalised patients with neurological manifestations of HIV/AIDS at the Nairobi hospital. East Afr Med J. 2007;84:67–76. doi: 10.4314/eamj.v84i2.9506. [DOI] [PubMed] [Google Scholar]
- 22.Mielke J. Neurological complications of human immunodeficiency virus infection in Zimbabwe—2005. J Neurovirol. 2005;11(Suppl 3):23–5. doi: 10.1080/13550280500511766. [DOI] [PubMed] [Google Scholar]
- 23.Parry O, Mielke J, Latif AS, Ray S, Levy LF, Siziya S. Peripheral neuropathy in individuals with HIV infection in Zimbabwe. Acta Neurol Scand. 1997;96:218–22. doi: 10.1111/j.1600-0404.1997.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 24.Robertson K, Liner J, Hakim J, et al. NeuroAIDS in Africa Conference Participants. NeuroAIDS in Africa. J Neurovirol. 2010;16:189–202. doi: 10.3109/13550284.2010.489597. Review. PMID: 20500018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson K, Liner J, Heaton R. Neuropsychological assessment of HIV-infected populations in international settings. Neuropsychol Rev. 2009;19:232–49. doi: 10.1007/s11065-009-9096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright EJ, Nunn M, Joseph J, Robertson K, Lal L, Brew BJ. NeuroAIDS in the Asia pacific region. J Neurovirol. 2008;14:465–73. doi: 10.1080/13550280802235932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.