This study suggests that for older patients hospitalized with pneumonia, use prior to hospitalization of statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers may be potentially beneficial. In addition, continuing these medications during hospitalization may also be potentially beneficial for patients with pneumonia.
Abstract
Background. Studies suggest that statins and angiotensin-converting enzyme (ACE) inhibitors might be beneficial for the treatment of infections. Our purpose was to examine the association of statin, ACE inhibitor, and angiotensin II receptor blocker (ARB) use with pneumonia-related outcomes.
Methods. We conducted a retrospective cohort study using Department of Veterans Affairs data of patients aged ≥65 years hospitalized with pneumonia. We performed propensity-score matching for 3 medication classes simultaneously.
Results. Of 50 119 potentially eligible patients, we matched 11 498 cases with 11 498 controls. Mortality at 30 days was 13%; 34% used statins, 30% ACE inhibitors, and 4% ARBs. In adjusted models, prior statin use was associated with decreased mortality (odds ratio [OR], 0.74; 95% confidence interval [CI], .68–.82) and mechanical ventilation (OR, 0.81; 95% CI, .70–.94), and inpatient use with decreased mortality (OR, 0.68; 95% CI, .59–.78) and mechanical ventilation (OR, 0.68; 95% CI, .60–.90). Prior (OR, 0.88; 95% CI, .80–.97) and inpatient (OR, 0.58; 95% CI, .48–.69) ACE inhibitor use was associated with decreased mortality. Prior (OR, 0.73; 95% CI, .58–.92) and inpatient ARB use (OR, 0.47; 95% CI, .30–.72) was only associated with decreased mortality. Use of all 3 medications was associated with reduced length of stay.
Conclusions. Statins, and to a lesser extent ACE inhibitors and ARBs, are associated with improved pneumonia-related outcomes. Prospective cohort and randomized controlled trials are needed to examine potential mechanisms of action and whether acute initiation at the time of presentation with these infections is beneficial.
Pneumonia and influenza together are the eighth leading cause of death and the leading causes of infectious death in the United States [1]. Despite decreases in hospitalizations and deaths due to pneumonia in all other age groups, for those aged ≥65 years the rates of hospitalizations and deaths due to pneumonia are stable [2]. Despite this, only a few new classes of antibiotics have been added to the armamentarium for treating pneumonia in the last 20 years.
Recent studies have demonstrated that HMG-CoA reductase inhibitors (“statins”) and angiotensin-converting enzyme (ACE) inhibitors have significant immunomodulatory effects and reduce systemic cytokine levels [3, 4]. Cytokines play an important role in host defense mechanisms for patients with pneumonia but under certain conditions may lead to septic shock or acute respiratory distress syndrome [5]. Studies evaluating the impact of these medications on outcomes for those with clinical infections have given conflicting results. Several studies have demonstrated that for patients hospitalized with bacteremia, diabetic lower extremity infections, or community-acquired pneumonia, statin or ACE inhibitor use was associated with decreased odds of death or decreased rates of severe sepsis [6, 7]. Conversely, other studies have shown either no association with mortality or increased mortality with the use of these medications in those with active infections [7, 8].
Although several studies have examined these issues [7–12], no definitive randomized clinical trials are available that provide high-level evidence regarding the proposed protective effects of these agents. Prior studies including our own were limited by examining use at single sites, in highly selected populations, having low rates of medication use, and/or not examining statins and ACE inhibitors/angiotensin II receptor blockers (ARBs) in the same models.
Therefore, the aims of our study were to assess the associations of the prior and inpatient use of statins, ARBs, and ACE inhibitors on important clinical outcomes, including mortality, length of hospital stay, and need for mechanical ventilation, for patients aged >65 years hospitalized with pneumonia using the extensive administrative databases of the Department of Veterans Affairs (VA).
METHODS
For this retrospective, population-based study we used data from the VA Health Care System administrative databases. These databases are the repositories of clinical data from more than 150 VA hospitals and 850 outpatient clinics. The Institutional Review Board of the University of Texas Health Science Center at San Antonio approved this study under expedited review.
Inclusion Criteria
Patients included in this study:
Were hospitalized during fiscal years 2002–2007 (October 2001–September 2007).
Had a previously validated discharge diagnosis of pneumonia with either a primary discharge diagnosis of pneumonia/influenza (International Classification of Diseases, Ninth Revision [ICD-9] codes 480.0–483.99 or 485–487) [13] or a secondary discharge diagnosis with a primary diagnosis of respiratory failure (ICD-9 code 518.81) or sepsis (ICD-9 code 038.xx).
Were age 65 or older on the date of admission.
Had at least one VA outpatient clinic visit in the year preceding the index admission.
Received at least one active and filled outpatient medication from a VA pharmacy within 90 days of admission.
Received at least one dose of antimicrobial therapy within the first 48 hours of admission.
If a patient was admitted more than once during the study period, only the first hospitalization was included.
Data
We used inpatient and outpatient demographic, utilization, and comorbidity data from the National Patient Care Database, pharmacy data from the Decision Support System National Data Extracts and Pharmacy Benefits Management, and vital status information from the vital status file, which incorporates data from veterans’ death benefits claims, inpatient deaths, Medicare vital status files, and the Social Security Administration's death master file. Encrypted patient identifiers linked information across these databases.
Race and ethnicity categories included white, black, Hispanic, and other/unknown. To infer current tobacco use and/or smoking cessation efforts, we identified ICD-9 codes for tobacco use (305.1, V15.82), smoking cessation clinic use, and/or use of medications for the treatment of nicotine dependence (Zyban, nicotine replacement, or varenicline). Alcohol abuse was defined using ICD-9 codes 291, 303, and 305.0, and illicit drug use using ICD-9 codes 292, 304, and 305 (excluding 305.0–305.1). We used the Charlson-Deyo comorbidity methodology to classify other preexisting comorbid conditions [14].
Patients were considered a current user of a given medication if they had a supply of a given medication to last until the date of hospitalization assuming an 80% compliance rate. To further control for potential confounding by medications, a count of unique drugs in each of the following classes was calculated for drugs filled within 90 days of presentation: cardiac (excluding statins, ACE inhibitors, ARBs), pulmonary, and diabetic medications. In addition, a dichotomized variable was created to identify those with intravenous or oral corticosteroid use.
Definition of Exposures
Medications classified as statins were atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, and simvastatin. Medications classified as ACE inhibitors were benazepril, captopril, enalapril, fosinopril, lisinopril, moexipril, quinapril, and ramipril. Medications classified as ARBs were candesartan, irbesartan, valsartan, losartan, telmisartan, eprosartan, and olmesartan. We created dichotomous variables to identify prior use of statins, ACE inhibitors, and ARBs, which we defined as a filled prescription for the medication of interest within 90 days of presentation, with a sufficient supply to overlap the date of admission, assuming 80% compliance. We also created individual dichotomous indicators of inpatient use for each of these medication classes, in which we defined a patient as exposed if the patient received that medication for at least the first 48 hours after admission. For all analyses where we examined inpatient use, we restricted our analyses to only those who were taking one or more other oral medications during the first 48 hours of admission. Finally, to examine the effect of dose of the most common statin (simvastatin) and ACE inhibitor (lisinopril) on the outcomes of interest, we categorized the average dose over the last 90 days by 20-mg increments.
Outcomes
We used 30-day all-cause mortality as the primary outcome for this study. Previous research has demonstrated that 30-day mortality is primarily due to the pneumonia [15]. Mortality was assessed through 1 October 2007 using the VA vital status file, which has been demonstrated to have a sensitivity of approximately 98% [16]. Secondary outcomes were use of invasive mechanical ventilation, length of hospital stay, and vasopressor use (for statins only).
Statistical Analyses
Propensity Score Creation and Matching
We attempted to address the potential problem of confounding by indication by creating separate propensity scores for the probability of being on a statin, ACE inhibitor, or ARB. Propensity scores were generated using logistic regression models with prior statin, ACE inhibitor, or ARB use at admission as the dependent variable. We selected potential confounder variables and prognostic variables from lists of candidate variables available in the VA administrative database. Our selection was based on candidate variables (Table 1) identified in the pneumonia literature that have been demonstrated to be associated with our outcomes or the use of the medications of interest.
Table 1.
Comparison of Patients and Controls Hospitalized With Pneumonia
| Variable | Patients (n = 11 498) | Controls (n = 11 498) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, years, mean (SD) | 74.8 (6.5) | 74.8 (6.8) | .77 |
| Sex, male | 11 291 (98.2) | 11 291 (98.2) | .77 |
| Race | |||
| White | 9693 (84.3) | 9612 (83.6) | .17 |
| Black | 1219 (10.6) | 1253 (10.9) | .43 |
| Hispanic | 701 (6.1) | 701 (6.1) | .89 |
| Other | 586 (5.1) | 621 (5.4) | .25 |
| Married | 6462 (56.2) | 6186 (53.8) | <.001 |
| Characteristics of hospitalization | |||
| ICU admission | 1518 (13.2) | 1644 (14.3) | .009 |
| Comorbid conditions | |||
| Current tobacco use | 4381 (38.1) | 4392 (38.2) | .95 |
| Alcohol abuse | 414 (3.6) | 414 (3.6) | 1.000 |
| Illicit drug abuse | 103 (0.9) | 138 (1.2) | .02 |
| Myocardial infarction | 839 (7.3) | 828 (7.2) | .76 |
| Congestive heart failure | 3127 (27.2) | 3081 (26.8) | .48 |
| Peripheral vascular disease | 1886 (16.4) | 1794 (15.6) | .09 |
| Chronic obstructivepulmonary disease | 6358 (55.3) | 6324 (55.0) | .61 |
| Rheumatologic disease | 345 (3.0) | 322 (2.8) | .32 |
| Mild liver disease | 34 (0.3) | 34 (0.3) | .80 |
| Dementia | 540 (4.7) | 506 (4.4) | .28 |
| Diabetes | 3748 (32.6) | 3783 (32.9) | .68 |
| Diabetes withcomplications | 1161 (10.1) | 1115 (9.7) | .33 |
| Moderate liver disease | 80 (0.7) | 69 (0.6) | .80 |
| Hemiplegia | 184 (1.6) | 149 (1.3) | .14 |
| Renal disease | 1495 (13.0) | 1506 (13.1) | .92 |
| Any prior malignancy | 2794 (24.3) | 2783 (24.2) | .94 |
| Metastatic solid tumor | 414 (3.6) | 379 (3.3) | .20 |
| AIDS | 23 (0.2) | 23 (0.2) | .87 |
| Other medications | |||
| Cardiovascular, mean (SD) | 1.95 (1.5) | 1.93 (1.6) | .26 |
| Respiratory, mean (SD) | 1.47 (2.0) | 1.45 (2.0) | .50 |
| Diabetic, mean (SD) | 0.33 (0.65) | 0.31 (0.6) | .02 |
| Corticosteroids | 2990 (26.0) | 2975 (25.8) | .82 |
Data are No. (%) unless otherwise specified.
Abbreviations: ICU, intensive care unit; SD, standard deviation.
We then used a multivariate matching procedure based on a Mahalanobis scoring algorithm to match cases (eg, a patient who received any combination of statins, ACE inhibitors, and/or ARBs) and controls simultaneously based on the logits of the 3 propensity scores. The Mahapick procedure was used to match the cases and controls on the logits of all 3 propensity scores simultaneously. Matched pairs were created and those with Mahalanobis scores >0.5 units were removed. Two-group t tests for mean comparisons and 2-group tests of differences in proportions were used to compare patients who received one or more of the medications of interest with their respective matched controls on all the potential confounders and prognostic variables.
Clustering of Sites
Because we planned to enter site of hospitalization as a fixed effect in our regression models, we used cluster analysis to reduce the number of inpatient sites. We based clustering on the number of medical/surgical intensive care units, cardiovascular units, presence of an emergency department, inpatient days per year, outpatient visits per year, approved resident training programs, and medical school affiliation. A hierarchical agglomerative clustering algorithm based on the Lance-Wallace flexible beta method was used to identify the number of clusters. Five dummy-coded variables representing a VA inpatient site's membership in a cluster were entered as fixed effects in all regression models. Clustering routines were developed by Kaufman and Rousseeuw [17].
Regression Models
Generalized linear models (GLMs) were used to evaluate the relationship of prior or inpatient use of statins, ACE inhibitors, and ARBs on the outcomes of interest. A logit link function was used for the binary endpoints and a Poisson function for length of stay. For the length of stay analyses we excluded those who died during the hospitalization. All analyses were based (clustered) on matched pairs of patients based on prior statin, ACE inhibitor, and/or ARB use, and adjusted for factors that prior literature suggests may be potentially associated with the outcomes, including sociodemographic variables, and receipt of guideline concordant antibiotics [18], comorbid conditions, and other medications.
Statistical significance was defined as a 2-tailed P value of ≤.05. Stata software, version 11.0 (StataCorp, College Station, Texas) was used for all analyses.
RESULTS
Baseline Characteristics
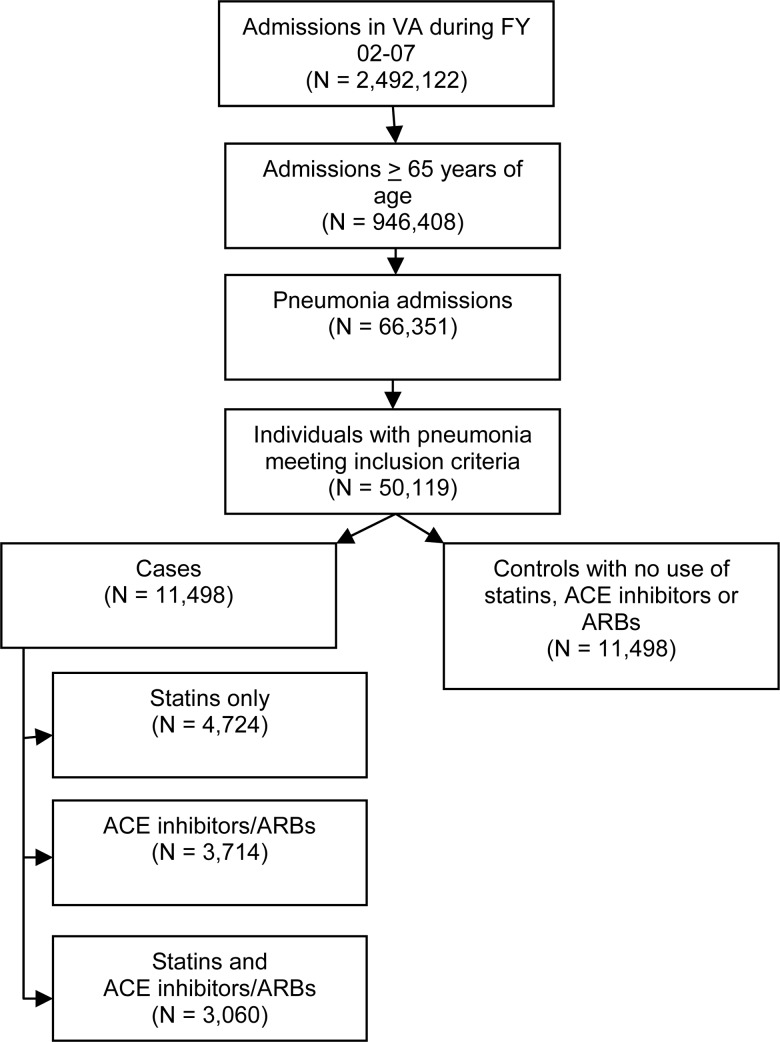
Our final analytic cohort was composed of 22 996 patients; 11 498 cases and 11 498 matched controls of the 50 119 patients who met the study criteria (Figure 1). Patients' mean age was 74.8 years (SD, 6.7 years), 55% of patients were married, and 98.2% were male. Overall, 12.9% of the patients died within 30 days of presentation. In our analytic cohort, 33.8% (n = 7763) were on statins, 29.5% (n = 6774) were on ACE inhibitors, and 3.7% (n = 839) were on ARBs. There were 3318 patients on ACE inhibitors only, 396 on ARBs only, 4724 on statins only, and 3060 who received statins along with ACE inhibitors and/or ARBs. Table 1 shows the balance between key variables after propensity matching. Although several variables reached statistical significance, there were no clinically significant differences after propensity matching. Table 2 demonstrates variable balance by statin, ACE inhibitor/ARB use, statin in addition to ACE inhibitor/ARB use, and nonuse.
Figure 1.
Flowchart for selection of cases and controls. Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.
Table 2.
Characteristics of Subjects (n = 22 996) Hospitalized With Pneumonia by Medication Use Prior to Hospitalization
| Variables | Statins (n = 7763) | ACE Inhibitors/ARBs (n = 6774) | Statins/ACE Inhibitors/ARBs (n = 3039) | No Statins/ACE Inhibitors/ARBs (n = 11 498) |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, mean (SD) | 74.4 (6.3) | 75.0 (6.6) | 74.0 (6.2) | 74.8 (6.8) |
| Male sex | 7623 (98.2) | 6666 (98.4) | 2999 (98.7) | 11291 (98.2) |
| Race | ||||
| White | 6637(85.5) | 5643 (83.3) | 2592 (85.3) | 9612 (83.6) |
| Black | 745 (9.6) | 772 (11.4) | 298 (9.8) | 1253 (10.9) |
| Hispanic | 450 (5.8) | 440 (6.5) | 185 (6.1) | 7014 (6.1) |
| Other | 380 (4.9) | 359 (5.3) | 149 (4.9) | 621 (5.4) |
| Married | 4495 (57.9) | 3760 (55.5) | 1784 (58.7) | 6186 (53.8) |
| Characteristics of hospitalization | ||||
| ICU admission | 970 (12.5) | 935 (13.8) | 389 (12.8) | 1644 (14.3) |
| Comorbid conditions | ||||
| Current tobacco use | 3028 (39.0) | 2493 (36.8) | 1152 (37.4) | 4392 (38.2) |
| Alcohol abuse | 225 (2.9) | 264 (3.9) | 85 (2.8) | 414 (3.6) |
| Illicit drug abuse | 54 (0.7) | 68 (1.0) | 18 (0.6) | 138 (1.2) |
| Myocardial infarction | 660 (8.5) | 447 (6.6) | 270 (8.9) | 828 (7.2) |
| Congestive heart failure | 2049 (26.4) | 2032 (30.0) | 960 (31.6) | 3081 (26.8) |
| Peripheral vascular disease | 1359 (17.5) | 1084 (16.0) | 556 (18.3) | 1794 (15.6) |
| COPD | 4308 (55.5) | 3651 (53.9) | 1602 (52.7) | 6324 (55.0) |
| Rheumatologic disease | 210 (2.7) | 203 (3.0) | 70 (2.3) | 322 (2.8) |
| Mild liver disease | 16 (0.2) | 20 (0.3) | 3 (0.1) | 34 (0.3) |
| Dementia | 342 (4.4) | 312 (4.6) | 115 (3.8) | 506 (4.4) |
| Diabetes | 2593 (33.4) | 2466 (36.4) | 1304 (42.9) | 3783 (32.9) |
| Diabetes with complications | 792 (10.2) | 779 (11.5) | 413 (13.6) | 1115 (9.7) |
| Moderate liver disease | 31 (0.4) | 61 (0.9) | 12 (0.4) | 69 (0.6) |
| Hemiplegia | 124 (1.6) | 102 (1.5) | 46 (1.5) | 149 (1.3) |
| Renal disease | 1071 (13.8) | 854 (12.6) | 422 (13.9) | 1506 (13.1) |
| Any prior malignancy | 1863 (24.0) | 1585 (23.4) | 656 (21.6) | 2783 (24.2) |
| Metastatic solid tumor | 272 (3.5) | 230 (3.4) | 88 (2.9) | 379 (3.3) |
| AIDS | 16 (0.2) | 7 (0.1) | 3 (0.1) | 23 (0.2) |
| Other medications by class | ||||
| Cardiovascular, mean (SD) | 2.0 (1.5) | 2.0 (1.5) | 2.3 (1.5) | 1.9 (1.6) |
| Respiratory, mean (SD) | 1.50 (2.0) | 1.40 (2.0) | 1.4 (2.0) | 1.5 (2.0) |
| Diabetic, mean (SD) | 0.34 (0.7) | 0.38 (0.7) | 0.48 (0.8) | 0.31 (0.6) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; SD, standard deviation.
Results of GLM Regression Models
Table 3 illustrates the association between statin, ACE inhibitor, and ARB use and the outcomes measured. Prior statin use (odds ratio [OR], 0.74; 95% confidence interval [CI], .68–.82) was significantly associated with decreased 30-day mortality, decreased mechanical ventilation (OR, 0.81; 95% CI, .70–.94), and decreased length of stay (incident rate ratio [IRR], 0.79; 95% CI, .73–.86). Continued use of statins was also associated with decreased 30-day mortality (OR, 0.68; 95% CI, .59–.78), decreased mechanical ventilation (OR, 0.68; 95% CI, .60–.90), and decreased length of stay (IRR, 0.77; 95% CI; .68–.86). Neither prior use nor continued use of statins was associated with vasopressor use. For those who were receiving the most common statin, simvastatin, there were significant associations between prior outpatient dose and 30-day mortality: 1–19 mg/day (OR, 0.78; 95% CI, .69–.90), 20–39 mg/day (OR, 0.75; 95% CI, .64–.88), 40–59 mg/day (OR, 0.75; 95% CI, .64–.88), and 60–80 mg/day (OR, 0.63; 95% CI, .49–.80).
Table 3.
Results of Generalized Linear Models Examining the Association of Statin, Angiotensin-Converting Enzyme Inhibitor, and Angiotensin II Receptor Blocker Use on Outcomes
| Variable | 30-Day Mortality, OR (95% CI) | Mechanical Ventilation, OR (95% CI) | Vasopressor Use, OR (95% CI) | Length of Stay, IRR (95% CI) |
|---|---|---|---|---|
| Statin | ||||
| Prior use | 0.74 (.68–.82) | 0.81 (.70–.94) | 1.17 (.94–1.45) | 0.79 (.73–.86) |
| Inpatient use | 0.68 (.59–.78) | 0.68 (.60–.90) | 0.83 (.65–1.03) | 0.77 (.68–.86) |
| ACE inhibitor | ||||
| Prior use | 0.88 (.80–.97) | 1.04 (.89–1.21) | … | 0.90 (.83–.99) |
| Inpatient use | 0.58 (.48–.69) | 0.92 (.72–1.18) | … | 0.69 (.60–.79) |
| ARBs | ||||
| Prior use | 0.73 (.58–.92) | 1.39 (1.00–1.93) | … | 0.78 (.63–.97) |
| Inpatient use | 0.47 (.30–.72) | 0.96 (.50–1.82) | … | 0.51 (.34–.77) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CI, confidence interval; IRR, incident rate ratio; OR, odds ratio.
Prior ACE inhibitor use was associated with decreased 30-day mortality (OR, 0.88; 95% CI, .80–.97), and decreased length of stay (IRR, 0.90; 95% CI, .83–.99). Continued ACE inhibitor use was also associated with decreased 30-day mortality (OR,0.58; 95% CI, .48–.69), and decreased length of stay (IRR, 0.69; 95% CI, .60–.79). However, neither prior nor continued ACE inhibitors use was associated with need of mechanical ventilation. For those who were receiving the most common ACE inhibitor, lisinopril, we found no significant association for those receiving 1–19 mg/day (OR, 0.93; 95% CI, .83–1.05), whereas there were significant associations for those receiving 20–39 mg/day (OR,0.80; 95% CI, .68–.94), and 40–80 mg/day (OR,0.77; 95% CI, .65–.92).
In the case of ARBs, prior and continued use were associated with decreased 30-day mortality (OR, 0.73; 95% CI, .58–.92 and OR, 0.47; 95% CI, .30–.72, respectively). Both prior and continued ARBs use were also associated with decreased length of stay (IRR, 0.78; 95% CI, .63–.97 and IRR, 0.51; 95% CI, .34–.77, respectively). However, ARB use was not associated with need of mechanical ventilation.
Secondary Analyses
When we restricted our analysis of 30-day mortality to only those who had no prior diagnosis of cardiovascular disease (n = 9722), findings were similar to those for statins (OR, 0.67; 95% CI, .58–.77) and ARBs (OR, 0.61; 95% CI, .38–.96), but not ACE inhibitors (OR, 0.97; 95% CI, .84–1.12), still significantly associated with decreased 30-day mortality. When we included those with a prior diagnosis of chronic obstructive pulmonary diseases (n = 12 677), statins (OR, 0.78; 95% CI, .69–.88) and ACE inhibitors (OR, 0.87; 95% CI, .77–.996), but not ARBs (OR, 0.84; 95% CI, .62–1.14), were still associated with 30-day mortality.
DISCUSSION
Our results suggest that the receipt of statins and ARBs, and to a lesser extent ACE inhibitors, was associated with improved outcomes, notably 30-day mortality and length of stay, for patients aged ≥65 years hospitalized with pneumonia. Although statins were consistently associated with improved outcomes, our results for ARBs were much less clear, potentially owing to the much smaller number of patients on ARBs. Our findings suggest that prior statin use is associated with a 37% reduction in mortality, 21% reduction in length of stay, and 29% reduction in mechanical ventilation use. Further studies, including randomized controlled trials, are needed to examine the impact of statins, ACE inhibitors and ARBs, both prehospitalization and when initiated on presentation, for patients hospitalized with pneumonia, yet the rigorous approach employed in the current study may constitute the “next best” evidence reported to date.
The present study supports the findings of previous studies [6] demonstrating decreased 30-day mortality in patients hospitalized with pneumonia who receive statins prior to admission. The first published paper examining the effects of statin use on infectious disease–related outcomes, was by Liappis et al [7]. Their study included 388 patients hospitalized with bacteremia, and they found a protective effect of in-hospital statin use on in-hospital mortality (6% vs 28%, P < .002). After adjusting for confounding factors, not being on a statin (OR, 7.6; 95% CI, 1.01–57.5) was associated with increased mortality. Since then, other studies have shown decreased in-hospital or 30-day mortality associated with statin use in patients hospitalized with serious infections [6].
The results of the present study do not support the study by Majumdar et al [8], who concluded that statins were not associated with improved pneumonia-related outcomes. Although their study was based on a prospective cohort of 3415 subjects hospitalized with pneumonia, it contained several limitations, including a questionable composite outcome of in-hospital mortality and intensive care unit admission, and very low rates of statin use in key populations as compared with other studies of statin and infections. Paradoxically increasing age and prior history of coronary artery disease were associated with decreased mortality.
Although little research has been done on potential mechanisms of ACE inhibitors or ARBs on reducing pneumonia-related mortality [4], a substantial body of research suggests that the cardiovascular benefits of statins go beyond just lowering cholesterol [19]. Studies suggest that a secondary mechanism of decreasing atherosclerosis is by decreasing inflammatory responses, which are also triggered in pneumonia [20]. An appropriate inflammatory response is critical to human health, so that pathogens are contained and eliminated without causing excessive damage. Inflammation is mediated by proinflammatory cytokines such as interleukin 1, interleukin 6, and tumor necrosis factor α, and anti-inflammatory cytokines such as interleukin 10 and transforming growth factor β. These cytokines are necessary for proper function of the immune system, but the inappropriate elevated expression of proinflammatory cytokines can result in sepsis, tissue destruction, or death [21]. Studies have documented changes in cytokine release following treatment with statins in those with sepsis [22].
There are several additional pathways through which statins may affect the body including protecting against cardiovascular disease, changes in antioxidant activities, coagulation, cellular apoptosis, inflammatory cell signaling, leukocyte-endothelial cell adhesion, and nitric oxide balance [23]. In addition, statins have been shown to have antibacterial or antifungal effects [24, 25] and also to affect microvascular permeability [26]. Statins have been shown to affect the body in many ways, yet it is unclear if any of these processes are responsible for the improved outcomes we have seen with statin use.
Our study has several limitations. Although our study was a large database analysis and subject to recognized limitations of such studies, we carefully assembled our cohort from complete patient discharge data to avoid ascertainment bias. Our sample was predominantly men owing to our use of VA administrative data, and further research is needed to examine these issues in women. In addition, we only examined elderly patients who were ≥65 years of age so as to have a relatively homogenous cohort that would be potentially exposed to the medications of interest. Future studies should also examine other age groups. In addition, our study cannot examine the association of these medications for those who are not hospitalized with pneumonia. We are also unable to examine specific bacterial or viral pathogens because of our use of administrative data. Most importantly, as an observational study we can only report associations, and cannot prove that use of statins, ACE inhibitors, and ARBs cause improved outcomes for these patients. Nevertheless, we believe our matching procedures and analytic process strengthen the hypothesis that these medications are beneficial in elderly patients with pneumonia. Indeed, because patients receiving these medications have numerous medical conditions significantly associated with increased mortality, one would expect worse outcomes, especially since our analyses were adjusted for factors associated with patient frailty or healthy user bias.
In conclusion, our study demonstrates that prior and inpatient use of statins, and to a lesser extent ACE inhibitors and ARBs, are associated with improved outcomes for patients hospitalized with pneumonia. These results suggest that there may be additional benefits of these medications to the already compelling data for their use in patients with vascular disease, renal disease, and diabetes. Prospective cohort studies and randomized controlled trials are needed to determine if the chronic use or acute initiation of these medications will improve outcomes for patients with pneumonia and to elucidate the mechanism(s) by which they may work.
Notes
Financial support. This work was supported by the National Institute of Nursing Research (grant number R01NR010828). This material is the result of work supported with resources and the use of facilities at the South Texas Veterans Health Care System.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. Funding agencies had no role in conducting the study, or role in the preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kung HC, Hoyert DL, Xu JQ, Murphy SL. Centers for Disease Control and Prevention. Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2008. Deaths: final data for 2005. [PubMed] [Google Scholar]
- 2.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294:2712–9. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 3.Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–5. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 4.Gullestad L, Aukrust P, Ueland T, et al. Effect of high- versus low-dose angiotensin converting enzyme inhibition on cytokine levels in chronic heart failure. J Am Coll Cardiol. 1999;34:2061–7. doi: 10.1016/s0735-1097(99)00495-7. [DOI] [PubMed] [Google Scholar]
- 5.Bauer TT, Monton C, Torres A, et al. Comparison of systemic cytokine levels in patients with acute respiratory distress syndrome, severe pneumonia, and controls. Thorax. 2000;55:46–52. doi: 10.1136/thorax.55.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakashima B, Restrepo MI, Anzueto A, Mortensen EM. The potential role of statins in pneumonia. Curr Respir Med Rev. 2010;6:155–61. [Google Scholar]
- 7.Liappis AP, Kan VL, Rochester CG, Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–7. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 8.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novack V, Macfadyen J, Malhotra A, Almog Y, Glynn RJ, Ridker PM. The effect of rosuvastatin on incident pneumonia: results from the JUPITER trial. CMAJ. 2012;184:E367–72. doi: 10.1503/cmaj.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruger PS, Harward ML, Jones MA, et al. Continuation of statin therapy in patients with presumed infection: a randomized controlled trial. Am J Resp Crit Care Med. 2011;183:774–81. doi: 10.1164/rccm.201006-0955OC. [DOI] [PubMed] [Google Scholar]
- 11.Chalmers JD, Short PM, Mandal P, Akram AR, Hill AT. Statins in community acquired pneumonia: evidence from experimental and clinical studies. Respir Med. 2010;104:1081–91. doi: 10.1016/j.rmed.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima B, Restrepo MI, Anzueto A, Mortensen EM. The potential role of statins in pneumonia. Curr Respir Med Rev. 2010;6:1–7. [Google Scholar]
- 13.Meehan TP, Fine MJ, Krumholz HM, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278:2080–4. [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 2002;162:1059–64. doi: 10.1001/archinte.162.9.1059. [DOI] [PubMed] [Google Scholar]
- 16.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2–12. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman L, Rousseeuw P. Finding groups in data: an introduction to cluster analysis. New York: Wiley; 1990. [Google Scholar]
- 18.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenig W. Heart disease and the inflammatory response. BMJ. 2000;321:187–8. doi: 10.1136/bmj.321.7255.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 21.Nathan C. Points of control in inflammation. Nature. 2002;420:846. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 22.Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. 2000;294:1043–6. [PubMed] [Google Scholar]
- 23.Hothersall E, McSharry C, Thomson NC. Potential therapeutic role for statins in respiratory disease. Thorax. 2006;61:729–34. doi: 10.1136/thx.2005.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerwood S, Cohen J. Unexpected antimicrobial effect of statins. J Antimicrob Chemother. 2008;61:362–4. doi: 10.1093/jac/dkm496. [DOI] [PubMed] [Google Scholar]
- 25.Sun HY, Singh N. Antimicrobial and immunomodulatory attributes of statins: relevance in solid-organ transplant recipients. Clin Infect Dis. 2009;48:745–55. doi: 10.1086/597039. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Pendyala S, Natarajan V, Garcia JG, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol Lung Cell Mol Physiol. 2008;295:L575–83. doi: 10.1152/ajplung.00428.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]