Abstract
Chronic hepatitis C virus (HCV) infection has become a major threat to the survival of human immunodeficiency virus (HIV)–infected persons in areas where antiretroviral therapy is available. In coinfection, viral eradication has been difficult to attain, and HCV therapy is underused. Novel therapies may be particularly beneficial for this population, yet studies lag behind those for HCV monoinfection. Increasingly, incident HCV among HIV-infected men who have sex with men is associated with sexual risk behavior further research should be performed to refine understanding of the causal mechanism of this association. The phenomenon of aggressive hepatic fibrogenesis when HIV infection precedes HCV acquisition requires longer-term observation to ensure optimal timing of HCV therapy. Medical management in coinfection will be improved by enhancing HCV detection, with annual serologic testing, screening with HCV RNA to detect acute infection, and HIV testing of HCV-infected individuals; by addressing HCV earlier in coinfected persons; and by universal consideration for HCV therapy. HCV drug trials in individuals coinfected with HIV should be expedited. HIV/HCV coinfection remains a growing and evolving epidemic; new developments in therapeutics and improved care models offer promise.
Hepatitis C virus (HCV) infection is often prevalent among human immunodeficiency virus (HIV)–infected populations, with one-third of HIV-infected Americans, and 7 million worldwide being coinfected [1, 2]. Chronic HCV infection is now the leading cause of death, after AIDS-related complications, among HIV-infected individuals in areas where highly active antiretroviral therapy (HAART) is available [3]. HIV coinfection exacerbates HCV disease, increasing the likelihood of cirrhosis and HCV-related mortality [4, 5]. The biologic basis for this is incompletely understood but may be related to impaired T-cell responses to HCV, to HIV's effect on hepatic cells, and to amplified microbial translocation promoting hepatic fibrosis [6, 7]. Although achievement of sustained virologic response (SVR) reduces the risk of liver-related outcomes and deaths in coinfection [8], historically, HCV therapy has been underused in this patient group [9]. New oral therapies offer promise. This review focuses on emerging issues in HIV/HCV coinfection.
A CHANGING EPIDEMIOLOGY
The prevalence of HCV coinfection varies, depending on the mode of HIV transmission. The most efficient means of HCV transmission is percutaneous exposure to blood, with transmission efficiency 10 times higher for HCV than for HIV. The principal route of HCV spread is injection drug use (IDU); HCV coinfection rates often exceed 90% among HIV-infected individuals who use injection drugs [10, 11]. Increasingly, incident HCV among HIV-infected men who have sex with men (MSM) is associated with sexual risk behavior [12–16].
Over the past decade, reports from Europe, the United States, and Australia described increasing detection of HCV among HIV-infected MSM [17–19]. The majority of these individuals had well-controlled HIV infection and did not report IDU [20]. Incident HCV has also been detected among HIV-uninfected men, although less commonly. The few cases of HCV infection detected among HIV-uninfected MSM have been generally among those who have engaged in high-risk sexual activities with HIV-infected male partners [15, 20–22].
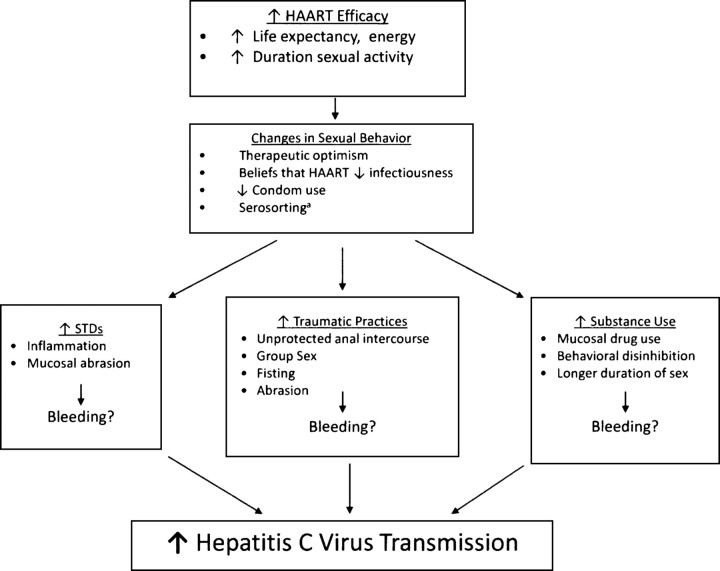
The reasons for the increased sexual transmission of HCV appear to be complex (Figure 1). Epidemiologic studies have identified HIV as an independent factor in HCV transmission and acquisition since the earliest days of the HIV epidemic [23]. HIV-infected individuals are less likely to spontaneously clear HCV, and their HCV RNA set point tends to be higher, making them more infectious to their partners than HCV-monoinfected individuals [24]. One study found that coinfected men were more likely than HIV-uninfected men to shed HCV RNA in semen [25], although this was not substantiated in other studies [26]. HIV-infected individuals may have compromised gastrointestinal mucosal barriers and may be more likely to have chronic inflammation, facilitating HCV transmission. HIV-infected MSM who engage in unprotected sex, often with partners who have the same HIV serostatus (termed serosorting), may be at increased risk for sexually transmitted diseases (STDs), which may upregulate HCV in the genital tract [27]. Multiple studies of the sexual transmission of HCV have found associations with STDs [15, 17–19, 21, 28].
Figure 1.
Biobehavioral factors associated with increased hepatitis C virus transmission among HIV-infected men who have sex with men. aDefined as the practice of engaging in unprotected sex with individuals who have the same human immunodeficiency virus serostatus.
The increased detection of sexually transmitted HCV infection since 1996 coincides with the expanded availability of HAART [29]. In addition to extending the lifespan of HIV-infected individuals, HIV-infected persons may perceive unprotected sex to be less risky for their partners because of their virologic control [30]. Serosorting would tend to concentrate the HCV epidemic among HIV-infected individuals. Over 50% of HIV-infected MSM in one San Francisco report described serosorting activity [31], which was corroborated in a United Kingdom study [32]. Serosorting has been associated with increased bacterial STDs [33], which could increase susceptibility to HCV infection.
Specific sexual practices may facilitate HCV transmission. In addition to unprotected anal intercourse, the manual insertion of digits in the rectum, known as “fisting,” and other traumatic sexual practices may potentiate the role of STDs in HCV transmission [34]. Mucosal sexual trauma is frequently associated with bleeding, impacting HCV transmission [35]. In some subpopulations of HIV-infected MSM, certain drugs, such as crystal methamphetamine, may be nasally or rectally administered. These drugs may enhance risk-taking behavior and may select for a group of individuals who are likely to engage in traumatic sexual practices [36, 37]. The use of these drugs may result in behavioral disinhibition and a decreased pain threshold, allowing sex to be longer and more intensive, resulting in mucosal bleeding and inflammation. Studies from the United States [38] and Germany [35] have identified the important role of the use of methamphetamines and other party drugs as predictors of HCV transmission. Exceedingly low transmission rates among HCV-discordant monogamous heterosexual couples followed prospectively over many years have established that HCV is not readily spread via heterosexual sexual transmission [39]. Supporting this is the fact that HCV infection does not follow the same heterosexual sexual transmission patterns as classic STDs, such as gonorrhea and chlamydial infection. However, certain behaviors, such as anal intercourse and group sex, could result in HCV transmission among heterosexuals.
One cross-sectional study of prevalent HCV antibody found that HIV-infected women were almost twice as likely as HIV-uninfected women to acquire HCV (adjusted odds ratio, 1.9; 95% confidence interval, 1.2–2.9 [40]). Among women reporting no history of IDU, sex with a male drug injector was independently associated with prevalent HCV antibody, suggesting that HIV-infected women may be more susceptible than HIV-uninfected women to sexually acquired HCV. It is possible that these women engaged in unreported unprotected anal intercourse associated with mucosal trauma and bleeding and that HIV infection increased their susceptibility to HCV infection. However, it is not feasible to designate sexual transmission as the cause of HCV infection because data were not collected about all blood exposures, such as sharing straws used to snort drugs, and IDU could have been underreported [14, 41].
Questions remain regarding the precise role of HIV infection in HCV sexual transmission. Although factors associated with sexually acquired HCV have been documented, further research is required to refine understanding of the causal mechanism of this association. It is important for clinicians to create a supportive environment for coinfected and at-risk patients so that questions regarding sexual risk behavior and prevention can be discussed.
HEPATIC FIBROSIS PROGRESSION: DOES THE ORDER OF HIV AND HCV ACQUISITION MAKE A DIFFERENCE?
One area of uncertainty is whether HCV-associated disease progresses more rapidly when HCV is acquired after established HIV infection, a factor that, once determined, may influence decisions about the timing of HCV therapy. Immunologic control of HCV infection may differ if HCV is acquired when there is a preexisting defect in cellular immunity. For example, accelerated fibrogenesis is observed among patients taking immunosuppressive medications following orthotopic transplantation [42]. A study of serial liver biopsy specimens obtained from HIV-infected men in New York who had sexually acquired acute HCV infection demonstrated accelerated hepatic fibrosis [19]. A European study that used transient elastography to evaluate fibrosis progression after acute HCV infection among HIV-infected MSM challenged these findings [43]. While high fibrosis progression rates were observed, the shorter the period from the estimated time of acute HCV infection to the elastography examination, the higher the calculated fibrosis progression rate. Vogel et al [43] concluded that these rates are influenced by brief follow-up periods and high inflammatory activity in the acute phase of infection, which may not persist when chronicity develops; linear progression estimated by rates calculated during acute infection may be misleading. The potential for aggressive hepatic fibrosis progression when HIV infection precedes HCV acquisition requires longer-term observation to ensure optimal timing of HCV therapy.
MEDICAL MANAGEMENT OF HCV/HIV-COINFECTED PATIENTS
Screening and Diagnosis
Lack of consensus about HCV screening in public health and treatment guidelines may contribute to suboptimal screening and late diagnosis in HIV-infected populations (Figure 2). Screening should not be deferred because of absence of symptoms, signs, or elevated hepatic transaminase levels, as HCV is often clinically silent until late stages, and transaminase levels may not be significantly elevated to trigger screening. All HIV-infected individuals should be screened for HCV with an anti-HCV antibody test on entry into HIV care. Individuals with a positive result of an anti-HCV antibody test should undergo confirmatory HCV RNA testing by polymerase chain reaction and have a second HCV RNA test performed 4–6 months later to diagnose chronic HCV infection and exclude spontaneous clearance after acute infection [44].
Figure 2.

Hepatitis C virus (HCV) screening, HCV evaluation, and ongoing care in HIV/HCV coinfection. Information is adapted from [44]. aGenetic variations in the gene encoding interleukin 28B, located on chromosome 19, strongly correlate with spontaneous and treatment-induced HCV clearance in coinfection. Appendix B, Table 7 [44a]. Abbreviations: Ab, antibody; ALT, alanine aminotransferase; ANC, absolute neutrophil count; AP, alkaline phosphatase; AST, aspartate aminotransferase; CPT, Child-Pugh-Turcotte; Cr, creatinine; ddI, didanosine; D4T, stavudine; EGD, esophagogastroduodenoscopy; HCC, hepatocellular carcinoma; HCT, hematocrit; Hg, hemoglobin; IL-28B, interleukin 28B; INR, international normalized ratio; MELD, model for end-stage liver disease; PE, physical examination; PT, prothrombin time; RUQ, right upper quadrant.
Coinfected individuals with percutaneous exposure are usually infected with HCV before HIV acquisition. This may be part of the rationale for endorsing HCV antibody testing only at the time of HIV diagnosis [45]. HCV-seronegative patients are not routinely screened subsequently in many locales, although individuals may acquire HCV later in their HIV disease course [14]. Without routine rescreening, incident infections may be missed. HCV serologic testing beyond entry into HIV care is often based on assessment of risk behaviors. However, patients do not always disclose HCV risk behaviors, and physicians are not always aware of all HCV risks.
Similar to many other screening tools used in HIV care, little evidence is available to guide decisions about the periodicity of HCV serologic rescreening. There is precedent for annual routine screening of HIV-infected patients for common co-occurring infections, such as syphilis and tuberculosis [46]. Although the optimal interval for routine HCV rescreening for patients with a negative result of a past anti-HCV antibody test has not been established, annual anti-HCV antibody rescreening has been deemed the standard of care in diverse international settings [47–50]. All HIV-infected, HCV antibody–negative individuals should have access to ongoing HCV testing irrespective of their perceived risk, since many physicians are often too busy or uncomfortable to discuss risks at each visit. Annual HCV antibody testing may be considered a minimum standard, with modifications based on a specific community and population. For example, HIV-infected MSM for whom STDs have been diagnosed and HIV-infected individuals who are active injection drug users should be screened for HCV every 3 months [48, 49].
For detection of acute HCV infection prior to seroconversion, HCV antibody–negative individuals with new elevations in transaminase levels or at recent risk for HCV infection should undergo testing for HCV RNA. Commercial HCV antibody tests measure prior exposure but do not detect reinfection. The incidence of reinfection after treatment of sexually acquired acute HCV infection in HIV-infected MSM in Amsterdam was recently reported to be 15.2 cases per 100 person-years, with a cumulative incidence of 33% within 2 years [51]. Thus, for HCV antibody–positive patients attaining SVR or spontaneous HCV clearance, annual HCV RNA screening is indicated.
All HCV-infected persons should be screened for HIV infection. Most of the US data on HIV testing among HCV-infected individuals originates from the Veterans Health Administration (VHA), the largest provider of HIV and HCV care in the United States [52]. The VHA has recommended HIV testing for all HCV-infected patients since 2003 [53]. In a retrospective study of veterans with HCV infection who underwent testing for HIV, 13.2% were found to have HIV infection [54]. A missed diagnosis of HIV infection may lead to misinformed treatment decisions and poorer outcomes for both HIV and HCV infections because dual infection alters management of both diseases.
Comprehensive HCV Care
While antiviral HCV therapies can eliminate virus, reduce morbidity and mortality, and decrease potential for secondary spread, use of these medications may be limited because of contraindications, adverse events, exorbitant costs, and drug interactions. Pharmacotherapy is not sufficient: other elements of care include addressing alcohol misuse, hepatitis A virus/hepatitis B virus (HBV) vaccination for susceptible individuals, management of hepatic steatosis, control of HIV infection, and attention to drug-induced liver injury.
While coinfection heightens the risk of HAART-related hepatotoxicity, some data suggest that fibrosis progression is slower in patients receiving HAART and that the benefits of HAART far outweigh its risk [55]. When sequencing HIV and HCV treatment, usually, HAART should precede HCV treatment, to decrease HIV-related morbidity and mortality and decrease HIV transmission [56]. Although a specific CD4+ cell count threshold for treating HCV with interferon-based therapy has not been established, most safety and efficacy data are based on observations of persons with CD4+ cell counts >200 cells/μL. Patients with portal hypertension may have splenic sequestration of CD4+ cells, resulting in low absolute CD4+ cell counts and higher CD4+ cell percentages, despite HIV RNA suppression [57]. HIV-infected drug users, who are at-risk for HCV coinfection, are less likely to be offered HAART, and initiate HAART at lower CD4+ cell counts than other subpopulations [58]. Although having lower nadir CD4+ cell counts may limit clinical responses, no data support withholding HCV treatment at any CD4+ cell level. Ideally, prior to initiating HCV therapy, HIV disease should be clinically stable. Treatments for HCV and HIV should not be started simultaneously, so that patients can adjust to each regimen sequentially. There are insufficient data to answer the question of whether successful virologic response to HAART improves the likelihood of SVR.
Antiretroviral drug-induced liver injury requires attention in coinfection. Didanosine and stavudine should be avoided as they may induce mitochondrial toxicity and microvesicular steatosis. Additionally, didanosine has been associated with noncirrhotic portal hypertension [59]. Didanosine and stavudine are no longer recommended as components of an antiretroviral regimen, even in resource-limited settings [60]. Coinfection may increase the risk of hepatotoxicity due to tipranavir or nevirapine.
Patients with cirrhosis require particular care. Hepatocellular carcinoma (HCC) incidence is rising among HIV-infected individuals, driven by chronic HCV and HBV infection, alcohol use, and tobacco use, warranting biannual ultrasonography [61, 62]. Swiss researchers recently found that HCC was the most common non-AIDS malignancy (12.7%) [63]. Nevertheless, many HIV-infected individuals are not being screened for HCC despite the survival benefit seen with screening [64].
HCV Treatment
HCV treatment decisions in coinfection are complex, with many factors to consider for each individual, including the status of HIV disease, the stage of hepatic fibrosis, the likelihood of SVR, the potential risks of therapy, and comorbidities. Development of direct-acting antivirals (DAAs) will lead to a major shift in HCV clinical management, particularly with the potential for interferon-free combination therapy, and higher cure rates. Overall, the urgency for treatment in coinfection is greater than in HCV monoinfection, and coinfected patients have less access to liver transplantation [44]. Thus all coinfected individuals should be considered for HCV treatment [44].
Studies of DAAs for coinfected patients are lagging behind those for patients with HCV monoinfection [65]. Therefore, a new standard of care is not yet ready to supplant pegylated interferon alpha plus ribavirin (PegIFN/RBV). PegIFN/RBV is effective in coinfection, although lower SVR rates than for HCV monoinfection have been reported [66, 67]. SVR rates among individuals coinfected with HCV genotype 1 have been dismal, ranging from 14% to 38% [66, 67].
The same pegIFN/RBV regimen should be used in coinfection and HCV monoinfection, with some notable differences. Weight-based RBV (1000 mg if <75 kg, 1200 mg if ≥75 kg, administered twice daily) is recommended irrespective of genotype [44, 68].
The planned duration of HCV treatment had been 48 weeks, independent of genotype; however, response-guided therapy based on on-treatment response and genotype is supported by a growing body of evidence [69]. For example, for patients infected with HCV genotype 2 or 3 with undetectable HCV RNA at week 4, some experts propose terminating treatment at week 24 [68, 70]. Certain antiretroviral agents are contraindicated with pegIFN/RBV. Lactic acidosis may develop with concomitant didanosine or stavudine therapy. Zidovudine should be avoided because its use may potentiate treatment-mediated anemia.
Coinfected patients who decline HCV therapy or for whom relative contraindications exist need formal re-evaluation at least annually [44]. Between visits, comorbid disease should be treated to permit HCV therapy commencement. Following SVR, patients require continued monitoring, at least annually, for reinfection, follow-up of steatosis, and care of advanced fibrosis [71].
Improving Treatment Delivery
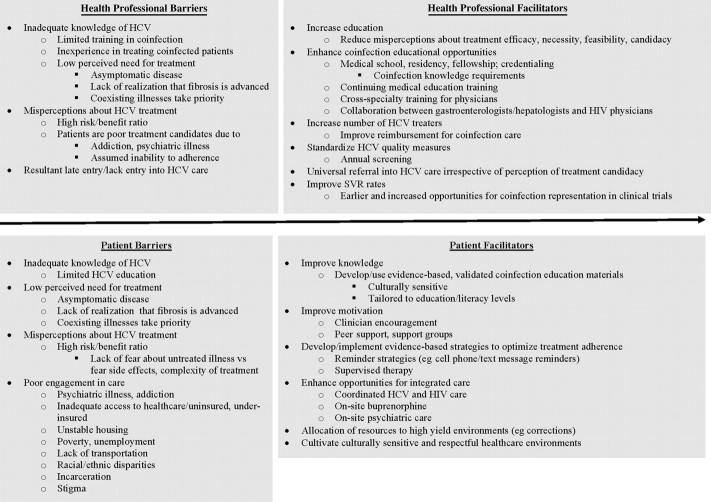
Coinfection is distinguished by many social and medical needs, stigma, and numerous individual and system-level problems with access (Figure 3). Challenges beyond those of HCV monoinfection include HIV/AIDS, the need for HAART, and polypharmacy. HIV infection increases the prevalence and severity of comorbidities (eg, accelerated atherosclerosis), which may pose contraindications to HCV treatment. Compared with HCV and HIV monoinfection, coinfection is associated with more severe psychiatric illness, ongoing drug use, poverty, homelessness, and incarceration [72]. Rates of health care use and disability are 70% greater in coinfection than in HCV monoinfection [73].
Figure 3.
Modifiable barriers and facilitators of treatment for hepatitis C virus (HCV) infection in individuals coinfected with human immunodeficiency virus (HIV) and HCV. Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; SVR, sustained virologic response.
Overcoming these barriers requires improved recognition among patients and physicians of the importance of HCV in coinfection and the benefits of treatment. Whether gastroenterologists, infectious disease specialists, or HIV primary care clinicians are responsible for HCV care of coinfected patients depends on local resources and knowledge. Coinfection care benefits from integrated, coordinated, multidisciplinary medical strategies; addiction treatment; social support; and treatment of coexisting medical and psychiatric conditions [74, 75].
DAAs IN COINFECTION
Many coinfected persons will require HCV treatment in the next few years, before effective, tolerable, and convenient oral DAA combinations are available. HCV therapeutic advances are slow to reach coinfected patients, despite great need. Although final SVR data from coinfected patients were not available as of early 2012, it is assumed that adding an HCV protease inhibitor to pegIFN/RBV will significantly increase treatment efficacy [65]. Indeed, 12 weeks after completion of treatment (SVR-12), 74% of coinfected people who received a telaprevir-based regimen and 60% of people who received a boceprevir-based regimen maintained an undetectable HCV RNA level, which is highly predictive of SVR-24. Safety profiles were similar to that for treatment of HCV monoinfection [76, 77].
Boceprevir and telaprevir are substrates and inhibitors of CYP 3A4. Drug-drug interactions between boceprevir and telaprevir and both antiretrovirals (Table 1) and other commonly used medications may limit their use in coinfected patients, who are more likely to require polypharmacy for management of comorbidities and may have multidrug-resistant virus, with few HIV treatment options [78]. Adherence may be difficult, since boceprevir-based and telaprevir-based regimens are demanding and inconvenient. The pill burden is significant: 6–9 tablets of telaprevir (a higher dose is needed when used with efavirenz), or 12 boceprevir capsules, plus twice-daily weight-based RBV, weekly pegIFN injections, and HAART. Boceprevir and telaprevir must be taken at 7–9-hour intervals, accompanied by food; in particular, telaprevir requires a meal containing at least 20 grams of fat. Food requirements are likely to be challenging, given the range of gastrointestinal side effects associated with HCV treatment and specific food requirements of certain antiretrovirals.
Table 1.
Antiretroviral Agents and Hepatitis C Virus (HCV) Protease Inhibitors (PIs)
| Antiretroviral Agents |
|||
|---|---|---|---|
| HCV PI | Evaluated in Clinical Trials | Coadministration Not Recommended | Prescribing Information |
| BOC | Tenofovir/emtricitabine or tenofovir/lamivudine, plus atazanavir/ritonavir or raltegravir | Efavirenz | BOC concentrations decreased with ritonavir, darunavir /ritonavir, and lopinavir/ritonavir. |
| AIDS Clinical Trials Group 5294 may permit use of additional antiretroviral agents. | BOC decreased concentrations of ritonavir, atazanavir/ritonavir, darunavir/ritonavir, and lopinavir/ritonavir. These medications can lose effectiveness when coadministered; close monitoring is recommended. | ||
| Plasma trough concentrations of BOC were decreased when BOC was coadministered with efavirenz, indicating a possible loss of therapeutic effect; avoid combination. | |||
| TEL | Tenofovir/emtricitabine or tenofovir/lamivudine, plus atazanavir/ritonavir; coadministration with efavirenz is possible with a higher dose of TEL (1125 mg 3 times daily) | Darunavir/ritonavir, fosamprenavir/ritonavir, and lopinavir/ritonavir | Concomitant administration of TEL and atazanavir/ritonavir resulted in reduced steady-state TEL exposure, while steady-state atazanavir exposure was increased. |
| Coadministration of TEL and raltegravir increases raltegravir exposure by 31%, but dose adjustment not necessary. | Concomitant administration of TEL and darunavir/ritonavir resulted in reduced steady-state exposures to TEL and darunavir. Coadministration is not recommended. | ||
| Concomitant administration of TEL and fosamprenavir/ritonavir resulted in reduced steady-state exposures to TEL and amprenavir. Coadministration is not recommended. | |||
| Concomitant administration of TEL and lopinavir/ritonavir resulted in reduced steady-state TEL exposure, while the steady-state exposure to lopinavir was not affected. Coadministration is not recommended. | |||
| Concomitant administration of TEL and efavirenz resulted in reduced steady-state exposures to TEL and efavirenz. | |||
| Concomitant administration of TEL and tenofovir resulted in increased tenofovir exposure. Increased clinical and laboratory monitoring are warranted. Tenofovir should be discontinued for patients who develop tenofovir-associated toxicities. | |||
FUTURE DIRECTIONS
The benefits of therapeutic advances will be limited for HIV/HCV-coinfected individuals until HCV care is enhanced on a large scale. Now is the time to rethink the HCV treatment standard. Previously, clinicians often waited until HIV infection, addiction, psychiatric illness, and other comorbidities were stabilized before considering treatment for HCV infection. The paradigm must shift so that both HCV and HIV infection are addressed soon after diagnosis. Adding annual HCV antibody testing to the US Federal Ryan White Program would permit earlier identification of undiagnosed HCV infection and create an instant widespread surveillance system, providing HCV incidence data.
In turn, HCV drug trials should be expedited for HIV/HCV-coinfected persons. Drug-drug interaction studies with antiretrovirals commonly used by treatment-naive and treatment-experienced HIV-infected patients should be performed as soon as possible, to facilitate early access trials and eventual use of DAA combinations in coinfection. Development of DAAs and combinations that are unlikely to interact with antiretrovirals, methadone, buprenorphine, psychotropics, and other commonly used medications should be prioritized to optimize treatment of coinfected persons. HIV/HCV coinfection remains a growing and evolving epidemic, but new developments in therapeutics and improved care models offer great promise.
Notes
Acknowledgments. We thank Andrea Karis and Michaela Maynard, MPH, for their administrative assistance.
Disclaimer. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute on Drug Abuse (K23DA020383-01 to L. E. T); the Lifespan/Tufts/Brown Center for AIDS Research, an NIH-funded program (P30 AI42853); the Center for Drug Abuse and AIDS Research, an NIH-funded program (P30 DA013868); and the Harvard Center for AIDS Research (to K. H. M., via P30 AI60354 to the principle investigator, B. Walker).
The Viral Hepatitis Action Coalition of the Centers for Disease Control and Prevention Foundation receives support from the following corporate sponsors: Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Janssen Therapeutics, Merck Sharp & Dohme, OraSure Technologies, and Vertex Pharmaceuticals.
Supplement sponsorship. This article was published as part of a supplement entitled “The Evolving Paradigm of Hepatitis C,” sponsored by an unrestricted grant from the Viral Hepatitis Action Coalition of the CDC Foundation.
Potential conflicts of interest. L. T. has received grant support and consulting fees from Vertex and has served on the speakers’ bureau for Genentech. T. S. has received educational grant support from Genentech, Tibotec, and Boehringer Ingelheim and has received consulting fees from Vertex and Merck. K. H. M. certifies no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(Suppl 1):S77–84. doi: 10.1086/313842. [DOI] [PubMed] [Google Scholar]
- 2.Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res. 2010;85:303–15. doi: 10.1016/j.antiviral.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4.de Ledinghen V, Barreiro P, Foucher J, et al. Liver fibrosis on account of chronic hepatitis C is more severe in HIV-positive than HIV-negative patients despite antiretroviral therapy. J Viral Hepat. 2008;15:427–33. doi: 10.1111/j.1365-2893.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 5.Holmberg SD, Kathleen L, Xing J, Klevens M, Jiles R, Ward JW. San Francisco, California: 2011. The growing burden of mortality associated with viral hepatitis in the United States, 1999–2007. Presented at: 62th Annual Meeting of the American Association for the Study of Liver Diseases. [Google Scholar]
- 6.Kim AY, Chung RT. Coinfection with HIV-1 and HCV—a one-two punch. Gastroenterology. 2009;137:795–814. doi: 10.1053/j.gastro.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuyama AC, Hong F, Saiman Y, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612–22. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berenguer J, Alvarez-Pellicer J, Martin PM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–13. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SH, Lucas GM, Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–9. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- 10.Garten RJ, Lai S, Zhang J, et al. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int J Epidemiol. 2004;33:182–8. doi: 10.1093/ije/dyh019. [DOI] [PubMed] [Google Scholar]
- 11.Quan VM, Go VF, Nam le V, et al. Risks for HIV, HBV, and HCV infections among male injection drug users in northern Vietnam: a case-control study. AIDS Care. 2009;21:7–16. doi: 10.1080/09540120802017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983–91. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 13.Urbanus AT, van de Laar TJ, Stolte IG, et al. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS. 2009;23:F1–7. doi: 10.1097/QAD.0b013e32832e5631. [DOI] [PubMed] [Google Scholar]
- 14.Danta M, Rodger AJ. Transmission of HCV in HIV-positive populations. Curr Opin HIV AIDS. 2011;6:451–8. doi: 10.1097/COH.0b013e32834b4974. [DOI] [PubMed] [Google Scholar]
- 15.Matthews GV, Pham ST, Hellard M, et al. Patterns and characteristics of hepatitis C transmission clusters among HIV-positive and HIV-negative individuals in the Australian trial in acute hepatitis C. Clin Infect Dis. 2011;52:803–11. doi: 10.1093/cid/ciq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 17.Browne R, Asboe D, Gilleece Y, et al. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sex Transm Infect. 2004;80:326–7. doi: 10.1136/sti.2003.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luetkemeyer A, Hare CB, Stansell J, et al. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:31–6. doi: 10.1097/01.qai.0000191281.77954.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fierer DS, Uriel AJ, Carriero DC, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–6. doi: 10.1086/590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Helm JJ, Prins M, del Amo J, et al. The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007. AIDS. 2011;25:1083–91. doi: 10.1097/QAD.0b013e3283471cce. [DOI] [PubMed] [Google Scholar]
- 21.van de Laar TJ, van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196:230–8. doi: 10.1086/518796. [DOI] [PubMed] [Google Scholar]
- 22.van de Laar TJ, Paxton WA, Zorgdrager F, Cornelissen M, de Vries HJ. Sexual transmission of hepatitis C virus in human immunodeficiency virus-negative men who have sex with men: a series of case reports. Sex Transm Dis. 2011;38:102–4. doi: 10.1097/OLQ.0b013e3181ec9de5. [DOI] [PubMed] [Google Scholar]
- 23.Riestra S, Fernandez E, Rodriguez M, Rodrigo L. Hepatitis C virus infection in heterosexual partners of HCV carriers. J Hepatol. 1995;22:509–10. doi: 10.1016/0168-8278(95)80120-0. [DOI] [PubMed] [Google Scholar]
- 24.Sherman KE, Shire NJ, Rouster SD, et al. Viral kinetics in hepatitis C or hepatitis C/human immunodeficiency virus-infected patients. Gastroenterology. 2005;128:313–27. doi: 10.1053/j.gastro.2004.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briat A, Dulioust E, Galimand J, et al. Hepatitis C virus in the semen of men coinfected with HIV-1: prevalence and origin. AIDS. 2005;19:1827–35. doi: 10.1097/01.aids.0000189847.98569.2d. [DOI] [PubMed] [Google Scholar]
- 26.Turner JM, Rider AT, Imrie J, et al. Behavioural predictors of subsequent hepatitis C diagnosis in a UK clinic sample of HIV positive men who have sex with men. Sex Transm Infect. 2006;82:298–300. doi: 10.1136/sti.2005.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dionne-Odom J, Osborn MK, Radziewicz H, Grakoui A, Workowski K. Acute hepatitis C and HIV coinfection. Lancet Infect Dis. 2009;9:775–83. doi: 10.1016/S1473-3099(09)70264-6. [DOI] [PubMed] [Google Scholar]
- 28.Matthews GV, Hellard M, Kaldor J, Lloyd A, Dore GJ. Further evidence of HCV sexual transmission among HIV-positive men who have sex with men: response to Danta et al. AIDS. 2007;21:2112–3. doi: 10.1097/QAD.0b013e3282ef3873. [DOI] [PubMed] [Google Scholar]
- 29.van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609–17. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dougan S, Evans BG, Elford J. Sexually transmitted infections in Western Europe among HIV-positive men who have sex with men. Sex Transm Dis. 2007;34:783–90. doi: 10.1097/01.olq.0000260919.34598.5b. [DOI] [PubMed] [Google Scholar]
- 31.Snowden JM, Raymond HF, McFarland W. Seroadaptive behaviours among men who have sex with men in San Francisco: the situation in 2008. Sex Transm Infect. 2011;87:162–4. doi: 10.1136/sti.2010.042986. [DOI] [PubMed] [Google Scholar]
- 32.Lattimore S, Thornton A, Delpech V, Elford J. Changing patterns of sexual risk behavior among London gay men: 1998–2008. Sex Transm Dis. 2011;38:221–9. doi: 10.1097/OLQ.0b013e3181f2ebe1. [DOI] [PubMed] [Google Scholar]
- 33.Marcus U, Schmidt AJ, Hamouda O. HIV serosorting among HIV-positive men who have sex with men is associated with increased self-reported incidence of bacterial sexually transmissible infections. Sex Health. 2011;8:184–93. doi: 10.1071/SH10053. [DOI] [PubMed] [Google Scholar]
- 34.Buchbinder SP, Katz MH, Hessol NA, Liu J, O'Malley PM, Alter MJ. Hepatitis C virus infection in sexually active homosexual men. J Infect. 1994;29:263–9. doi: 10.1016/s0163-4453(94)91128-2. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt AJ, Rockstroh JK, Vogel M, et al. Trouble with bleeding: risk factors for acute Hepatitis C among HIV-positive gay men from Germany—a case-control study. PLoS ONE. 2011;6:e17781. doi: 10.1371/journal.pone.0017781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darrow WW, Biersteker S, Geiss T, et al. Risky sexual behaviors associated with recreational drug use among men who have sex with men in an international resort area: challenges and opportunities. J Urban Health. 2005;82:601–9. doi: 10.1093/jurban/jti122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colfax G, Vittinghoff E, Husnik MJ, et al. Substance use and sexual risk: a participant- and episode-level analysis among a cohort of men who have sex with men. Am J Epidemiol. 2004;159:1002–12. doi: 10.1093/aje/kwh135. [DOI] [PubMed] [Google Scholar]
- 38.Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men–New York City, 2005–2010. MMWR Morb Mortal Wkly Rep. 2011;60:945–50. [PubMed] [Google Scholar]
- 39.Marincovich B, Castilla J, del Romero J, et al. Absence of hepatitis C virus transmission in a prospective cohort of heterosexual serodiscordant couples. Sex Transm Infect. 2003;79:160–2. doi: 10.1136/sti.79.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frederick T, Burian P, Terrault N, et al. Factors associated with prevalent hepatitis C infection among HIV-infected women with no reported history of injection drug use: the Women's Interagency HIV Study (WIHS) AIDS Patient Care STDS. 2009;23:915–23. doi: 10.1089/apc.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52:1497–505. doi: 10.1002/hep.23808. [DOI] [PubMed] [Google Scholar]
- 42.Berenguer M, Ferrell L, Watson J, et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673–84. doi: 10.1016/s0168-8278(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 43.Vogel M, Page E, Boesecke C, et al. Liver fibrosis progression after acute Hepatitis C virus infection in HIV-positive individuals. Clin Infect Dis. 2012;54:556–9. doi: 10.1093/cid/cir854. [DOI] [PubMed] [Google Scholar]
- 44.Sulkowski MS, Cheever L, Spach DH. A guide for evaluation and treatment of Hepatitis C in adults coinfected with HIV. Washington, DC: Department of Health and Human Services, Health Resources and Services Administration, 14; January 2011. [Google Scholar]
- 45.Department of Health and Human Services (DHHS) Antiretroviral dosing recommendations in patients with renal or hepatic insufficiency panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington, DC: DHHS, 26 January 2012; Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf . [Google Scholar]
- 46.Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–81. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. quiz CE1-4. [PubMed] [Google Scholar]
- 48.Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS. 2011;25:399–409. doi: 10.1097/QAD.0b013e328343443b. [DOI] [PubMed] [Google Scholar]
- 49.Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82–8. doi: 10.1111/j.1468-1293.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 50.Institute NYSDoHA. HIV clinical resource update: hepatitis C virus. New York State Department of Health AIDS Institute in collaboration with Johns Hopkins University Division of Infectious Diseases. Available at: http://www.hivguidelines.org . Accessed 16 April 2012. [Google Scholar]
- 51.Lambers FA, Prins M, Thomas X, et al. Alarming incidence of hepatitis C virus re-infection after treatment of sexually acquired acute hepatitis C virus infection in HIV-infected MSM. AIDS. 2011;25:F21–7. doi: 10.1097/QAD.0b013e32834bac44. [DOI] [PubMed] [Google Scholar]
- 52.Backus LI, Boothroyd D, Deyton LR. HIV, hepatitis C and HIV/hepatitis C virus co-infection in vulnerable populations. AIDS. 2005;19(Suppl 3):S13–9. doi: 10.1097/01.aids.0000192065.09281.01. [DOI] [PubMed] [Google Scholar]
- 53.Department of Veterans Affairs. VA treatment recommendations for patients with chronic hepatitis C (version 5.0) Federal Practitioner. 2003;20(Suppl 5):1–33. [Google Scholar]
- 54.Fuller BR, Veronica L, Linke A, Hauser P. HIV co-testing among veterans with chronic hepatitis C in the veterans health administration. The Open Infectious Diseases Journal. 2011;5:91–6. [Google Scholar]
- 55.Brau N, Salvatore M, Rios-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431–7. doi: 10.1086/509580. [DOI] [PubMed] [Google Scholar]
- 58.Wood E, Montaner JS, Tyndall MW, Schechter MT, O'Shaughnessy MV, Hogg RS. Prevalence and correlates of untreated human immunodeficiency virus type 1 infection among persons who have died in the era of modern antiretroviral therapy. J Infect Dis. 2003;188:1164–70. doi: 10.1086/378703. [DOI] [PubMed] [Google Scholar]
- 59.Schiano TD, Uriel A, Dieterich DT, Fiel MI. The development of hepatoportal sclerosis and portal hypertension due to didanosine use in HIV. Virchows Arch. 2011;458:231–5. doi: 10.1007/s00428-010-1004-7. [DOI] [PubMed] [Google Scholar]
- 60.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf . Accessed on: 26 January 2012. [Google Scholar]
- 61.MacDonald DC, Nelson M, Bower M, Powles T. Hepatocellular carcinoma, human immunodeficiency virus and viral hepatitis in the HAART era. World J Gastroenterol. 2008;14:1657–63. doi: 10.3748/wjg.14.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garcia-Samaniego J, Rodriguez M, Berenguer J, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:179–83. doi: 10.1111/j.1572-0241.2001.03374.x. [DOI] [PubMed] [Google Scholar]
- 63.Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–9. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 64.Nunez M, Kikuchi L, Barreiro P, et al. Screening for Hepatocellular Carcinoma (HCC) in HIV/HCV-coinfected patients: impact on staging, therapy and survival. 2010 San Francisco, CA: 17th Conference on Retroviruses and Opportunistic Infections. [Google Scholar]
- 65.Thomas DL, Bartlett JG, Peters MG, Sherman KE, Sulkowski MS, Pham PA. Provisional guidance on the use of hepatitis C virus protease inhibitors for treatment of hepatitis C in HIV-infected persons. Clin Infect Dis. 2012;54:979–83. doi: 10.1093/cid/cir882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laguno M, Murillas J, Blanco JL, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS. 2004;18:F27–36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 68.Reiss P, Battegay M, Clumeck N, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. European AIDS Clinical Society, October 2011, Version 6. [Google Scholar]
- 69.Crespo M, Esteban JI, Ribera E, et al. Utility of week-4 viral response to tailor treatment duration in hepatitis C virus genotype 3/HIV co-infected patients. AIDS. 2007;21:477–81. doi: 10.1097/QAD.0b013e328012b5ba. [DOI] [PubMed] [Google Scholar]
- 70.EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–64. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 71.Innes HA, Hutchinson SJ, Allen S, et al. Excess liver-related morbidity of chronic hepatitis C patients, who achieve a sustained viral response, and are discharged from care. Hepatology. 2011;54:1547–58. doi: 10.1002/hep.24561. [DOI] [PubMed] [Google Scholar]
- 72.Rosenberg SD, Drake RE, Brunette MF, Wolford GL, Marsh BJ. Hepatitis C virus and HIV co-infection in people with severe mental illness and substance use disorders. AIDS. 2005;19(Suppl 3):S26–33. doi: 10.1097/01.aids.0000192067.94033.aa. [DOI] [PubMed] [Google Scholar]
- 73.Linas BP, Wang B, Smurzynski M, et al. The impact of HIV/HCV co-infection on health care utilization and disability: results of the ACTG Longitudinal Linked Randomized Trials (ALLRT) Cohort. J Viral Hepat. 2011;18:506–12. doi: 10.1111/j.1365-2893.2010.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehta SH, Thomas DL, Sulkowski MS, Safaein M, Vlahov D, Strathdee SA. A framework for understanding factors that affect access and utilization of treatment for hepatitis C virus infection among HCV-mono-infected and HIV/HCV-co-infected injection drug users. AIDS. 2005;19(Suppl 3):S179–89. doi: 10.1097/01.aids.0000192088.72055.90. [DOI] [PubMed] [Google Scholar]
- 75.Dore GJ, Thomas DL. Management and treatment of injection drug users with hepatitis C virus (HCV) infection and HCV/human immunodeficiency virus coinfection. Semin Liver Dis. 2005;25:18–32. doi: 10.1055/s-2005-864779. [DOI] [PubMed] [Google Scholar]
- 76.Dieterich D, Soriano V, Sherman K, et al. on behalf of the Study 110 Team. Telaprevir in combination with pegylated interferon-a-2a+RBV in HCV/HIV-co-infected patients: a 24-week treatment interim analysis [abstract 46] 2012 Presented at: 9th Annual Conference on Retroviruses and Opportunistic Infections, Seattle, Washington. [Google Scholar]
- 77.Sulkowski M, Pol S, Cooper C, et al. Boceprevir + Pegylated Interferon + Ribavirin for the treatment of HCV/HIV-co-infected patients: end of treatment (week-48) interim results [abstract 47] 2012 Presented at: 9th Annual Conference on Retroviruses and Opportunistic Infections, Seattle, Washington. [Google Scholar]
- 78.Food and Drug Administration. FDA Drug Safety Communication: Important drug interactions between Victrelis (boceprevir) and ritonavir-boosted human immunodeficiency virus (HIV) protease inhibitor drugs. 8 February 2012. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm291119.htm#HCPs . Accessed 11 March 2012. [Google Scholar]
- 79.Hulskotte E, Feng H-P, Xuan F, et al. Pharmacokinetic interaction between the HCV protease inhibitor boceprevir and ritonavir-boosted HIV-1 protease inhibitors atazanavir, lopinavir, and darunavir [abstract 771 LB] 2012 doi: 10.1093/cid/cis968. Presented at: 19th Annual Conference on Retroviruses and Opportunistic Infections, Seattle, Washington. [DOI] [PubMed] [Google Scholar]
- 80.Kasserra C, Hughes E, Treitel M, et al. Clinical pharmacology of BOC: metabolism, excretion, and drug-drug interactions [abstract 118] Presented at: 18th Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, 27 February–2 March 2011. [Google Scholar]
- 81.Sherman KE, Rockstroh JK, Dieterich DT, et al. Telaprevir combination with peginterferon alfa-2a/ribavirin in HCV/HIV coinfected patients: 24-week treatment interim analysis [abstract LB-8] Presented at: 62nd Annual Meeting of the American Association for the Study of Liver Disease, San Francisco, California, 4–8 November 2011. [Google Scholar]
- 82.van Heeswijk R, Vandevoorde A, Boogaerts G, et al. Pharmacokinetic interactions between ARV agents and the investigational HCV protease inhibitor TVR in healthy volunteers [abstract 119] Presented at: 18th Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, 27 February–2 March 2011. [Google Scholar]
- 83.van Heeswijk R, Garg V, Boogaerts G, et al. The pharmacokinetic interaction between telaprevir and raltegravir in healthy volunteers [abstract A1-1738a]. Presented at: Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Illinois, 17–20 September 2011. [Google Scholar]
- 84.Marzolini C, Elzi L, Gibbons S, et al. Prevalence of comedications and effect of potential drug-drug interactions in the Swiss HIV Cohort Study. Antivir Ther. 2010;15:413–23. doi: 10.3851/IMP1540. [DOI] [PubMed] [Google Scholar]