Abstract
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability. Patients with FXS do not only suffer from cognitive problems, but also from abnormalities/deficits in procedural memory formation. It has been proposed that a lack of fragile X mental retardation protein (FMRP) leads to altered long-term plasticity by deregulation of various translational processes at the synapses, and that part of these impairments might be rescued by the inhibition of type I metabotropic glutamate receptors (mGluRs). We recently developed the Erasmus Ladder, which allows us to test, without any invasive approaches, simultaneously, both procedural memory formation and avoidance behavior during unperturbed and perturbed locomotion in mice. Here, we investigated the impact of a potent and selective mGluR5 inhibitor (Fenobam) on the behavior of Fmr1 KO mice during the Erasmus Ladder task. Fmr1 KO mice showed deficits in associative motor learning as well as avoidance behavior, both of which were rescued by intraperitoneal administration of Fenobam. While the Fmr1 KO mice did benefit from the treatment, control littermates suffered from a significant negative side effect in that their motor learning skills, but not their avoidance behavior, were significantly affected. On the basis of these studies in the FXS animal model, it may be worthwhile to investigate the effects of mGluR inhibitors on both the cognitive functions and procedural skills in FXS patients. However, the use of mGluR inhibitors appears to be strongly contraindicated in healthy controls or non-FXS patients with intellectual disability.
Keywords: Avoidance behavior, cue recognition, Erasmus Ladder, Fmr1 KO, Fragile X syndrome, locomotion, mGluR5 inhibitor, motor learning, procedural memory formation
Fragile X syndrome (FXS) is the most common genetic form of mental impairment (WHO 1996), affecting approximately 1 in 4000 males (Crawford et al. 2002; de Vries et al. 1997; Patsalis et al. 1999; Youings et al. 2000) and 1 in 6000 females worldwide (Crawford et al. 2001). In nearly all cases, the observed mutation is an expansion of a CGG trinucleotide repeat (>200) in the 5′-untranslated region (UTR) region of the fragile X mental retardation gene (FMR1) (Oberle et al. 1991; Verkerk et al. 1991). As a consequence, the FMR1 gene is methylated and cannot be transcribed into mRNA, causing the absence of fragile X mental retardation protein (FMRP) (Oostra & Willemsen 2009). Besides physical characteristics such as macro-orchidism and facial features (Pfeiffer & Huber 2009), the symptoms of FXS include general deficits in cognitive processing (Van der Molen et al. 2010), abnormalities in procedural memory formation (Koekkoek et al. 2005), social anxiety and autistic-like behavior (Sabaratnam et al. 2003).
FMRP, which is an RNA binding protein (Schaeffer et al. 2003), is present in the postsynaptic compartment and locally synthesized upon mGluR activation (Weiler et al. 1997). As an RNA binding protein, FMRP is thought to repress the translation of target mRNAs that are important for receptor recycling in the postsynaptic dendritic spines (Levenga et al. 2010; Pfeiffer & Huber 2009). The absence of FMRP induces increased translation of a subset of mRNAs, which results in altered receptor trafficking dynamics. Internalization of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors may facilitate long-term plasticity and is stimulated by the synthesis of novel proteins after the activation of mGluRs (Snyder et al. 2001). Accordingly, the ‘mGluR theory of FXS' suggests that the neurobiological and psychiatric symptoms of FXS result from an exaggerated AMPA receptor internalization triggered by mGluR activation (Bear et al. 2004). As a consequence the mGluR theory has directed research toward the use of mGluR antagonists to treat FXS.
A ladder rung task provides comprehensive assessment for skilled limb movements in mice (Farr et al. 2006; Hunsaker et al. 2011). As FXS patients suffer from both motor abnormalities and cognitive deficits (Koekkoek et al. 2005; Sabaratnam et al. 2003; Van der Molen et al. 2010), we subjected Fmr1 KO mice to the Erasmus Ladder test, which allows a quantitative assay for both categories of symptoms. With regard to the motor abnormalities, the Erasmus Ladder test offers sensitive measurements for locomotion learning controlled by the olivocerebellar system (Renier et al. 2010; Van Der Giessen et al. 2008; Van der Vaart et al. 2011). For example, blockage of electrotonic coupling in the inferior olive results in impaired learning-dependent timing of locomotion steps during classical delay conditioning (Van Der Giessen et al. 2008). With regard to avoidance behavior, which is mainly controlled by limbic and basal ganglia systems (Ermisch et al. 1986; Ursin 1965), the Erasmus Ladder task can test the ability of mice to temporarily prevent their exposure to the stressful situation on the ladder that is created by unexpectedly lowering or rising one of the rungs; because of the presence of such an unconditioning stimulus (US), mice try to avoid the US by waiting inside the shelter box as long as possible and thus inhibit their reaction to the cues of departure (Ursin (1965)). Moreover, different from other tests such as eyeblink conditioning, in which Fmr1 KO mice also show a phenotype (Chen & Toth 2001; Paylor et al. 2008; Spencer et al. 2006), the Erasmus Ladder test does not require any surgical intervention and allows drug screening at an automated, medium-throughput level. Thus, because of the technical advantages, we tried to test the ‘mGluR theory of FXS' by investigating the impact of a specific mGluR negative modulator, Fenobam, on the behavior of mice lacking FMRP (‘fragile X mental retardation 1 knockouts' or ‘Fmr1 KO mice’) using the multifunctional, motor-cognitive assay on the Erasmus Ladder.
Methods
Animals
Fmr1 KO mice were obtained by crossing FVB/Ant x het Fmr1 KO(2) to test hybrid mice with 50% FVB/Ant and 50% C57Bl/6 contribution. Because the FVB/Ant strain is pigmented and devoid of the genetic predisposition to retinal degeneration of the FVB/N strain, these mice show clear visual evoked potential in the presence of normal eye histology and improved performance in the Morris water maze test (Errijgers et al. 2007). The Fmr1 KO(2) line, unlike the first generation of Fmr1 KO model, does not express any FMRP and lacks detectable Fmr1 transcripts (Mientjes et al. 2006). Both lines were inbred (>10 times backcrossed). All mice were male between 12 and 26 weeks of age and were single housed. Mice were allowed to have free access to standard laboratory food and water. They were left on a 12 h light/dark cycle. As required by Dutch legislation, all experiments were approved in advance by the Institutional Animal Welfare Committee (Erasmus MC, Rotterdam, The Netherlands).
Treatment
Fenobam [N-(3-chlorophenyl)-N’-(4,5-dihydro-1-methyl-4-oxo-1H-imi dazole-2-yl)urea], which is a clinically validated non-benzodiazepine anxiolytic drug, is a selective and potent mGluR5 receptor antagonist acting at an allosteric modulatory site shared with 2-methyl-6-(phenylethynyl)-pyridine (MPEP) (Porter et al. 2005). Similar to MPEP, Fenobam acts in a noncompetitive manner and shows inverse agonist properties, blocking 66% of mGluR5 receptor basal activity given at a dose of 10–30 mg/kg orally. Fenobam (Sigma-Aldrich, St. Louis, MO, USA) was injected intraperitoneally at a dose of 30 mg/kg 30 min before each associative motor learning session, using methyl cellulose (MC) as dissolvent. For the motor learning and avoidance discrimination tasks Fmr1 KO and wild-type (WT) mice were assigned to different groups: one treated with Fenobam in MC and another one treated with the vehicle (MC only). In addition, we also tested the performance of Fmr1 KO and WT littermates without any application as control.
The Erasmus Ladder
The Erasmus Ladder is a fully automated test for detecting motor performance, associative motor learning deficits and cognitive phenotypes in mouse models. The Erasmus Ladder consists of a horizontal ladder in between two shelter boxes, which are equipped with a bright white LED spotlight in the roof and two pressurized air outlets (Pneumax, Gosport, UK). Both, light and air stimuli are used as cues for departure. In addition, one of the air outlets is used to control the speed of the mice and to prevent them from leaving the shelter box at the opposite side and crossing the ladder at unwanted moments (which we call ‘escape’). The ladder has 2 × 37 rungs for the left and right side. All rungs are equipped with pressure sensors (produced at Erasmus MC), which are continuously monitored and which can be used to register and analyze the walking pattern of the mouse instantaneously. Moreover, based upon the prediction of the walking pattern, the rungs can be moved up or down by a high-speed pneumatic slide (Pneumax) with a maximum of 13 mm at any moment in time. The computer system (National Instruments, Austin, TX, USA), which runs the real-time system recording sensor data, adjusts air pressure, predicts future touches, calculates interventions, repositions slides and stores data, operates in a fixed cycle of 2 ms. Details of the device and its operations have been published (Van Der Giessen et al. 2008).
During the first 4 days (‘unperturbed sessions'), mice were trained with the even-numbered rungs on the left side and the odd-numbered rungs on the right side in a descended position so as to create an alternated stepping pattern with 30-mm gaps. Mice were trained to walk the ladder for 72 runs per day. We calculated the number of missteps that were sensed by the descended rungs, and steptime, which is defined as the time needed to place one of the front paws from one rung to the other (i.e. onset of touch until onset of following touch). Associative motor learning trials (‘perturbed sessions') started on day 5 using a 15 kHz tone as conditioning stimulus (CS; gradually increasing over 20 ms to 100 dB and lasting up to 300 ms; Voltcraft, Barking, UK) and a rising rung as the US (ascending 12 mm). The interstimulus interval was fixed at 285 ms. To keep this time period constant, we observed in real-time the speed of the mouse and calculated which rung should rise. Mice typically learn that increasing walking speed avoids being hit by the rung, so mice will decrease their pre-steptime (nearest steptime before the onset of the US) and post-steptime (nearest steptime after the onset of the US; i.e. not the average of all steptimes after onset of US) through the sessions.
Apart from motor coordination deficits, the Erasmus Ladder is able to detect cognitive phenotypes in mice. Cognition is tested by determining the capability of mice to respond to the cues of departure (light or air) and to modify this response under certain circumstances. Thereby, unperturbed sessions that are neutral in the beginning of the experiment (Fig. 1), turn into unpleasant, perturbed sessions (Figs 2 and 3), which will reverse the initial response of mice to the given cues. Indeed, in the attempt to reduce their exposure to the US stimulus during perturbed sessions, mice inhibit the reaction to the cues of departure (‘avoidance behavior’). At the beginning of each session a mouse was placed in the starting box and after a period (randomly varying from 9 to 11 seconds) the shelter light is turned on (first cue). At this stage the mouse is supposed to leave the box. In case the mouse left the box before the light turned on (so-called ‘escape behavior’), a strong air puff from the opposite box drives the mouse back into the shelter, and a new cycle begins. If the mouse does not leave the box within 3 seconds after the onset of the light, a strong air puff is given from the pressurized air outlets in the box (second cue) so as to push the mouse out of the box within 20 seconds. When the mouse arrives at the opposite shelter, the pressurized air outlets and light are switched off, and after a period randomly varying from 9 to 11 seconds, the cycle is repeated. Thus, each trial of the training paradigm described above can result in one out of four possible outcomes: (1) the mouse leaves the box before the light is turned on (‘escape response’) (Fig. 3a1); (2) the mouse responds to the light and leaves the box on time (‘light response’) (Fig. 3a2); (3) the mouse does not respond to the light, but responds to the strong air pressure and leaves the box on time (‘air response’) (Fig. 3a3) and (4) the mouse neither responds to the light nor to the air within the allotted time period and has to wait for another cycle (‘waiting response’). A schematic description of the possible outcomes and their interactions on time is depicted in Fig. 3a4. During each session, we quantified the percentage of light responses, air responses and escape, and used them to assess the mouse's cue response capabilities and avoidance behavior.
Figure 1. Motor performance of Fmr1 KO mice.
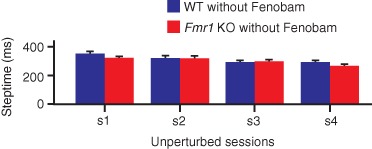
Motor performance was tested with the use of the Erasmus Ladder by calculating the average steptimes for every unperturbed session (s1–s4). Fmr1 KO (n = 35) and their WT (n = 41) littermates did not show significant differences in steptimes (P = 0.810; repeated measures anova). Error bars represent standard error of the mean.
Figure 2. Deficits of procedural memory formation in Fmr1 KO mice and rescue by Fenobam.
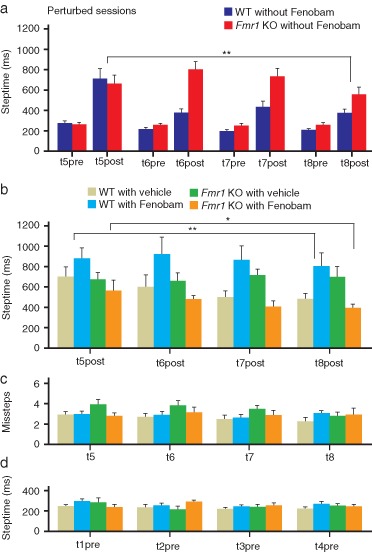
(a) Motor learning was tested with the use of the Erasmus Ladder by calculating for every associative motor learning session (‘perturbed sessions'; (t5post to t8post) the average duration of the step that immediately followed the onset of the US (‘post-steptime’). As control, we also calculated the average duration of the step that immediately preceded the onset of the US (‘pre-steptime’). Fmr1 KO (n = 16) mice showed a specific deficit in procedural memory formation in that they showed longer post-steptimes to an auditory conditioned stimulus than their WT littermates (n = 17) (P = 0.002; repeated measures anova). The differences in post-steptimes between Fmr1 WT and KO mice did not depend on differences in the pre-steptime values (P = 0.085; repeated measures anova). (b) Fmr1 KO mice injected with Fenobam (n = 10) showed faster post-steptime responses than Fmr1 KO mice that receive vehicle (n = 9) (P = 0.046; LSD post hoc test). Moreover, they were indistinguishable from vehicle-treated WT mice (n = 11) (P = 0.303; LSD post hoc test). In contrast, Fenobam had a negative side effect on WT mice (n = 13) in that it increased their post-steptimes compared with the animals that were injected with vehicle (P = 0.004; LSD post hoc test). (c), (d) Interestingly, the number of missteps and pre-steptime responses during the same period of training were not affected by the administration of Fenobam neither in Fmr1 KO nor in WT mice (missteps P = 0.234; pre-steptime P = 0.463; repeated measures anova). Error bars represent standard error of the mean. Asterisks indicate level of significance: * stands for P < 0.05; ** stands for P < 0.01; *** stands for P < 0.001.
Figure 3. Avoidance discrimination impairments of Fmr1 KO mice measured with the Erasmus Ladder.
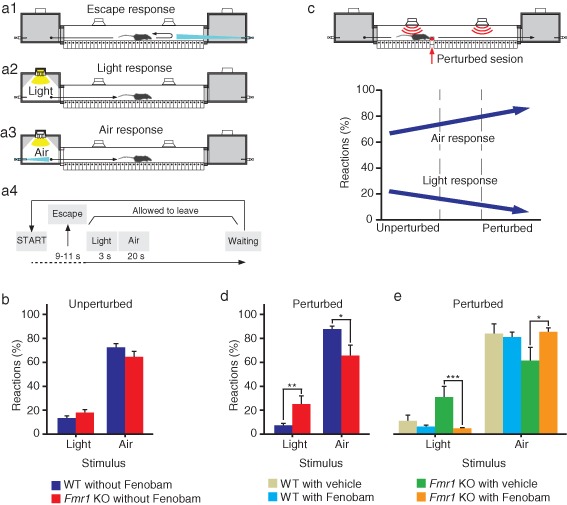
Light and air stimuli are used to control the moment of departure. (a1) When the mouse leaves the starting box before the light turns on, the crosswind is turned on at full force (30 km/h) from the opposite box. This wind usually causes the mouse to immediately return to the starting shelter. (a2) When the shelter light is turned on the mouse is allowed to walk on the ladder. The light will remain on until the mouse reaches the end box. Permitted ladder crossing is accompanied by a tailwind that is kept constant at 16 km/h at the actual position of the mouse. (a3) If the mouse does not leave the starting box within 3 seconds after the light is turned on, an air puff comes from the pressurized air outlets in this shelter. Normally, the air puff encourages the mouse to leave the starting box. (a4) A schematic representation of the temporal order of events is mentioned under a1–a3. (b) During unperturbed sessions there was no difference in the percentage of reactions, neither to the light nor to the air stimuli, between Fmr1 KO mice (n = 32) and their WT littermates (n = 41) (light: P = 0.136; air: P = 0.154; one-way anova). (c) The percentage of times that WT mice properly responded to the light stimulus decreased when they were transferred from the unperturbed to the perturbed sessions. The opposite occurred with the percentage of reactions to the air stimulus. (d) Fmr1 KO mice (n = 13) reacted significantly more to the light and less to the air stimuli than WT mice (n = 17) (light: P = 0.006; air: P = 0.007; one-way anova). Fenobam-treated Fmr1 KO mice (n = 10) decreased their response to the light and increased their response to the air stimuli with respect to vehicle-treated Fmr1 KO mice (n = 9) (light: P = 0.001; air: P = 0.013, LSD post hoc tests); furthermore, their responses to light and air stimuli were similar to that of the vehicle-treated WT mice (n = 11) (light: P = 0.309; air: P = 0.975, LSD post hoc tests). Error bars represent standard error of the mean. Asterisks indicate level of significance: * stands for P < 0.05; ** stands for P < 0.01; *** stands for P < 0.001.
Statistical analysis
Off-line analyses of motor coordination and avoidance behavior were performed using custom written software in Labview (National Instruments) and the results were stored in a relational database (MySQL). Statistical tests were performed with SPSS Statistics (IBM Corporation, New York, NY, USA). Data were compared using one-way analysis of variance (anova) or two-way repeated measures anova, as appropriate. If a significant difference was found, post hoc analysis was performed using Fisher's least significant difference (LSD) test, unless stated otherwise. In total, 39 Fmr1 KO and their 41 WT littermates were measured in the Erasmus Ladder. Four mice were excluded from the analysis of motor performance and motor learning because the computer failed capturing several post-steptime values that could have changed the averaged post-steptime values. Three mice were excluded from the avoidance behavior analysis because they were not measured properly.
Results
Fmr1 KO mice show no deficits in motor performance
To rule out the potential caveat that possible differences in motor learning or avoidance discrimination are partly due to differences in motor performance, we started out by testing the overall motor performance level on the Erasmus Ladder. Unperturbed sessions (s1–s4), that were used to evaluate motor performance capabilities, showed that Fmr1 KO mice (n = 35) and their WT littermates (n = 41) did not show significant differences in steptimes (genotype: F1,74 = 0.06, P = 0.810; repeated measures anova) (Fig. 1). In addition, the number of missteps in Fmr1 KO mice was not significantly different from that in WTs (genotype: F1,74 = 1.65, P = 0.204; repeated measures anova) (data not shown).
Fmr1 KO mice show deficits in procedural memory formation, which can be rescued by Fenobam
In contrast to WT mice (n = 17), which learned to adjust their walking pattern to the CS over the sessions, Fmr1 KO (n = 16) mice did not learn to do so (genotype × session: F3,93 = 5.33, P = 0.002; repeated measures anova) (Fig. 2a). Already during the second perturbed session, WT mice showed decreased post-steptimes (t6post: 374.24 ± 37 ms vs. t5post: 711.12 ± 99 ms, P < 0.001; one-way anova with Dunnett's post hoc test). The Fmr1 KO mice did not show any motor learning during the perturbed sessions (F3,60 = 0.16, P = 0.160; one-way anova) (Fig. 2a). Even during the fourth perturbed session, Fmr1 KO mice did not show a clear reduction in post-steptimes compared with the first perturbed session (t8post: 557.97 ± 72 ms vs. t5post: 663.84 ± 82 ms). The difference in post-steptimes between WT and Fmr1 KO mice did not depend on a difference in steptimes before the onset of the CS–US stimuli as shown by the pre-steptime values which were not statistically different (genotype × session: F3,93 = 2.27, P = 0.085; repeated measures anova) (Fig. 2a).
To explore the effect of an mGluR5 inhibitor on procedural memory formation we administrated (30 min before each perturbed session) Fenobam or MC as vehicle to both Fmr1 KO and WT mice. The interaction genotype × treatment was statistically different (genotype × treatment: F1,39 = 9.29, P = 0.004; repeated measures anova). Fmr1 KO mice treated with Fenobam (n = 10) showed faster post-steptime responses during the perturbed sessions than those that were injected with vehicle (n = 9) (P = 0.046; LSD post hoc test) (Fig. 2b). Moreover, they were indistinguishable from WT mice treated with vehicle (n = 11) (P = 0.303; LSD post hoc test) (Fig. 2b). This improvement was not due to the injection itself, because vehicle-treated Fmr1 KO were not significantly different from those that did not received treatment (P = 0.955; LSD post hoc test). The overall level of motor performance remained intact during the perturbed sessions in that there was no significant interaction genotype × treatment neither in the number of (genotype × treatment: F1,39 = 1.46, P = 0.234; repeated measures anova) (Fig. 2c) nor in pre-steptime responses (genotype × treatment: F1,39 = 0.549, P = 0.463; repeated measures anova) (Fig. 2d).
Fenobam elicits negative side effects in wild types
In contrast, the motor learning in Fenobam-treated WT (n = 13) was severely impaired. In this group, post-steptimes increased significantly compared with animals that were not treated (P < 0.001; LSD post hoc test) or that received vehicle (P = 0.004; LSD post hoc test) (Fig. 2b). Again, this was not due to the injection, because vehicle-treated WT mice showed normal post-steptimes compared with nontreated animals (P = 0.305; LSD post hoc test) (data not shown).
Fmr1 KO mice show abnormal avoidance behavior, which can be rescued by Fenobam
During unperturbed sessions WT (n = 41) and Fmr1 KO (n = 32) mice used light and air as cues to leave the box and to start to walk on the ladder at a similar level (light genotype: F1,71 = 2.27, P = 0.136; air genotype: F1,71 = 2.07, P = 0.154; one-way anova) (Fig. 3b). However, during perturbed sessions, WT mice reacted significantly less to light and significantly more to air stimuli than during the unperturbed sessions (light phase: F1,56 = 5.86, P = 0.019; air phase: F1,56 = 11.79, P = 0.001; one-way anova). These changes reflect avoidance behavior in order to delay the exposure to the perturbation. In Fmr1 KO mice this avoidance behavior did not occur; they did not show any change in their reactions, neither to the light nor to the air stimuli (light phase: F1,43 = 0.99, P = 0.326; air phase: F1,43 = 0.009, P = 0.763; one-way anova) (Fig. 3b,d); in line with these results, they also reacted more to the light and less to the air stimuli than their WT littermates during the perturbed sessions (light genotype: F1,28 = 8.67, P = 0.006; air genotype: F1,28 = 8.41, P = 0.007; one-way anova) (Fig. 3d). Importantly, the administration of Fenobam rescued the abnormal avoidance behavior of Fmr1 KO mice. The interaction genotype × treatment was significant for both light and air stimuli (light genotype × treatment: F1,39 = 4.85, P = 0.034; air genotype × treatment: F1,39 = 4.36, P = 0.043; one-way anova). Fmr1 KO mice treated with Fenobam decreased the percentage of occasions that they reacted to the light stimulus with respect to vehicle-treated Fmr1 KO mice from 29.78% ± 8.72 to 4.46% ± 0.73 (P = 0.001; LSD post hoc test) and significantly increased their percentage of responses to the air stimulus from 57.77% ± 10.11 to 82.35% ± 4.17 (P = 0.013; LSD post hoc test) during perturbed sessions; moreover, they were not significantly different from vehicle-treated WT mice (light: P = 0.309; air: P = 0.975; LSD post hoc test) (Fig. 3e). This improvement was due to the administration of Fenobam and not due to the injection itself, because vehicle-treated Fmr1 KO mice showed the same response to the light and air as Fmr1 KO mice that did not receive any treatment (light: P = 0.407; air: P = 0.540; LSD post hoc tests).
Discussion
Subjecting Fmr1 KO mice to treatment with Fenobam while performing locomotion conditioning tasks showed that the Erasmus Ladder can be used to test different types of learning simultaneously and to assess the impact of different drugs at a medium to high throughput level. Our main findings are that Fmr1 KO mice show deficits in both associative motor learning and avoidance behavior and that Fenobam, which is a selective mGluR5 inhibitor, can rescue both deficits. In addition, we show that Fenobam treatment of WT mice showed profound side effects in the motor coordination task. Together, these findings are in line with the mGluR hypothesis, and they offer, as will be discussed below, impetus to potential therapies of both motor and cognitive symptoms in FXS patients.
Fmr1 KO mice showed a marked deficit in procedural memory formation in that they did not reduce significantly their post-steptime response during the perturbation training sessions. Given that there were no differences in steptimes during the unperturbed sessions, we can conclude that the higher values of post-steptimes of Fmr1 KO mice were not due to motor performance deficits. The deficits in locomotion conditioning on the Erasmus Ladder agree with the findings by Koekkoek et al. (2005) in which it was shown that Fmr1 KO mice and FXS patients show deficits in classical delay eyeblink conditioning. The abnormalities of the Fmr1 KO mice in locomotion conditioning were rescued after administration of 30 mg/kg of Fenobam, 30 min before the learning task started. In fact, Fmr1 KO mice were able to reduce significantly their post-steptime response to an auditory CS, achieving values that were close to those of WT mice. As application of vehicle (MC alone) did not significantly affect motor behavior in the Fmr1 KO mice, it is parsimonious to conclude that the therapeutic impact of Fenobam in Fmr1 KO mice on procedural memory formation was not due to the induction of stress or other sham effects that might have been induced by the injection itself.
Fmr1 KO mice also showed a marked deficit in avoidance behavior in that they did not show the normal waiting reaction inside the box; they did not delay the exposure to the suddenly occurring aversive stimulus, i.e. the perturbation due to the rising rung. WT mice tend to remain inside the boxes as long as possible during the unpleasant perturbation sessions. They usually do not leave the box when the light is turned on and they need the air stimulus significantly more often to force them out of the box as compared to the unperturbed performance task. Fmr1 KO mice instead did not modify their responses to the light and air departure cues after they were transferred from the unperturbed to the perturbed sessions. It appeared as if they did not fully perceive and/or did not know how to react to a stressful situation. This type of apathetic reaction might point toward a decrease in anxiety and/or a decrease in fear memory. This possibility is in line with other FXS mouse model studies in which a general lower level of anxiety and/or general deficits in fear memory formation were observed (Liu & Smith 2009). Importantly, administration of Fenobam also rescued this cognitive phenotype. These results agree with the reports that showed positive effects of mGluR5 inhibitors on abnormalities in prepulse inhibition (PPI) in FXS patients (Berry-Kravis et al. 2009) and Fmr1 KO mice (de Vrij et al. 2008).
While Fmr1 KO mice improved both their procedural memory formation and avoidance discrimination after administration of Fenobam, WT mice showed a clear negative side effect in that their procedural memory formation was severely impaired. Similarly, Jacob et al. reported impairments in the passive avoidance test, the Morris water maze and contextual fear conditioning following administration of similar dosages of Fenobam (Jacob et al. 2009). These findings emphasize the critical status of a proper diagnosis for patients with intellectual disability. While patients with FXS may benefit from mGluR5 inhibitors without overt negative side effects (Berry-Kravis et al. 2009), other patients with different forms of intellectual disability may suffer profoundly from inadequate treatment with drugs like Fenobam.
Conclusions
Testing locomotion conditioning and avoidance discrimination in mutant mice can be performed reliably and noninvasively with the use of the Erasmus Ladder, and the impact of drugs on these tests can be screened at a medium to high throughput level. Fmr1 KO mice show deficits in both procedural memory formation and avoidance discrimination and both deficits can be rescued with Fenobam. On the basis of this mouse model study, it can be inferred that the use of mGluR inhibitors may be beneficial for procedural memory formation and avoidance discrimination in FXS patients, but it appears contraindicated for healthy controls or non-FXS patients with intellectual disability.
Acknowledgments
We kindly thank the Dutch Organization for Medical Sciences (ZonMw 91207022; RW, BAO, CIDZ), Life Sciences (ALW; CIDZ), Senter (Neuro-Bsik; CIDZ), Prinses Beatrix Fonds (CIDZ), and the SENSOPAC, CEREBNET and C7 programs of the European Community (CIDZ) for their financial support. We thank M. Valk and C. Martin for technical assistance.
References
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103:1043–1050. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, Shubek L, Holmgreen P, Yeargin-Allsopp M, Boyle C, Sherman SL. Prevalence of the fragile X syndrome in African-Americans. Am J Med Genet. 2002;110:226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- Ermisch A, Landgraf R, Mobius P. Vasopressin and oxytocin in brain areas of rats with high or low behavioral performance. Brain Res. 1986;379:24–29. doi: 10.1016/0006-8993(86)90251-9. [DOI] [PubMed] [Google Scholar]
- Errijgers V, Van Dam D, Gantois I, Van Ginneken CJ, Grossman AW, D’Hooge R, De Deyn PP, Kooy RF. FVB.129P2-Pde6b+ Tyr c−ch/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes Brain Behav. 2007;6:552–557. doi: 10.1111/j.1601-183X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Farr TD, Liu L, Colwell KL, Whishaw IQ, Metz GA. Bilateral alteration in stepping pattern after unilateral motor cortex injury: a new test strategy for analysis of skilled limb movements in neurological mouse models. J Neurosci Methods. 2006;153:104–113. doi: 10.1016/j.jneumeth.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, von Leden RE, Ta BT, Goodrich-Hunsaker NJ, Arque G, Kim K, Willemsen R, Berman RF. Motor deficits on a ladder rung task in male and female adolescent and adult CGG knock-in mice. Behav Brain Res. 2011;222:117–121. doi: 10.1016/j.bbr.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob W, Gravius A, Pietraszek M, Nagel J, Belozertseva I, Shekunova E, Malyshkin A, Greco S, Barberi C, Danysz W. The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning. Neuropharmacology. 2009;57:97–108. doi: 10.1016/j.neuropharm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Levenga J, de Vrij FM, Oostra BA, Willemsen R. Potential therapeutic interventions for fragile X syndrome. Trends Mol Med. 2010;16:516–527. doi: 10.1016/j.molmed.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, Smith CB. Dissociation of social and nonsocial anxiety in a mouse model of fragile X syndrome. Neurosci Lett. 2009;454:62–66. doi: 10.1016/j.neulet.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mientjes EJ, Nieuwenhuizen I, Kirkpatrick L, Zu T, Hoogeveen-Westerveld M, Severijnen L, Rife M, Willemsen R, Nelson DL, Oostra BA. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol Dis. 2006;21:549–555. doi: 10.1016/j.nbd.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas M, Mandel J. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Oostra BA, Willemsen R. FMR1: a gene with three faces. Biochim Biophys Acta. 2009;1790:467–477. doi: 10.1016/j.bbagen.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsalis PC, Sismani C, Hettinger JA, Boumba I, Georgiou I, Stylianidou G, Anastasiadou V, Koukoulli R, Pagoulatos G, Syrrou M. Molecular screening of fragile X (FRAXA) and FRAXE mental retardation syndromes in the Hellenic population of Greece and Cyprus: incidence, genetic variation, and stability. Am J Med Genet. 1999;84:184–190. [PubMed] [Google Scholar]
- Paylor R, Yuva-Paylor LA, Nelson DL, Spencer CM. Reversal of sensorimotor gating abnormalities in Fmr1 knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122:1371–1377. doi: 10.1037/a0013047. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15:549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Buttelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Muhlemann A, Gatti S, Mutel V, Malherbe P. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315:711–721. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Renier N, Schonewille M, Giraudet F, Badura A, Tessier-Lavigne M, Avan P, De Zeeuw CI, Chedotal A. Genetic dissection of the function of hindbrain axonal commissures. PLoS Biol. 2010;8:e1000325. doi: 10.1371/journal.pbio.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaratnam M, Murthy NV, Wijeratne A, Buckingham A, Payne S. Autistic-like behaviour profile and psychiatric morbidity in Fragile X Syndrome: a prospective ten-year follow-up study. Eur Child Adolesc Psychiatry. 2003;12:172–177. doi: 10.1007/s00787-003-0333-3. [DOI] [PubMed] [Google Scholar]
- Schaeffer C, Beaulande M, Ehresmann C, Ehresmann B, Moine H. The RNA binding protein FMRP: new connections and missing links. Biol Cell. 2003;95:221–228. doi: 10.1016/s0248-4900(03)00037-6. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Serysheva E, Yuva-Paylor LA, Oostra BA, Nelson DL, Paylor R. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between Fragile X-related proteins. Hum Mol Genet. 2006;15:1984–1994. doi: 10.1093/hmg/ddl121. [DOI] [PubMed] [Google Scholar]
- Ursin H. Effect of amygdaloid lesions on avoidance behavior and visual discrimination in cats. Exp Neurol. 1965;11:298–317. doi: 10.1016/0014-4886(65)90050-6. [DOI] [PubMed] [Google Scholar]
- Van Der Giessen RS, Koekkoek SK, van Dorp S, De Gruijl JR, Cupido A, Khosrovani S, Dortland B, Wellershaus K, Degen J, Deuchars J, Fuchs EC, Monyer H, Willecke K, De Jeu MT, De Zeeuw CI. Role of olivary electrical coupling in cerebellar motor learning. Neuron. 2008;58:599–612. doi: 10.1016/j.neuron.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Van der Molen MJ, Huizinga M, Huizenga HM, Ridderinkhof KR, Van der Molen MW, Hamel BJ, Curfs LM, Ramakers GJ. Profiling Fragile X Syndrome in males: strengths and weaknesses in cognitive abilities. Res Dev Disabil. 2010;31:426–439. doi: 10.1016/j.ridd.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Van der Vaart T, van Woerden GM, Elgersma Y, de Zeeuw CI, Schonewille M. Motor deficits in neurofibromatosis type 1 mice: the role of the cerebellum. Genes Brain Behav. 2011;10:404–409. doi: 10.1111/j.1601-183X.2011.00685.x. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- de Vries BB, van den Ouweland AM, Mohkamsing S, Duivenvoorden HJ, Mol E, Gelsema K, van Rijn M, Halley DJ, Sandkuijl LA, Oostra BA, Tibben A, Niermeijer MF. Screening and diagnosis for the fragile X syndrome among the mentally retarded: an epidemiological and psychological survey. Collaborative Fragile X Study Group. Am J Hum Genet. 1997;61:660–667. doi: 10.1086/515496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci U S A. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 1996. ICD-10 Guide for Mental Retardation WHO/MNH/96.3, Geneva.
- Youings SA, Murray A, Dennis N, Ennis S, Lewis C, McKechnie N, Pound M, Sharrock A, Jacobs P. FRAXA and FRAXE: the results of a five year survey. J Med Genet. 2000;37:415–421. doi: 10.1136/jmg.37.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


