To the editor
Life expectancy (LE) and tumor characteristics are clinical factors affecting the likelihood of benefit from curative treatment of prostate cancer. Treatment of patients with shorter LE may contribute to additional costs or complications without a commensurate improvement in quality of life or survival [1–3]. The National Comprehensive Cancer Network Practice Guidelines in Oncology recommends active surveillance as an alternative to curative therapy (CTx) for patients with low-risk tumor characteristics who have LE<10 years. For patients with intermediate-risk cancers and a LE≥10 years, curative therapy (radical prostatectomy or radiation therapy) is recommended [4]. Although treatment options for prostate cancer patients have expanded considerably in recent years, little is known about whether the management of men with early stage prostate cancer has evolved [5–7]. It is unclear whether patterns of care correspond to the likelihood of clinical benefit from treatment, as determined by LE and tumor characteristics. We therefore assessed trends in the use of CTx across strata of potential clinical benefit.
Methods
Using the Surveillance, Epidemiology and End Results-Medicare linked database, we identified men ages 67–84 with localized prostate cancer diagnosed in 1998–2007, including only patients from registries that existed prior to the 2000 SEER expansion [8]. We defined low-risk tumors as those with SEER grade 1 or 2 and stage T1 or T2a and moderate-risk tumors as those with SEER grade 3 or 4 or stage T2b–T2c.
A standard life table approach was used to estimate LE as a function of non-cancer comorbidity [9]. Specifically, we used a sample of patients without a cancer diagnosis recorded in SEER from the Medicare 5% random sample to determine annual mortality rates for each age and comorbidity stratum, and then used these rates to estimate LE. Patients with LE <5, 5–<10 and ≥10 years were classified as having a short, intermediate and long LE. The 10-year survival of these groups was 19.3%, 51.6% and 76.1%, respectively.
We defined CTx as receipt of radiation or prostatectomy within 9 months of cancer diagnosis, as defined by International Classification of Diseases-9 procedure codes and Healthcare Common Procedure Coding System codes (Appendix 1).
Appendix 1.
Prostate Cancer Treatment Billing Codes.
| Surgery | Minimally Invasive | HCPCS: 55866 | |
| Open | HCPCS: 55801, 55810, 55812, 55815, 55821, 55831, 55840, 55842, 55845 ICD-9 Procedure: 60.3, 60.4, 60.5, 60.6, 60.62, 60.69 |
||
| Radiation | External | Beam | HCPCS: 77402, 77403, 77404, 77406, 77407, 77408, 77409, 77411, 77412, 77413, 77414, 77416 |
| IMRT | HCPCS: 77301, 77418, 0073T, G0174 | ||
| Proton | HCPCS: 77520, 77522, 77523, 77525 | ||
| SRS | HCPCS: 77371, 77372, 77373, 0082T, G0173, G0243, G0251, G0339, G0340 | ||
| Brachytherapy | High-dose | HCPCS: 77781, 77782, 77783, 77784, 77799 | |
| Low-dose | HCPCS: 77776, 77777, 77778, G0256, G0261 | ||
Multivariable logistic regression was used to model the receipt of CTx controlling for age, race, marital status, comorbidity, and SEER registry. Chi-square tests were used to ascertain bivariate associations between the independent variables and receipt of CTx. Interactions between LE and diagnosis year were assessed in low-and moderate-risk tumor groups.
Results
The study sample was composed of 39,270 patients; 43.2% had moderate-risk tumors. Nearly 83% of patients were white and 9% were black. The median age was 74 years; 55% had no comorbid conditions, 34.3% had 1–2 conditions and 10.4% had ≥3 conditions. Of patients in the full sample, 64.3% received CTx. There was a strong association between LE and receipt of CTx. Approximately 39.1% of 3,557 patients with a short LE, 62.7% of 23,721 patients with intermediate LE and 75.1% of 11,992 patients with long LE received CTx (p<.001).
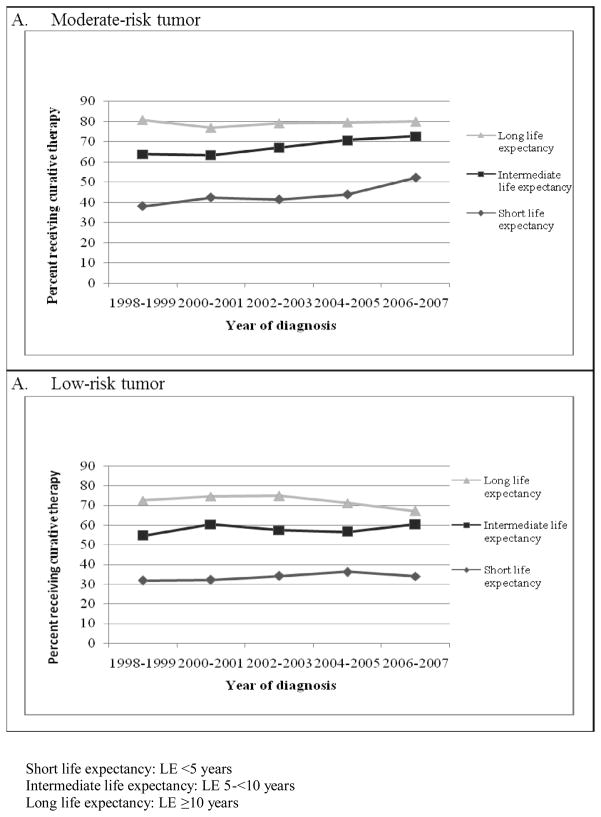
Prostate cancer treatment rates increased over time. Overall, CTx increased from 61.2% to 67.6% from 1998 through 2007 (p<.001). Within each tumor-risk category, the increase in CTx use differed across LE groups. Among men with moderate-risk prostate cancer, there was a substantial increase in CTx rates in the short LE group (from 38.0% in 1998–1999 to 52.1% in 2006–2007; Figure). Conversely, the use of CTx decreased from 80.7% to 80.0% among men with long LE (LE*Time interaction: p=0.015). Among men with low-risk tumors, the use of CTx trended downward for men in the long LE category, but increased for men in the short and intermediate LE categories (Time*LE interaction: p<0.001)
Figure.
Percent of patients with moderate-risk (A) and low-risk (B) tumor characteristics receiving curative therapy over time, stratified by life expectancy.
Comment
Men with localized prostate cancer may not receive CTx in accordance with clinical benefit. During our study period, there was increasingly aggressive treatment of patients with low likelihood of clinical benefit, without a commensurate increase in the treatment of patients with high likelihood of clinical benefit. While not treating potentially fatal cancer can reflect poor-quality care, aggressive management of disease unlikely to progress puts patients at risk for morbidity and increases cost without medical benefits [1–3]. Given widespread concerns about the rate of increase in Medicare expenditures, it is notable that the most substantial increase in our sample was noted among patients who are least likely to benefit. Possible explanations include financial incentives, emergence of new therapies with perceived lower side-effect profiles, and changes in patient preferences. The use of cancer therapies should be informed by clinical evidence and guided by patient preferences. Future work should explore how to better incorporate both cancer characteristics and patient life expectancy into decision-making.
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare Database. This study used the SEER-Medicare linked database. The interpretation and reporting of this data are the sole responsibility of the authors. The authors would also like to acknowledge the National Cancer Institute (5R01CA149045) and generous financial support from the James G. Hirsch Medical Student Research Fellowship.
Footnotes
Financial disclosures: None
References
- 1.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–8. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 2.Miller DC, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772–80. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 3.Potosky AL, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96(18):1358–67. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 4.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8(2):145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 5.Bolenz C, et al. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol. 2010;57(3):453–8. doi: 10.1016/j.eururo.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Cahlon O, Hunt M, Zelefsky MJ. Intensity-modulated radiation therapy: supportive data for prostate cancer. Semin Radiat Oncol. 2008;18(1):48–57. doi: 10.1016/j.semradonc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen PL, et al. J Clin Oncol. 2011. Cost Implications of the Rapid Adoption of Newer Technologies for Treating Prostate Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute N.C. Surveillance, Epidemiology, and End Results. 2009. [Google Scholar]
- 9.Elixhauser A, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]