Abstract
The neural circuits that mediate behavioral choices must not only weigh internal demands and environmental circumstances, but also select and implement specific actions, including associated visceral or neuroendocrine functions. Coordinating these multiple processes suggests considerable complexity. As a consequence, even circuits that support simple behavioral decisions remain poorly understood. Here we show that the environmentally sensitive wing expansion decision of adult fruit flies is coordinated by a single pair of neuromodulatory neurons with command-like function. Targeted suppression of these neurons using the Split Gal4 system abrogates the fly's ability to expand its wings in the face of environmental challenges, while stimulating them forces expansion by coordinately activating both motor and neuroendocrine outputs. The arbitration and implementation of the wing expansion decision by this neuronal pair may illustrate a general strategy by which neuromodulatory neurons orchestrate behavior. Interestingly, the decision network exhibits a plasticity that is unmasked under conducive environmental conditions in flies lacking the function of the command-like neuromodulatory neurons. Such flies can often expand their wings using a motor program distinct from that of wild-type animals and controls. This compensatory program may be the vestige of an ancestral, environmentally insensitive program used for wing expansion that existed before the evolution of the environmentally adaptive program currently used by Drosophila and other cyclorrhaphan flies.
Introduction
How and where in the nervous system behavioral decisions are made is unknown with any degree of precision for most behaviors. Studies in primates reveal a critical role for forebrain structures in decisions that require memory and motor planning (Gold and Shadlen, 2007; Kable and Glimcher, 2009), but decerebrate animals without these structures make basic behavioral decisions regarding survival and reproduction, indicating that there are additional decision-making loci, probably involving the hypothalamus and brainstem (Swanson, 2000; Humphries et al., 2007). Decisions that require these structures are often accompanied by changes in neuroendocrine or visceral function that support the behaviors being executed, but very little is known about the mechanisms coordinating either the motor patterns that constitute behavioral programs or the concomitant autonomic changes.
More is known about the implementation of behavioral decisions in invertebrates, where motor pattern generators have been characterized with cellular and molecular resolution (Marder and Bucher, 2001; Kristan et al., 2005; Katz and Hooper, 2007). In keeping with the overall similarity of motor control in vertebrates and invertebrates (Kien and Altman, 1992; Büschges et al., 2011; Mullins et al., 2011), these pattern generators have been shown in numerous cases to be regulated by more rostrally disposed higher-order neurons (Kupfermann and Weiss, 2001) capable of initiating behavioral components of escape (Edwards et al., 1999), locomotion (Friesen and Kristan, 2007), feeding (Kupfermann and Weiss, 2001; Elliott and Susswein, 2002), mating (Hedwig, 2006; von Philipsborn et al., 2011), and postural change (Larimer and Moore, 2003; Mesce et al., 2008). As in vertebrates, however, it is generally not understood how component motor patterns are integrated to produce coherent whole-animal behavior.
Neuromodulators, with their diverse targets and capacity to reconfigure pattern-generating networks, are attractive candidates for accomplishing behavioral integration (Nusbaum et al., 2001; Dickinson, 2006) and are known to elicit basic behaviors when introduced into the CNS (Kow et al., 1994; Leibowitz and Wortley, 2004). Recent work using genetic techniques to target specific cell populations confirms that neuromodulatory neurons can coordinate complex motor output. For example, by selectively activating hypothalamic neurons that express agouti-related peptide, a known, positive modulator of feeding, Aponte et al. (2011) demonstrated the robust induction of eating behavior in mice. Likewise, stimulation of neurons in Drosophila that express the neuropeptide CCAP (i.e., crustacean cardioactive peptide) and other factors (Kim et al., 2006) induces the motor program underlying wing expansion in newly emerged adults (Peabody et al., 2009). Interestingly, the latter manipulation concomitantly triggers hormonal release to facilitate somatic changes that support expansion (Reynolds, 1977; Luan et al., 2006b), making this system a useful model for studying the coordination of behavioral and neuroendocrine output in behavioral decisions.
Here we dissect the architecture of command in the CCAP network. We find that a single pair of neuromodulatory neurons located in the subesophageal ganglion coordinates the activation of the wing expansion motor program and the neuroendocrine output neurons in inhibitory environments. Surprisingly, in noninhibitory environments, the suppression of behavioral command can be compensated for by other motor patterns.
Materials and Methods
Fly culture/crosses.
All flies were grown on cornmeal–molasses medium and maintained at 25°C in a constant 12 h light-dark cycle. The following lines used in this study have been described previously: w;CCAP-Gal4;+ (Park et al., 2003), w; UAS-TRPM8C4D;+; w; +; UAS-TRPM8C4A (Peabody et al., 2009), w; Burs-Gal4;+ (Peabody et al., 2008), yw;+;UAS-Kir2.1 (Paradis et al., 2001); w;+;UAS-EGFP (Halfon et al., 2002). We thank Deborah Hursh (FDA, Bethesda, MD) for the UAS-hid line. The UAS-pburs-RNAi line (#27141) and the UAS-dicer-2 line (#60014) used to facilitate RNAi-mediated knockdown in the BAG neurons were from the Vienna Drosophila RNAi Center (Dietzl et al., 2007).
Generation of the BSEG and BAG Split-Gal4 hemidrivers.
The BursGal4DBD construct, which is designed to express the Gal4 DNA-binding domain under the control of the bursicon α-subunit (i.e. burs) promoter, was made from the previously described elavGal4DBD construct (Luan et al., 2006a). The elav promoter was first removed by EcoRI and NotI digestion and replaced with the 252 bp burs promoter described previously (Peabody et al., 2008). Transgenic lines were made by Rainbow Transgenic Flies and a third chromosome insert (i.e., BursGal4DBD-U6A1) was used.
VP16AD enhancer trap lines were generated by p-element mobilization in previously published lines (Luan et al., 2006a) using the transposase-bearing line, w;+;Δ2–3 (a gift from Dr. Yuzhong Cheng; NICHD, Bethesda, MD) and standard methods (Grigliatti, 1998). Approximately 400 ETVP16AD lines were made and subsequently screened for expression within the bursicon-expressing neurons by crosses to a line carrying both the BursGal4DBD and UAS-EGFP transgenes. The ETVP16AD-99 line, with principal expression in the two bursicon-expressing neurons of the subesophageal ganglion (BSEG) and the ETVP16AD-N9A88A line, with expression in the 14 bursicon-expressing neurons of the abdominal ganglion (BAG) were used in this study. Both lines carry second chromosome inserts of the transgene. Consensus patterns of UAS-EGFP expression for these hemidrivers paired with BursGal4DBD were determined as described by Luan et al. (2006b). While strong and exclusive expression in all 14 BAG neurons was consistently observed with the ETVP16AD-N9A88A ∩ BursGal4DBD combination, developmental expression profiles indicated that the ETVP16AD-99 ∩ BursGal4DBD combination drove UAS-EGFP expression in at least a subset of BAG neurons during metamorphosis in addition to the BSEG. In approximately two-thirds of newly eclosed adults, there was persistent EGFP expression in one BAG neuron, and weaker labeling was sometimes detectable in up to two other BAG neurons in one-third of animals. Low-frequency (i.e., in less than one-third of animals), low-level expression of EGFP was also seen in a nonbursicon-expressing neuron located posteriorly in the abdominal ganglion. However, exclusive expression within the BSEG was observed when driving UAS-TRPM8, due either to lower sensitivity of anti-TRPM8 immunostaining relative to EGFP fluorescence or to reduced perdurance of the TRPM8 protein versus EGFP.
Behavioral observations and analysis.
Behavioral observations of unperturbed flies were made on single animals transferred as pupae into two types of noninhibitory environment, either a food vial filled with 5 ml of cornmeal–molasses medium, or a 1 × 1 × 3 cm plastic spectrophotometer cuvette filled with 250 μl of 1% agarose. Pupae were transferred ∼18 h before eclosion and posteclosion behavior was scored either directly by eye for animals in food vials, or by videorecording and later analysis for animals in cuvettes. Videorecording was conducted until the completion of wing expansion, or, in the absence of expansion, for up to 7 h. Walking, grooming, and abdominal flexion bouts were scored. The time to wing expansion and/or flexion was calculated as the time from eclosion until flexion was completed and the abdomen assumed its normal posture, or until the splayed wings had returned to their unsplayed condition. This condition coincided well with the end of abdominal flexion and was easier to score. Mild perturbation was accomplished by gently tapping single animals from the food vial in which they had just eclosed into a fresh food vial and observing them by eye without further perturbation. The inhibitory environment has been described before (Peabody et al., 2009) and consisted of a 0.3 cm diameter × 0.7 cm length cylindrical glass minichamber. A single animal was placed into a minichamber within 3 min of eclosion for videorecording and subsequent analysis. For neuronal activation experiments, the chambers were set at 18°C for 15 min to activate the TRPM8 channel and then transferred to a Peltier plate maintained at 25°C for videorecording. Videorecording was accomplished with a Sony HDR-FX7 digital video camera. Even wild-type flies in the minichamber sometimes failed to expand their wings after delayed execution of expansional behaviors, likely due to progressive stiffening of the cuticle due to secondary tanning processes (Cottrell, 1962). For convenience, the time to complete flexion and the time to complete wing expansion (which are equivalent in most flies) are collectively labeled in figures as the time of expansional delay.
Air swallowing was analyzed as described previously (Peabody et al., 2008) by measuring the volume of air present in the gut after the termination of wing expansion and/or abdominal flexion. Whereas all other behavioral observations were made on either males or females without discrimination, air swallowing measurements were performed independently on males and females because of the systematic differences in the volumes of swallowed air between the two sexes. Final wing phenotypes were typically scored at least 24 h after eclosion using previously described criteria (Luan et al., 2006b). For the various manipulations described, statistical differences between experimental and control groups of flies were assessed by two-tailed t test.
Immunohistochemistry and immunoblotting.
Whole mounts of the CNS from either males or females were prepared, stained, and analyzed by confocal microscopy as described previously (Luan et al., 2006b) using the following primary antibodies: rabbit anti-burs (anti-bursicon α-subunit; 1:5000), rat anti-pburs (anti-bursicon β-subunit; 1:500; kind gift from Aaron Hsueh; Stanford University, Stanford, CA), and goat anti-TRPM8 (1:2000; Everest Biotech). In all cases, donkey serum was used in the blocking solution. Kir2.1 expression was analyzed by EGFP fluorescence, taking advantage of the N-terminal EGFP tag on the UAS-Kir2.1 construct (Baines et al., 2001).
To analyze anti-burs immunoreactivity in the BAG fibers of the abdominal nerves and the BSEG fibers of thoracic segment, T2, whole-animal fillets were prepared and analyzed as reported previously (Peabody et al., 2008). For box-and-whisker plots of immunohistochemistry and behavioral data, the boxes represent the interquartile range of the data separated by the median value, and the whiskers (error bars) indicate the 10th and 90th percentiles of the data. Outliers are represented as x's.
Hemolymph collection and Western blotting were performed essentially as described by Peabody et al. (2009). Experimental flies of mixed sex were collected within 5 min of eclosion at room temperature (∼24°C) and put into the inhibitory environment. Within 10 min, they were subjected to a 15 min temperature shift to 18°C and then returned to room temperature for 15 min before hemolymph collection. Negative control flies were handled in parallel, but without temperature shift (i.e., they spent 30 min at room temperature) and hemolymph for positive control samples was collected from unperturbed flies within an hour of eclosion. In all cases, samples were pooled from 14–17 flies to collect 1 μl of hemolymph. For 3 μl of hemolymph, 45–50 flies were needed. Anti-burs antibody was used at 1:2000.
Results
Neuromodulatory command of wing expansion in adverse environments
Upon emergence from the pupal case, adult fruit flies will perch if the environment is suitable and expand their wings. This process is accomplished by forcing blood into the folded wing pads. The increase in blood pressure required for expansion results from the simultaneous execution of two motor patterns, one an adapted feeding behavior in which the fly swallows air, and the other a tonic radial constriction and downward flexion of the abdomen that lasts ∼15 min (Denlinger and Zdarek, 1994; Baker and Truman, 2002; Peabody et al., 2009). These two motor patterns, which serve together to drive blood out of the abdomen and into the wings, must be accompanied by physiological changes in wing properties for expansion and subsequent hardening to occur (Honegger et al., 2008).
If newly emerged flies find themselves in an adverse environment, something that likely happens regularly in the wild and can be accomplished in the laboratory by placing them in a tightly confining chamber, they will search for a more suitable perch and delay wing expansion for up to several hours. Even in an inhibitory environment, however, perching and subsequent wing expansion can be forced by stimulating a set of ∼50 central neurons that express the neuropeptide CCAP (Peabody et al., 2009). Although CCAP itself plays no known role in wing expansion, a subset of the CCAP-expressing neurons coexpresses the heterodimeric hormone bursicon, which is an essential molecular determinant of that process (Luan et al., 2006b). Flies with genetic lesions in the bursicon signaling pathway fail to expand their wings, as do animals in which the bursicon-expressing neurons have been suppressed (Baker and Truman, 2002; Dewey et al., 2004; Peabody et al., 2008).
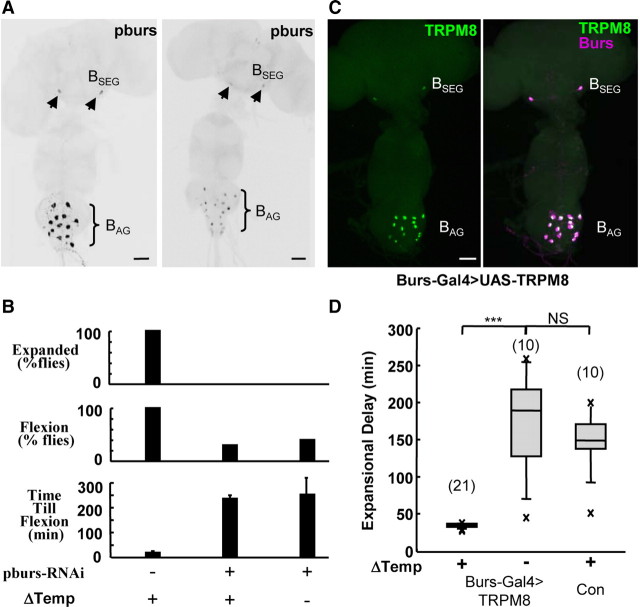
Given bursicon's critical role in wing expansion, we reasoned that at least part, and perhaps all, aspects of the induction of wing expansion by stimulation of the CCAP-expressing neurons was mediated by this molecule. To directly test the role of bursicon in the induction of wing expansion, we selectively reduced bursicon levels in these neurons by RNAi-mediated knock-down of the essential bursicon subunit pburs (Fig. 1A) (Luo et al., 2005; Mendive et al., 2005) and examined the ability of neuronal stimulation to induce expansion. Newly emerged flies were placed in a confined environment, and the CCAP-expressing neurons, which expressed the cold-activated channel TRPM8 in addition to the pburs-RNAi construct, were then selectively stimulated by briefly shifting the animals from 25°C to 18°C. To assess induction, we scored animals for persistent abdominal constriction and downward flexion (henceforth referred to as abdominal flexion), one of the two behavioral signatures of wing expansion. Whereas all control animals rapidly flexed their abdomens in response to activation of the CCAP-expressing neurons, and expanded their wings as previously reported (Peabody et al., 2009), none of the animals expressing pburs-RNAi expanded (Fig. 1B, top), confirming the essential role of bursicon in that process. Wing expansion behavior was also eliminated in most flies, though approximately one-third of the stimulated animals did exhibit tonic abdominal flexion. However, they did so only after environmental delays similar to those of flies in which the CCAP-expressing neurons were not activated. We conclude that bursicon is required for the command-like function of the CCAP neurons, and that bursicon levels must exceed some threshold to be able to trigger wing expansion behavior in the face of environmental inhibition.
Figure 1.
Bursicon and bursicon-expressing neurons are critical for the induction of wing expansion in adverse environments. A, CNS whole mounts from newly eclosed flies expressing either TRPM8 alone (left) or TRPM8 plus an RNAi construct directed against the bursicon subunit pburs (right) in CCAP-expressing neurons were labeled with an anti-pburs antibody. pburs-RNAi expression substantially reduced anti-pburs immunostaining in both groups of bursicon-expressing neurons, the BSEG (arrowheads) and the BAG (brackets). Scale bar, 50 μm. B, Bar graphs showing that flies expressing pburs-RNAi did not expand their wings (top). Unlike control flies not expressing pburs-RNAi, ∼30% of flies in which pburs levels were knocked down exhibited expansional behavior (middle), but only after environmental delays in the time till abdominal flexion (bottom), even when the CCAP-expressing neurons were stimulated (Δtemperature). C, Burs-Gal4 drives UAS-TRPM8 expression in both subsets of bursicon-expressing neurons, the BAG and the BSEG, as revealed by double-labeling with anti-burs (magenta) and anti-TRPM8 (green) immunolabeling. Scale bar, 50 μm. D, Box plots show that under adverse conditions, a 15 min temperature shift to 18°C from 25°C (ΔTemp) rapidly induced wing expansion in Burs-Gal4>TRPM8 flies (+) compared with control animals either of the same genotype and not subjected to temperature shift (−) or lacking the Burs-Gal4 driver [control (Con)]. The no-temperature-shift control plot includes two animals that tonically flexed their abdomens, but did not fully expand their wings. As noted in Materials and Methods, this sometimes happens after prolonged delays. In these cases, “Expansional Delay” indicates the time until termination of abdominal flexion. ***p < 0.001; NS, not significant (p > 0.05); evaluated by t test. The number of animals in each group is in parentheses above each plot. Error bars are SD.
To directly determine whether the bursicon-expressing neurons alone could command wing expansion, we selectively targeted expression of TRPM8 to the bursicon-expressing neurons using the Burs-Gal4 driver (Fig. 1C) (Peabody et al., 2008) and stimulated them as before by briefly shifting animals confined within an inhibitory environment from 25°C to 18°C. We found that activation of the bursicon-expressing neurons quickly forced expansion compared with control animals not subjected to cold or lacking the Burs-Gal4 driver (Fig. 1D). The effect is essentially identical to that produced by stimulation of the CCAP-expressing neurons and demonstrates that the bursicon-expressing neurons alone can command wing expansion.
The BSEG neurons have command-like function
The bursicon-expressing neurons consist of two anatomically distinct groups, which have been differentially implicated in mediating the somatic and behavioral aspects of wing expansion (Luan et al., 2006b; Peabody et al., 2008). A set of 14 bilaterally paired cells in the abdominal ganglion (i.e., the BAG; Fig. 1A,C) are known to release bursicon into the blood where it is thought to promote, first, plasticization and, later, tanning of the wing cuticle (Honegger et al., 2008), whereas a single pair of cells in the subesophageal ganglion (i.e., the BSEG) have been proposed to act centrally to govern the motor program underlying wing expansion based on indirect evidence. To directly assess the function of the BSEG in promoting the wing expansion motor program, we first used the Split Gal4 system (Luan et al., 2006a) to create hemidriver combinations that permitted selective transgene targeting to the BSEG. Screening a library of enhancer-trap VP16AD hemidriver lines with a BursGal4DBD hemidriver, which expresses the Gal4 DNA-binding domain specifically in the bursicon-expressing neurons, yielded lines that permit UAS-EGFP expression in newly eclosed adults in the BSEG (Fig. 2A–A″) or the BAG (Fig. 2B–B″).
Figure 2.
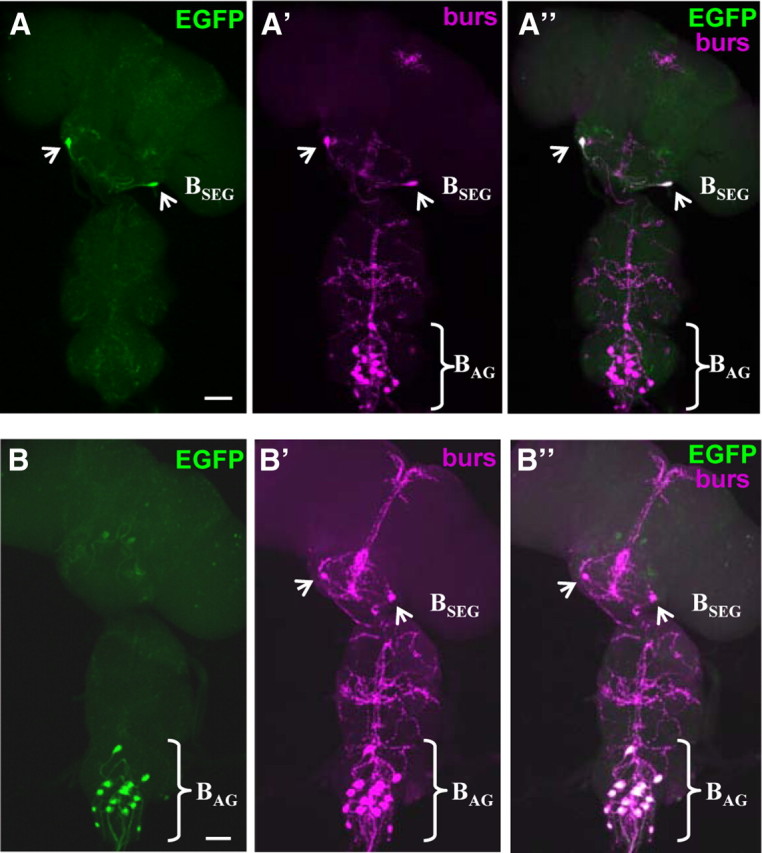
UAS transgenes can be selectively targeted to the BSEG and BAG neurons. A, B, UAS transgene expression (green, UAS-EGFP) can be targeted to the two subsets of bursicon-expressing neurons (magenta, anti-burs) using the BSEG-specific (i.e., ETVP16AD-99 ∩ Burs-Gal4DBD-U6A1; A) or the BAG-specific (i.e., ETVP16AD-N9A88A ∩ Burs-Gal4DBD-U6A1; B) hemidriver combinations. Images are volume-rendered fluorescent confocal micrographs of CNS whole mounts. Scale bars, 50 μm. A′, B′, The corresponding patterns of anti-bursicon α-subunit labeling for the preparations shown in A and B, respectively. A″, B″, Merged images of the patterns of EGFP and anti-bursicon labeling for the two preparations.
We used these lines to activate TRPM8 in either the BSEG or BAG in animals placed in an inhibitory environment. We found that only stimulation of the BSEG induced the abdominal motor pattern characteristic of wing expansion (Fig. 3A; Table 1). In contrast, stimulation of the BAG alone did not accelerate performance of the wing expansion motor program, which was executed only after a delay of 2–3 h, similar to controls. This is consistent with previous results indicating that these neurons have no role in governing behavior, but instead are responsible for bursicon release into the blood. Indeed, Western blot analysis of blood obtained from BAG>TRPM8 animals confirmed the presence of bursicon in the blood after a temperature shift to 18°C (Fig. 3B, top), though interestingly the amount of hormone released was reduced relative to that seen under conditions of normal expansion. This stimulated release of hormone was likely responsible for the notable change in wing morphology that followed BAG activation, in which the wings became malleable and partially unfolded in response to occasional grooming by the hindlegs (Fig. 3C, left).
Figure 3.
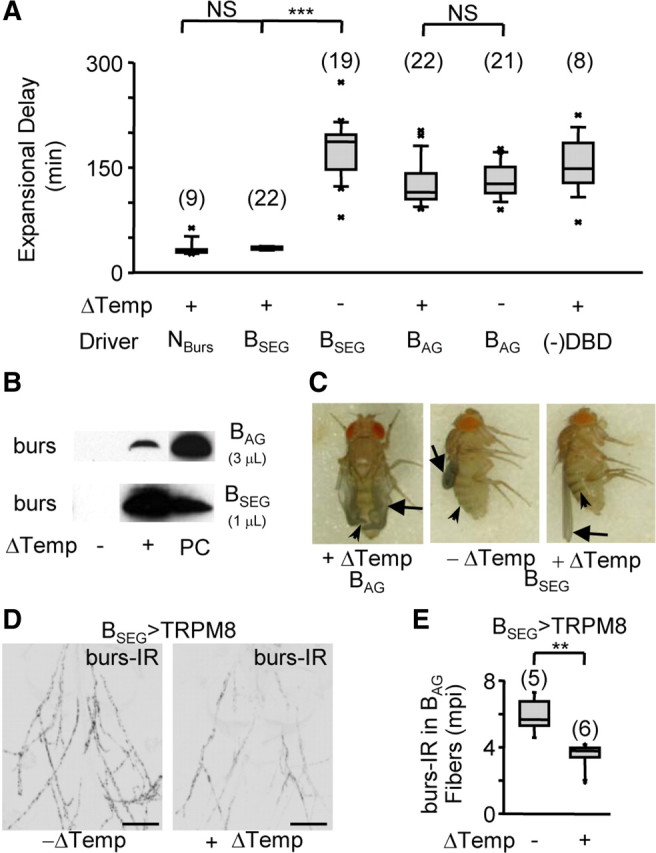
The BSEG neurons command wing expansion in Drosophila. A, Box plots showing the time required to complete expansional behaviors (i.e., Expansional Delay) for flies expressing TRPM8 in the indicated cell group (Driver) with or without stimulation (Δtemperature). (−)DBD, controls lacking the BursGalDBD-U6A1 hemidriver. As noted in the legend of Figure 1, some flies that attempted expansion after prolonged delays failed to expand their wings, in which case the expansional delay represents the time they took to complete abdominal flexion. The number of flies tested in each group is indicated in parentheses above the bars; the results of t test comparisons between groups are indicated by brackets [NS, not significant (p > 0.05); ***p < 0.001]. B, Western blots showing blood titers of bursicon (i.e., anti-burs immunostaining) resulting from stimulation (Δtemperature) of the BAG (top) or BSEG (bottom). PC, burs-positive control indicates release during normal expansion. Volumes of blood loaded per sample are indicated. C, Wing morphology (arrows) and tanning (arrowheads) of flies expressing TRPM8 in either the BAG or BSEG evaluated 3 h after eclosion. D, Abdominal nerves from BSEG>TRPM8 animals with (right) or without (left) BSEG stimulation immunostained for bursicon using anti-burs antibodies. Scale bars, 50 μm. E, Box plot showing quantified burs-IR data from abdominal nerves of the indicated number of animals of each kind shown in D, demonstrating release of bursicon from these nerves in response to BSEG stimulation. mpi; Mean pixel intensity (background-subtracted). **p < 0.01 by t test. Error bars are SEM.
Table 1.
Summary of manipulations and results
| Cells manipulated/phenotype | Activation (TRPM8) | Suppression (Kir2.1) |
Knockdown (pburs-RNAi) | ||
|---|---|---|---|---|---|
| Strong perturbation | No perturbation | Mild perturbation | Strong perturbation | No perturbation | |
| BSEG | |||||
| Unexpanded wings (% flies) | 0% | 2% | 78% | 100% | |
| Air swallowing | — | Little | No | — | |
| Abdominal flexion | Yes, acute | No | No | No | |
| Bursicon release | |||||
| BAG | High | Yes | — | — | |
| BSEG | — | No | — | — | |
| Tanning | Yes | Yes | No | No | |
| BAG | |||||
| Unexpanded wings (% flies) | 22% | 2% | 17% | 71% | 47% |
| Air swallowing | — | Yes | — | — | Yes |
| Abdominal flexion | Yes, but delayed | Yes | Yes | Yes | 2× |
| Bursicon release (by IR) | |||||
| BAG | Low | — | — | — | — |
| BSEG | — | — | — | — | — |
| Tanning | Yes | No | No | — | — |
The table summarizes the effects of the three major manipulations performed: electrical activation or suppression of the individual groups of bursicon-expressing neurons (i.e. the BSEG and the BAG) or block of bursicon formation in the BAG by knocking down expression of the bursicon subunit pburs. Column subheadings indicate the environmental conditions under which each manipulation was performed. The effects analyzed include: wing expansion deficits (reported here as the percentage of flies with unexpanded wings), behavioral deficits (in either air swallowing or tonic abdominal flexion), neuroendocrine deficits (in terms of bursicon release, measured directly by either immunoblot or by depletion of bursicon immunoreactivity from BAG or BSEG fibers), and cuticle tanning. —, Not determined.
Surprisingly, stimulation of the BSEG induced not just the wing expansion motor program, which alone is insufficient for expansion (Luan et al., 2006b), but induced full wing expansion on time scales similar to those of animals in which all CCAP- or bursicon-expressing neurons were stimulated (Fig. 3A,C, right). The BSEG thus retain the full command potential of the larger cell groups in inducing wing expansion. Consistent with this, we observed that BSEG stimulation induced robust bursicon secretion from the BAG as assayed by Western blot (Fig. 3B, bottom; Table 1). To directly confirm that the BAG were the source of the released hormone, we monitored the depletion of bursicon immunoreactivity (burs-IR) from the abdominal nerves, which contain the BAG axons and act as neurohaemal release sites (Peabody et al., 2008). We found that the abdominal nerves of BSEG>TRPM8 animals had ∼40% lower burs-IR after BSEG stimulation compared with unstimulated control animals (Fig. 3D,E). We conclude that BSEG activation causes bursicon release into the blood from the BAG, thus accounting for the ability of BSEG-stimulated animals to plasticize and tan normally (Fig. 3C, right) despite the lack of direct BAG activation by TRPM8.
The BSEG are required for wing expansion in adverse environments
Our TRPM8 stimulation experiments suggest that the BSEG can orchestrate the entire wing expansion process, and thus effect the expansion decision even in the face of environmental perturbation. To examine whether these neurons are normally required for that function and whether their activity is essential for activation of the BAG, we selectively inhibited the BSEG using the potent inward rectifier K+ channel, Kir2.1 (Baines et al., 2001; Paradis et al., 2001) and evaluated the behavioral consequences of this manipulation. Observation of BSEG-suppressed flies in the inhibitory environment revealed that these animals neither expanded their wings nor initiated the phase of tonic abdominal flexion that normally drives expansion (n = 10), thus providing strong evidence that the BSEG are required for performance of wing expansion behavior. A puzzling observation, however, was that the wing expansion deficits seen in the inhibitory environment were largely absent in unperturbed siblings that expanded in the food vial in which they had pupariated (Fig. 4A, left). Most of the unperturbed animals exhibited only minor wing abnormalities such as mild wrinkling and splaying, and one-third of those with fully expanded wings were capable of flight (n = 14/43). Examination of the patterns of Kir2.1 expression and anti-bursicon immunolabeling in these animals confirmed that Kir2.1 was expressed in the BSEG and that no obvious compensatory changes in the bursicon network occurred, in that the full complement of BAG neurons were present (data not shown). In addition, ablation experiments confirmed that the absence of wing expansion deficits was not simply due to incomplete suppression of the BSEG neurons by Kir2.1, as similar results were observed when the BSEG were eliminated using the proapoptotic gene hid (data not shown).
Figure 4.
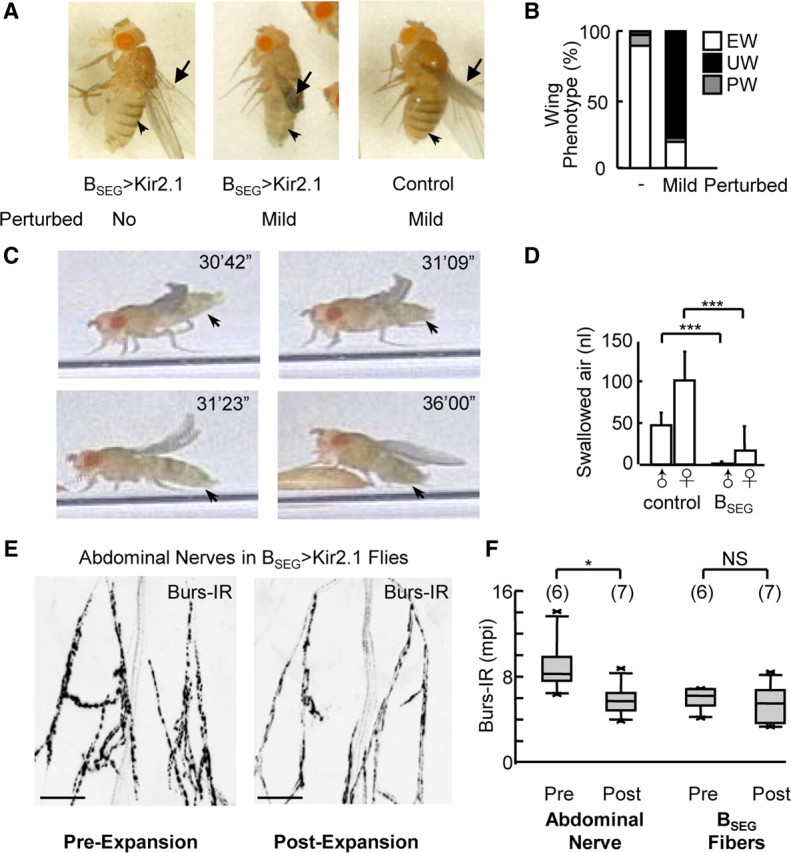
BSEG suppression inhibits wing expansion motor patterns and blocks expansion in adverse environments. A, Tanning (arrowhead) and wing expansion (arrow) are relatively normal in BSEG>Kir2.1 flies expanding unperturbed (left), but impaired in animals experiencing even mild perturbation (middle) compared with controls lacking the BSEG hemidriver (right). B, Wing expansion is differentially impaired in BSEG>Kir2.1 flies in unperturbed (−) and mildly perturbing (Mild) environments. EW, Expanded wings, UW, unexpanded wings; PW, partially expanded wings. C, Video frames of a BSEG>Kir2.1 fly expanding unperturbed showing abdominal movement (arrows) but not persistent flexion during the course of successful expansion. Time points are as indicated. D, Unperturbed BSEG>Kir2.1 flies (BSEG) swallow significantly less air during expansion than flies lacking the BSEG hemidriver (control).  , males; ♀, females. ***p < 0.001 by t test. E, Abdominal nerves from unperturbed BSEG>Kir2.1 flies immediately after eclosion (i.e., pre-expansion; left) or expansion (i.e., post-expansion; right) immunostained for bursicon using anti-burs antibodies. Scale bars, 50 μm. F, Box plots (left) show quantified burs-immunoreactivity data from abdominal nerves of each kind shown in E, demonstrating release of bursicon from the nerves in the absence of BSEG activity. In contrast, box plots on the right show no change in burs-IR in BSEG fibers in the second thoracic ganglion (BSEG Fibers), confirming absence of BSEG activity. Number of samples analyzed for each plot is indicated in parentheses. *p < 0.05; NS, not significant (p > 0.05), as determined by t test. Error bars are SD.
, males; ♀, females. ***p < 0.001 by t test. E, Abdominal nerves from unperturbed BSEG>Kir2.1 flies immediately after eclosion (i.e., pre-expansion; left) or expansion (i.e., post-expansion; right) immunostained for bursicon using anti-burs antibodies. Scale bars, 50 μm. F, Box plots (left) show quantified burs-immunoreactivity data from abdominal nerves of each kind shown in E, demonstrating release of bursicon from the nerves in the absence of BSEG activity. In contrast, box plots on the right show no change in burs-IR in BSEG fibers in the second thoracic ganglion (BSEG Fibers), confirming absence of BSEG activity. Number of samples analyzed for each plot is indicated in parentheses. *p < 0.05; NS, not significant (p > 0.05), as determined by t test. Error bars are SD.
The differences in wing phenotype observed in BSEG-suppressed flies allowed to expand in a large vial as opposed to a small chamber strongly suggested that environmental factors were important to success in expansion. To systematically test the role of environment, we therefore compared the behavior of single animals that emerged into a large food vial and remained completely unperturbed with those that experienced gentle perturbation immediately after emergence, in the form of transfer to a new food vial. Although the latter manipulation was without effect on control flies, it prevented most BSEG-suppressed flies (n = 21/27) from expanding (Fig. 4A, middle vs right; Table 1).
Consistent with their failure to expand, these gently perturbed animals also did not tonically flex their abdomens. Interestingly, this was also true of flies that expanded in the absence of environmental perturbation (Fig. 4B,C; Table 1). In addition, the latter flies exhibited impaired air swallowing, with most males swallowing little to no air and females swallowing, on average, 16% of the amount of air swallowed by control females (Fig. 4D; Table 1). The normal motor patterns required for wing expansion are thus largely or completely absent in BSEG-suppressed animals, regardless of environmental circumstances, indicating that the BSEG are not only sufficient, but also necessary for the initiation and/or maintenance of these behaviors. Careful observation revealed that the ability of BSEG-suppressed animals to expand in noninhibitory environments despite these deficits derives from their reliance on an alternate behavioral strategy. These animals expand their wings by vigorously pulsing their abdomens in both longitudinal and lateral dimensions, while repeatedly stroking their hindlegs backwards across their wings (Fig. 4C). Such wing stroking was occasionally observed in control animals, but almost always immediately after expansion when it appeared to transiently splay the wings (data not shown). Wing grooming may thus play an auxiliary role in expansion in normal flies, but in BSEG-suppressed flies it appears to be essential.
Because the wings of BSEG-suppressed animals were sufficiently plasticized to expand in response to repeated stroking, and because expansion in these animals was followed by tanning (Fig. 4A, left, arrowhead), we reasoned that wing grooming must be coordinated with bursicon release from the BAG. To determine whether unperturbed BSEG-suppressed flies do indeed release bursicon at the time of wing expansion, we measured bursicon immunoreactivity in the abdominal nerves both before wing expansion (i.e., immediately after emergence) and after its completion. We found that burs-IR levels were, on average, 35% lower in animals that had expanded (Fig. 4E,F), indicating substantial release of the hormone from BAG axons. In contrast, we detected no significant difference in burs-IR in the axons of the suppressed BSEG neurons (Fig. 4F; Table 1), consistent with the electrical silencing of these cells.
BSEG activation is independent of the BAG
The observation that the silenced BSEG do not release detectable bursicon suggests that BSEG-derived bursicon may be necessary for the normal execution of wing expansion motor patterns. To directly demonstrate this, we attempted to eliminate bursicon production in the BSEG using RNAi-mediated pburs-knockdown but found that this manipulation, which necessitated the coexpression of UAS-dicer-2, was not fully effective. However, we were able to selectively eliminate pburs expression in the BAG neurons by this means (data not shown) and used this manipulation, together with Kir2.1-mediated suppression of excitability, to examine the molecular and cellular contribution of the BAG to wing expansion. Consistent with previous results, we found that both BAG suppression and pburs knockdown within this cell group left behavioral performance intact (Fig. 5A,B; Table 1). Interestingly, however, most flies in which BAG pburs expression was blocked exhibited a robust second bout of abdominal flexion, a phenomenon that was also seen in attenuated form in some BAG>Kir2.1 animals. While the first bout of flexion in BAG> pburs-RNAi animals was similar to that of control animals in duration and time of onset relative eclosion (Fig. 5C), the second bout lasted approximately half as long as the first (7 ± 2 min vs 12 ± 4 min, SD; n = 7), and typically followed it after an interval of 13 min (±2 min, SD). These results confirm that BAG-derived bursicon is clearly not necessary for the performance of wing expansion behaviors, but suggest that it plays a role in negatively regulating them.
Figure 5.
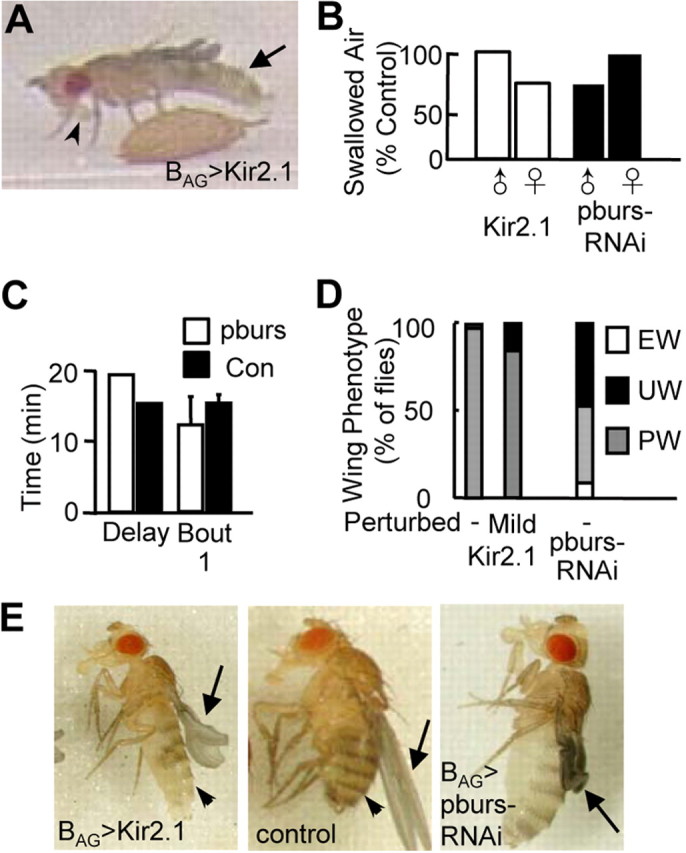
BAG suppression blocks somatic, but not behavioral, aspects of wing expansion. A, Video frame of a BAG>Kir2.1 fly showing that the execution of abdominal flexion (arrow) and proboscis extension required for air swallowing (arrowhead) are normal. B, Both BAG>Kir2.1 (left) and BAG>pburs-RNAi (right) flies swallow substantial amounts of air during the period of sustained abdominal flexion relative to matched controls (i.e., either flies lacking the DBD hemidriver or the UAS-pburs-RNAi transgene, respectively).  , males; ♀, females. C, Bar graphs showing the median time from eclosion to the onset of the first bout sustained abdominal flexion (Delay) and the average duration of the first bout (Bout 1) in BAG>pburs-RNAi flies (n = 7) versus matched controls (Con) lacking the UAS-pburs-RNAi transgene (n = 9). Error bars are SD. D, Wing expansion deficits observed in BAG>Kir2.1 flies in noninhibitory (−) and inhibitory (+) environments are similar (left) and somewhat less severe than those observed in BAG>pburs-RNAi flies (right). EW, Expanded wings, UW, unexpanded wings; PW, partially expanded wings. E, Left and middle, Wing expansion (arrow) and cuticle tanning (arrowhead) are impaired in BAG>Kir2.1 animals (left; note reduced pigmentation), relative to controls lacking the BAG hemidriver (middle). Right, the wings (arrow) of BAG>pburs-RNAi flies typically undergo little to no expansion during abdominal flexion.
, males; ♀, females. C, Bar graphs showing the median time from eclosion to the onset of the first bout sustained abdominal flexion (Delay) and the average duration of the first bout (Bout 1) in BAG>pburs-RNAi flies (n = 7) versus matched controls (Con) lacking the UAS-pburs-RNAi transgene (n = 9). Error bars are SD. D, Wing expansion deficits observed in BAG>Kir2.1 flies in noninhibitory (−) and inhibitory (+) environments are similar (left) and somewhat less severe than those observed in BAG>pburs-RNAi flies (right). EW, Expanded wings, UW, unexpanded wings; PW, partially expanded wings. E, Left and middle, Wing expansion (arrow) and cuticle tanning (arrowhead) are impaired in BAG>Kir2.1 animals (left; note reduced pigmentation), relative to controls lacking the BAG hemidriver (middle). Right, the wings (arrow) of BAG>pburs-RNAi flies typically undergo little to no expansion during abdominal flexion.
In addition, we confirmed that suppression of the BAG substantially inhibits bursicon release into the hemolymph, as indicated by the failure to tan within 3 h of eclosion (Fig. 5E, left vs middle). Surprisingly, suppression by Kir2.1 appeared not to be complete, in that during the period of persistent abdominal flexion BAG>Kir2.1, animals succeeded in partially unfolding their wings under the combined influence of pressure and wing stroking by the hindlegs (Fig. 5D,E). This effect was relatively insensitive to mild perturbation (Fig. 5D) and was considerably less evident in animals subjected to pburs knockdown in the BAG, many of which retained completely unexpanded wings (Fig. 5D,E, right). Kir2.1-mediated suppression thus appears to provide a slightly less effective block of bursicon release into the hemolymph than pburs knockdown in the BAG, which may also account for the behavioral differences described above. However, both manipulations confirm that the BAG are the principal source of bursicon released into the hemolymph and play an essential role in insuring normal wing tanning and morphology.
Discussion
Here we functionally dissect the previously described command system for wing expansion in Drosophila (Peabody et al., 2009) and find that stimulation of a single pair of neurons, the BSEG, is capable of commanding the entire wing expansion program under environmental conditions that would otherwise inhibit it. Because this program requires the simultaneous execution of at least two motor patterns and the hormonal release of bursicon from the BAG neurons, we conclude that BSEG stimulation coordinates the various processes required for expansion. Genetic evidence (Baker and Truman, 2002; Dewey et al., 2004), along with the RNAi-mediated knockdown results presented here, indicates that the neuromodulator bursicon is the primary effector in orchestrating the various aspects of wing expansion. Thus, our results illustrate how a neuromodulator, released from widely ramifying projection neurons, can orchestrate a complex response to environmental conditions within a simple decision circuit.
The neuroarchitecture of the wing expansion decision
The command-like function of the BSEG and their necessity for wing expansion in adverse environments suggests a critical role for these neurons in the decision-making network that governs wing expansion in Drosophila. A central question, however, is where in the hierarchy of the decision network these neurons lie. Our results, together, speak against the simplest possibility, namely that they are directly responsible for the wing expansion decision, receiving and integrating sensory inputs from the environment and executing the expansion decision by the release of bursicon. If this were the case, all effectors of wing expansion would necessarily lie downstream of the BSEG, including the BAG. While this interpretation is compatible with the effects of TRPM8-mediated activation of the BSEG, it is inconsistent with our finding that the BAG become activated when the BSEG are suppressed, at least in noninhibitory environments. That the BSEG are likewise activated when the BAG are suppressed is a strong argument that both groups of bursicon-expressing neurons, which are coordinately activated during normal wing expansion, are regulated by a common activation path. The existence of such a path is underscored by the fact that BSEG-suppressed flies can expand their wings using non-BSEG-mediated motor patterns (i.e., wing grooming), indicating that the drive to expand is represented by neurons outside the bursicon-expressing group. Although the identity of the putative activation path remains to be determined, it may include eclosion hormone-secreting neurons, which have been postulated to activate the wing expansion circuit (Truman, 2005) and are also required for wing expansion (McNabb et al., 1997).
If the BAG are not functionally downstream of the BSEG, how does stimulation of the BSEG induce bursicon release into the blood? Although previous anatomical evidence suggested that the BAG might receive inputs from the BSEG (Peabody et al., 2008), the evidence presented here is more consistent with a model in which the BAG are indirectly activated by the BSEG through de-repression of the activation pathway, which would account for the extreme environmental sensitivity of the wing expansion program when the BSEG are suppressed. Such a model would also predict distinct roles for the BSEG in regulating environmental inputs and wing expansion motor patterns. Just such a separation of function is suggested by the bursicon knock-down experiments presented in Figure 1B, where lowered levels of bursicon result in the inability to overcome environmental inhibition, but do not completely compromise the ability to execute wing expansion motor patterns.
If the BSEG feed back to modulate the environmental suppression of an activation path that governs both the BAG and BSEG, it might account for how the wing expansion network resolves the conflict between the internally motivated need to expand (represented by the activation path) and the externally motivated need to delay expansion (represented by the environmental inhibition path). As a fly moves into a more favorable environment, activity in the inhibitory pathway should decrease, thus increasing activity in the activation path, which is amplified by disinhibition via a positive feedback loop through the BSEG. With an appropriately set threshold, such a loop would promote the eventual concerted release of bursicon and the behavioral transition to wing expansion. The behavioral transition to larval ecdysis in Manduca and Drosophila is known to also rely on a positive feedback loop that drives the mutual release of ecdysis-triggering hormone and eclosion hormone (Ewer et al., 1997; Kingan et al., 1997; Clark et al., 2004); we postulate that similar positive feedback switches with neuromodulatory outputs may be used in other decision networks.
Compensatory mechanisms and environmental adaptation
As indicated above, our results suggest that the BSEG serve distinct functions in modulating environmental inputs and governing motor output. With respect to motor output, the BSEG satisfy two of the major criteria required of command neurons (Kupfermann and Weiss, 1978): they are necessary for initiating or sustaining the motor patterns normally required for wing expansion and are sufficient to elicit those patterns when stimulated. Given this role, it was surprising to find that the BSEG were dispensable for wing expansion in flies that remained completely unperturbed after eclosion.
What is the status of the BSEG-independent wing expansion program? Because this compensatory program is coordinated with bursicon release from the BAG, it is likely linked to the putative activation pathway. However, because the compensatory wing grooming patterns are not (or not necessarily) components of normal wing expansion, it is unclear how they are recruited in the absence of BSEG activity. It is possible that recruitment is due to rewiring of the wing expansion network, perhaps as a consequence of constitutive developmental suppression of the BSEG. Alternatively, the compensatory wing-grooming program may result from a change in the pattern of activity within the existing network, for example by the unmasking of motor patterns normally suppressed by the BSEG, or by the recruitment of backup motor patterns in response to proprioceptive input that indicates wing expansion is failing. A role for proprioception in wing expansion is likely given the observed recruitment of grooming during the expansional phase of BAG- as well as BSEG-suppressed flies, but further work will be required to further elucidate the mechanistic basis of the compensatory program.
Regardless of mechanism, the dependence of the compensatory wing expansion program on the BAG underscores the essential nature of these neurons. Without them, the wings would neither fully expand nor harden regardless of the expansion program used. It is interesting to speculate that the BAG may thus represent an evolutionarily more basal component of the wing expansion process than the BSEG, which insure expansion under conditions of environmental challenge. In this context, it is worth noting that only certain insect lineages, including cyclorrhaphan flies such as Drosophila, exhibit environmentally sensitive wing expansion programs as an evolutionary adaptation (Cottrell, 1964). Consistent with the proposed role of neuromodulation in circuit evolution (Katz and Harris-Warrick, 1999), this adaptation may have been accomplished by conscripting the BSEG neurons to modulate a novel environmentally governed inhibitory delay path. The compensatory wing expansion strategy used by flies in the absence of BSEG activity may correspondingly represent a vestige of an ancestral, hormonally governed and environmentally insensitive form of the wing expansion program. As more comparative studies become available, it will be interesting to see whether insects lacking environmentally adaptive wing expansion programs likewise lack BSEG-like neurons. Currently, BSEG homologues have been described outside of Drosophila only in the hawkmoth, Manduca, which also can delay wing expansion (Truman, 1973; Dai et al., 2008).
Neuromodulation and the integration of behavior
Bursicon's action in orchestrating wing expansion bridges the known roles of neuromodulation in the regulation of motor rhythms (Dickinson, 1995; Nusbaum et al., 2001; Brezina, 2010) and physiological state (Pfaff et al., 2008) and illustrates how neuromodulators, released by a small set of neurons, can coordinate secretomotor and somatomotor outputs in response to environmental conditions. In pointing to a critical integrative role for neuromodulatory neurons in motor control, our work complements other recent findings, including the identification of octopaminergic neurons in the fly subesophageal ganglion that mediate male aggressiveness (Zhou and Rao, 2008) and peptidergic neurons in the mouse hypothalamus that regulate feeding (Aponte et al., 2011). It is interesting in this regard to note that, like the neuromodulator-rich upper brainstem and hypothalamus in vertebrates (Swanson, 2000), the subesophageal ganglion has been implicated by brain lesion studies in the initiation and coordination of basic motor patterns in both insects and annelids (Ridgel and Ritzmann, 2005; Cornford et al., 2006). Based on the work presented here and previously (Brocard et al., 2005; Mesce et al., 2008; Mullins et al., 2011), it is interesting to speculate that vertebrates and invertebrates may share similar architectures of descending motor control in which neuromodulation acts as an organizing principle.
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Mental Health (Project 1ZIA-MH-002800-07). We thank Kevin HoWan, Sarrah Ben-Achour, and Michael Syme for technical assistance in generating and characterizing the enhancer-trap hemidriver lines. We thank Aaron Hsueh and Willi Honnegger for the anti-pburs antibody, Deborah Hursh for UAS-hid flies, and Grace Gray for critical comments on the manuscript. We would also like to express our profound gratitude to the late Howard Nash whose encouragement, generous advice, and thoughtful comments were invaluable throughout.
The authors declare no competing financial interest.
References
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JD, Truman JW. Mutations in the Drosophila glycoprotein hormone receptor, rickets, eliminate neuropeptide-induced tanning and selectively block a stereotyped behavioral program. J Exp Biol. 2002;205:2555–2565. doi: 10.1242/jeb.205.17.2555. [DOI] [PubMed] [Google Scholar]
- Brezina V. Beyond the wiring diagram: signalling through complex neuromodulator networks. Philos Trans R Soc Lond B Biol Sci. 2010;365:2363–2374. doi: 10.1098/rstb.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard F, Bardy C, Dubuc R. Modulatory effect of substance P to the brain stem locomotor command in lampreys. J Neurophysiol. 2005;93:2127–2141. doi: 10.1152/jn.00401.2004. [DOI] [PubMed] [Google Scholar]
- Büschges A, Scholz H, El Manira A. New moves in motor control. Curr Biol. 2011;21:R513–R524. doi: 10.1016/j.cub.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Clark AC, del Campo ML, Ewer J. Neuroendocrine control of larval ecdysis behavior in Drosophila: complex regulation by partially redundant neuropeptides. J Neurosci. 2004;24:4283–4292. doi: 10.1523/JNEUROSCI.4938-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford A, Kristan WB, 3rd, Malnove S, Kristan WB, Jr, French KA. Functions of the subesophageal ganglion in the medicinal leech revealed by ablation of neuromeres in embryos. J Exp Biol. 2006;209:493–503. doi: 10.1242/jeb.02030. [DOI] [PubMed] [Google Scholar]
- Cottrell CB. General observations on the imaginal ecdysis of blowflies. Trans R Entomol Soc London. 1962;114:317–333. [Google Scholar]
- Cottrell CB. Insect ecdysis with particular emphasis on cuticular hardening and darkening. New York: Academic; 1964. [Google Scholar]
- Dai L, Dewey EM, Zitnan D, Luo CW, Honegger HW, Adams ME. Identification, developmental expression, and functions of bursicon in the tobacco hawkmoth, Manduca sexta. J Comp Neurol. 2008;506:759–774. doi: 10.1002/cne.21575. [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Zdarek J. Metamorphosis behavior of flies. Annu Rev Entomol. 1994;39:243–266. doi: 10.1146/annurev.en.39.010194.001331. [DOI] [PubMed] [Google Scholar]
- Dewey EM, McNabb SL, Ewer J, Kuo GR, Takanishi CL, Truman JW, Honegger HW. Identification of the gene encoding bursicon, an insect neuropeptide responsible for cuticle sclerotization and wing spreading. Curr Biol. 2004;14:1208–1213. doi: 10.1016/j.cub.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Dickinson PS. Interactions among neural networks for behavior. Curr Opin Neurobiol. 1995;5:792–798. doi: 10.1016/0959-4388(95)80108-1. [DOI] [PubMed] [Google Scholar]
- Dickinson PS. Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr Opin Neurobiol. 2006;16:604–614. doi: 10.1016/j.conb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 1999;22:153–161. doi: 10.1016/s0166-2236(98)01340-x. [DOI] [PubMed] [Google Scholar]
- Elliott CJ, Susswein AJ. Comparative neuroethology of feeding control in molluscs. J Exp Biol. 2002;205:877–896. doi: 10.1242/jeb.205.7.877. [DOI] [PubMed] [Google Scholar]
- Ewer J, Gammie SC, Truman JW. Control of insect ecdysis by a positive-feedback endocrine system: roles of eclosion hormone and ecdysis triggering hormone. J Exp Biol. 1997;200:869–881. doi: 10.1242/jeb.200.5.869. [DOI] [PubMed] [Google Scholar]
- Friesen WO, Kristan WB. Leech locomotion: swimming, crawling, and decisions. Curr Opin Neurobiol. 2007;17:704–711. doi: 10.1016/j.conb.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Grigliatti T. Transposons-gene tagging and mutagenesis. In: Roberts D, editor. Drosophila: a practical approach. 2nd edition. Oxford: Oxford UP; 1998. pp. 85–108. [Google Scholar]
- Halfon MS, Gisselbrecht S, Lu J, Estrada B, Keshishian H, Michelson AM. New fluorescent protein reporters for use with the Drosophila Gal4 expression system and for vital detection of balancer chromosomes. Genesis. 2002;34:135–138. doi: 10.1002/gene.10136. [DOI] [PubMed] [Google Scholar]
- Hedwig B. Pulses, patterns and paths: neurobiology of acoustic behaviour in crickets. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:677–689. doi: 10.1007/s00359-006-0115-8. [DOI] [PubMed] [Google Scholar]
- Honegger HW, Dewey EM, Ewer J. Bursicon, the tanning hormone of insects: recent advances following the discovery of its molecular identity. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:989–1005. doi: 10.1007/s00359-008-0386-3. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Gurney K, Prescott TJ. Is there a brainstem substrate for action selection? Philos Trans R Soc Lond B Biol Sci. 2007;362:1627–1639. doi: 10.1098/rstb.2007.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Harris-Warrick RM. The evolution of neuronal circuits underlying species-specific behavior. Curr Opin Neurobiol. 1999;9:628–633. doi: 10.1016/S0959-4388(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Katz PS, Hooper SL. Invertebrate central pattern generators. In: North G, Greenspan RJ, editors. Invertebrate neurobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory; 2007. pp. 251–279. [Google Scholar]
- Kien J, Altman JS. Preparation and execution of movement: parallels between insect and mammalian motor systems. Comp Biochem Physiol Comp Physiol. 1992;103:15–24. doi: 10.1016/0300-9629(92)90236-j. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Kingan TG, Gray W, Zitnan D, Adams ME. Regulation of ecdysis-triggering hormone release by eclosion hormone. J Exp Biol. 1997;200:3245–3256. doi: 10.1242/jeb.200.24.3245. [DOI] [PubMed] [Google Scholar]
- Kow LM, Mobbs CV, Pfaff DW. Roles of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: a review. Neurosci Biobehav Rev. 1994;18:251–268. doi: 10.1016/0149-7634(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Jr, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol. 2005;76:279–327. doi: 10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Weiss KR. The command neuron concept. Behav Brain Sci. 1978;1:3–39. [Google Scholar]
- Kupfermann I, Weiss KR. Motor program selection in simple model systems. Curr Opin Neurobiol. 2001;11:673–677. doi: 10.1016/s0959-4388(01)00267-7. [DOI] [PubMed] [Google Scholar]
- Larimer JL, Moore D. Neural basis of a simple behavior: abdominal positioning in crayfish. Microsc Res Tech. 2003;60:346–359. doi: 10.1002/jemt.10273. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 2004;25:473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Luan H, Peabody NC, Vinson CR, White BH. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006a;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H, Lemon WC, Peabody NC, Pohl JB, Zelensky PK, Wang D, Nitabach MN, Holmes TC, White BH. Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J Neurosci. 2006b;26:573–584. doi: 10.1523/JNEUROSCI.3916-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CW, Dewey EM, Sudo S, Ewer J, Hsu SY, Honegger HW, Hsueh AJ. Bursicon, the insect cuticle-hardening hormone, is a heterodimeric cystine knot protein that activates G protein-coupled receptor LGR2. Proc Natl Acad Sci U S A. 2005;102:2820–2825. doi: 10.1073/pnas.0409916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol. 2001;11:R986–R996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- McNabb SL, Baker JD, Agapite J, Steller H, Riddiford LM, Truman JW. Disruption of a behavioral sequence by targeted death of peptidergic neurons in Drosophila. Neuron. 1997;19:813–823. doi: 10.1016/s0896-6273(00)80963-0. [DOI] [PubMed] [Google Scholar]
- Mendive FM, Van Loy T, Claeysen S, Poels J, Williamson M, Hauser F, Grimmelikhuijzen CJ, Vassart G, Vanden Broeck J. Drosophila molting neurohormone bursicon is a heterodimer and the natural agonist of the orphan receptor DLGR2. FEBS Lett. 2005;579:2171–2176. doi: 10.1016/j.febslet.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Mesce KA, Esch T, Kristan WB., Jr Cellular substrates of action selection: a cluster of higher-order descending neurons shapes body posture and locomotion. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:469–481. doi: 10.1007/s00359-008-0319-1. [DOI] [PubMed] [Google Scholar]
- Mullins OJ, Hackett JT, Buchanan JT, Friesen WO. Neuronal control of swimming behavior: comparison of vertebrate and invertebrate model systems. Prog Neurobiol. 2011;93:244–269. doi: 10.1016/j.pneurobio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Park JH, Schroeder AJ, Helfrich-Förster C, Jackson FR, Ewer J. Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development. 2003;130:2645–2656. doi: 10.1242/dev.00503. [DOI] [PubMed] [Google Scholar]
- Peabody NC, Diao F, Luan H, Wang H, Dewey EM, Honegger HW, White BH. Bursicon functions within the Drosophila CNS to modulate wing expansion behavior, hormone secretion, and cell death. J Neurosci. 2008;28:14379–14391. doi: 10.1523/JNEUROSCI.2842-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody NC, Pohl JB, Diao F, Vreede AP, Sandstrom DJ, Wang H, Zelensky PK, White BH. Characterization of the decision network for wing expansion in Drosophila using targeted expression of the TRPM8 channel. J Neurosci. 2009;29:3343–3353. doi: 10.1523/JNEUROSCI.4241-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Kieffer BL, Swanson LW. Mechanisms for the regulation of state changes in the central nervous system: an introduction. Ann N Y Acad Sci. 2008;1129:1–7. doi: 10.1196/annals.1417.031. [DOI] [PubMed] [Google Scholar]
- Reynolds SE. Control of cuticle extensibility in wings of adult manduca at time of eclosion: effects of eclosion hormone and bursicon. J Exp Biol. 1977;70:27–39. [Google Scholar]
- Ridgel AL, Ritzmann RE. Effects of neck and circumoesophageal connective lesions on posture and locomotion in the cockroach. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:559–573. doi: 10.1007/s00359-005-0621-0. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Truman JW. Physiology of insect ecdysis. 3. Relationship between hormonal-control of eclosion and of tanning in tobacco hornworm, Manduca-sexta. J Exp Biol. 1973;58:821–829. [Google Scholar]
- Truman JW. Hormonal control of insect ecdysis: endocrine cascades for coordinating behavior with physiology. Vitam Horm. 2005;73:1–30. doi: 10.1016/S0083-6729(05)73001-6. [DOI] [PubMed] [Google Scholar]
- von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]