Introduction
Vertebrate development is best studied in an intact embryo model, yet a robust interface between time-lapse microscopy and in vivo embryo health and maintenance is difficult to achieve in model systems that rely on external factors for life support. Here, we present a protocol for in ovo culture and time-lapse imaging of fluorescently labeled cells within living avian embryos, using a teflon membrane that is oxygen permeable and liquid impermeable. We discuss the teflon membrane assembly and interface with the egg window and an upright microscope and heated chamber. To demonstrate how the system works, we describe the protocol in use with chick embryos while following individual fluorescently labeled neural crest cells, a multipotent stem cell-like population that differentiate into a wide range of derivatives and travel extensively throughout the embryo. By combining the in ovo culture with confocal or two-photon 4D time-lapse imaging, we demonstrate that single neural crest cell behaviors may be visualized for long periods of time (~36hrs) and embryo health maintained for up to 5 days. This technique has been adapted to study somitogenesis and the teflon membrane assembly size changed to fit smaller quail eggs.
Materials
Reagents:
India Ink (Pelikan Fount, PLK 51822A143)
Ringer’s Solution [R]
Equipment:
Beeswax (Fisher Scientific, W25-500)
Digital thermometer (Fisher Scientific, 15-077-17A)
Egg candler
Egg incubator (VWR, WL51475)
Fertile White Leghorn chick eggs
Filter paper (Whatman, 1001185)
Forceps (Fine Science Tools, 11252-30)
Hotplate (VWR, Hotplate/Stirrer model 375)
Upright laser scanning confocal microscope (Zeiss LSM710 Pascal)
Kimax glass petri dish, 100 × 15 mm (Kimble, #23064 100015)
Needles (Becton Dickinson, 18 and 25 gauge)
Petri dishes (Becton Dickinson, 35 ×100mm)
Plastic ring (Chick: 26mm O.D., 22mm I.D.; Quail: 19mm O.D., 16mm I.D.)
Plastic transfer pipets (Samco, 202-205)
Rubber o-ring (Chick: 24mm O.D., 21mm I.D.; Quail: 17 O.D., 15mm I.D.)
Scissors (Fine Science Tools, 14063-09)
Syringes (VWR, 1 and 5 ml)
teflon high sensitivity membrane (YSI Inc., model 5794)
Transparent tape (3M, Corporate Express, MMM6001x72)
Methods
Egg preparation
* Time requirement = dependent on manipulations to be performed
-
1.
Rinse eggs with 70% alcohol and remove 3 ml of albumin from the caudal part of the egg with the 5 ml syringe and 27-gauge needle.
-
2.
Place the egg against the egg candler to locate the position of the blastoderm/embryo.
-
3.
Place the acrylic ring on top of the eggshell over the center of the blastoderm and draw a circle around it.
-
4.
Use scotch tape to cover the portion of the eggshell (circle) which will be cut out for the window.
-
5.
With scissors, cut a hole in the eggshell by following along the line drawn around the acrylic ring. Remove most of the air bubbles on the exposed surface.
-
6.
Inject the India ink solution under the blastoderm with the 25 gauge needle to visualize and stage the embryo.
-
7.
Perform any cell labeling or manipulation of the embryo.
Teflon membrane assembly
Preparation of teflon membrane ring:
-
*
Time requirement = ~20 minutes
-
*
Note: Prepared rings may be used for more than one imaging session if the teflon membrane and beeswax is removed and replaced
-
8.
Use the heat block to melt the beeswax in a glass petri dish. Adjust temperature of heat block to be high enough to melt beeswax, but low enough to touch when manipulating the acrylic ring on the surface of the heat block.
-
9.
To adhere the teflon membrane onto the acrylic ring, hold the acrylic ring horizontally with forceps and submerge the ring so that 3/4 of the ring is covered with warmed beeswax for about 10 seconds.
-
10.
Lay the ring on a rectangular piece of the teflon membrane so that the beeswax is in contact with the teflon.
-
11.
Flip the teflon membrane over so that the acrylic ring lies underneath the membrane.
-
12.
Place a rubber o-ring on top of the teflon and push it onto the ring such that the teflon membrane is taut and the o-ring is around the circumference of the ring.
-
13.
After 5 minutes, remove the o-ring and cut the excess teflon membrane away.
-
14.
There may be parts of the teflon membrane which do not adhere to the sides of the ring. In this case, roll the ring vertically along the heat block so that the beeswax between the ring and the teflon melts. Pull on the teflon membrane so that it is taut around the ring.
-
15.
Let the ring assembly cool for approximately 5 minutes.
-
16.
The goal is to stretch the teflon membrane across the acrylic ring, forming a taut drumhead.
-
8.
In ovo imaging preparation
*Time requirement = ~20 minutes
-
17.
After finishing any manipulations or labeling of the embryo, place the ring assembly into the hole cut in the eggshell such that it lays directly over the embryo. Be careful not to allow an excess air space between the embryo and teflon surface.
-
18.
To seal the ring assembly into the eggshell, use a metal spatula to dip into the warmed beeswax and gently wipe the beeswax into the gap between the eggshell and the ring assembly.
-
19.
Continue to place the beeswax along the circumference of the ring and eggshell border, checking to make sure there are no leaks of the egg contents.
-
20.
The egg is now ready to position on the microscope stage such that the embryo is visible with a low magnification 5X or 10X objective, or long-working-distance objectives, through the teflon membrane.
Heated chamber calibration:
* Time requirement = dependent on controller and temperature of room
* Note: unless the system is changed (temperature or components), calibration need only be performed once. The heated chamber can be self-built (see Kulesa and Kasemeier-Kulesa, CSH Protocols; 2007; doi:10.1101/pdb.prot4792) or commercially purchased.
-
21.
Insert the probe of a digital thermometer within the heated chamber. Tape the probe onto the microscope stage.
-
22.
Turn on the heated chamber until the temperature at the stage is maintained at 38°C.
-
23.
Record the time to reach equilibrium. This should be factored into the in ovo culture preparation to limit the time the egg is out of the incubator.
-
24.
Let system equilibrate 30 minutes before imaging.
Imaging parameters:
-
*
Time requirements = ~15 minutes
-
25.
A Plan-Neofluar 10×/NA 0.3 objective (for global field of view events) or higher magnification long-working-distance objective can be used for high-resolution imaging.
-
26.
For example, 488-nm and 543-nm laser lines are used to excite the green (EGFP) protein and DiI, respectively. Photoactivation of GFP or GFP variants can be performed in ovo (please see Stark et al., CSH Protocols; 2008; doi:10.1101/pdb.prot4975)
-
27.
Single track z-sections may be collected at appropriate time and space intervals to produce a 3D time-lapse movie.
-
28.
Refocus to center the embryo as necessary.
-
25.
Troubleshooting:
Problem: The acrylic ring is deformed after removing from the warmed beeswax.
[Steps 9–10]
Solution: The acrylic ring may have been in contact with the bottom of the glass dish holding the beeswax, which caused the ring to deform. Hold the ring in the beeswax, but do not let it touch the bottom of the dish.
Problem: The Teflon surface is wavy and not taut across the surface on the acrylic ring.
[Steps 14–16]
Solution: The Teflon should be stretched circumferentially around the acrylic ring. There is a bit of finesse to hold the ring with one hand, and pull the membrane tighter with the thumb and for finger by stretching over the ring. Gently warming the ring in the region of interest will help to melt the beeswax locally, causing the membrane to be stretched across the ring with ease.
Problem: There is wet albumen around the circumference of the teflon ring assembly after the ring has been sealed in place.
[Steps 17–20]
Solution: There must be a gap in the beeswax seal of the teflon ring assembly with the eggshell. Re-heat the beeswax and seal the hole using the spatula to apply the beeswax to the eggshell and ring.
Problem: The embryo is not visible when the ring is placed over the vitelline membrane.
[Step 17]
Solution: There is an air bubble between the embryo and teflon surface. Pick up the teflon membrane assembly and re-place it over the embryo.
Problem: The embryo drifts dramatically during time-lapse imaging.
[Steps 5; 25–28]
Solution: There may be a hole in the eggshell and leak. This causes the surface of the vitelline membrane and embryo to drop away from the teflon surface. Alternatively, an air bubble within the egg may have burst and changed the depth of the embryo surface. Refocus on the embryo and remember to remove nearly all air bubbles prior to placing the teflon membrane in the egg.
Problem: The objective is not compatible with the teflon ring assembly.
[Step 25]
Solution: The circumference of the teflon ring limits the diameter of the objective that can be used, unless the objective has a long-working-distance. One possible solution is to change to a long-working-distance objective. Alternatively, one can alter the height of the plastic ring by using a hacksaw or similar to cut the ring in half, through its height, before the teflon is attached.
Discussion
We have presented an in ovo technique developed for intravital, 4D in ovo time-lapse imaging of live avian embryos. The technique uses a teflon membrane window in the egg and is adaptable to upright widefield, confocal and two-photon microscopy. Previously, it had been difficult to image cell dynamics in living avian embryos in their natural, intact environment due to an inability to prevent the embryo surface from drying after exposing it to dry air through a window in the egg. The teflon membrane provides a solution since the teflon is oxygen permeable, liquid impermeable and optically transparent.
We have utilized our technique to study chick somite formation and neural crest cell migratory behaviors, including analysis of cell-cell and cell-environment interactions. We have applied the technique to start time-lapse imaging from Hamburger and Hamilton (1951) stage-9 embryos and continued to image embryos for 36hrs. We have re-incubated and periodically imaged chick embryos through a teflon window for up to 5 days. Embryo health was monitored separately by assessing somite development.
Avian development in ovo allows for more accurate assessment of morphogenesis since cell-tissue microenvironment interactions and physical forces within the embryo remain intact. In contrast, a typical embryo in ex ovo culture on a membrane insert or paper ring has approximately a 24hr duration of growth and embryo health must be maintained by external factors. Ex ovo avian embryo growth is typically limited by difficulties in embryo turning and pH changes in culture media. In ovo imaging circumvents these roadblocks.
One complex issue of in ovo imaging is that the embryo has more freedom of movement to grow, becomes rapidly 3-dimensional and heartbeat make it challenging to maintain a focused field of view. This is especially true as the decrease in yolk volume, due to normal embryo growth, lowers the surface of the embryo from the dorsal surface of the eggshell. Future designs may help to extend this time window without limiting the resolution by providing a means to maintain the embryo near the surface of the egg and teflon membrane. The framework for this technique has also been tested with different sized eggs, including smaller quail eggs, using a smaller diameter ring. Thus, the teflon membrane assembly and in ovo imaging technique, when combined with fluorescently labeled cells and molecular perturbation, offers a powerful tool to gain insight into developmental events in the avian embryo’s natural setting.
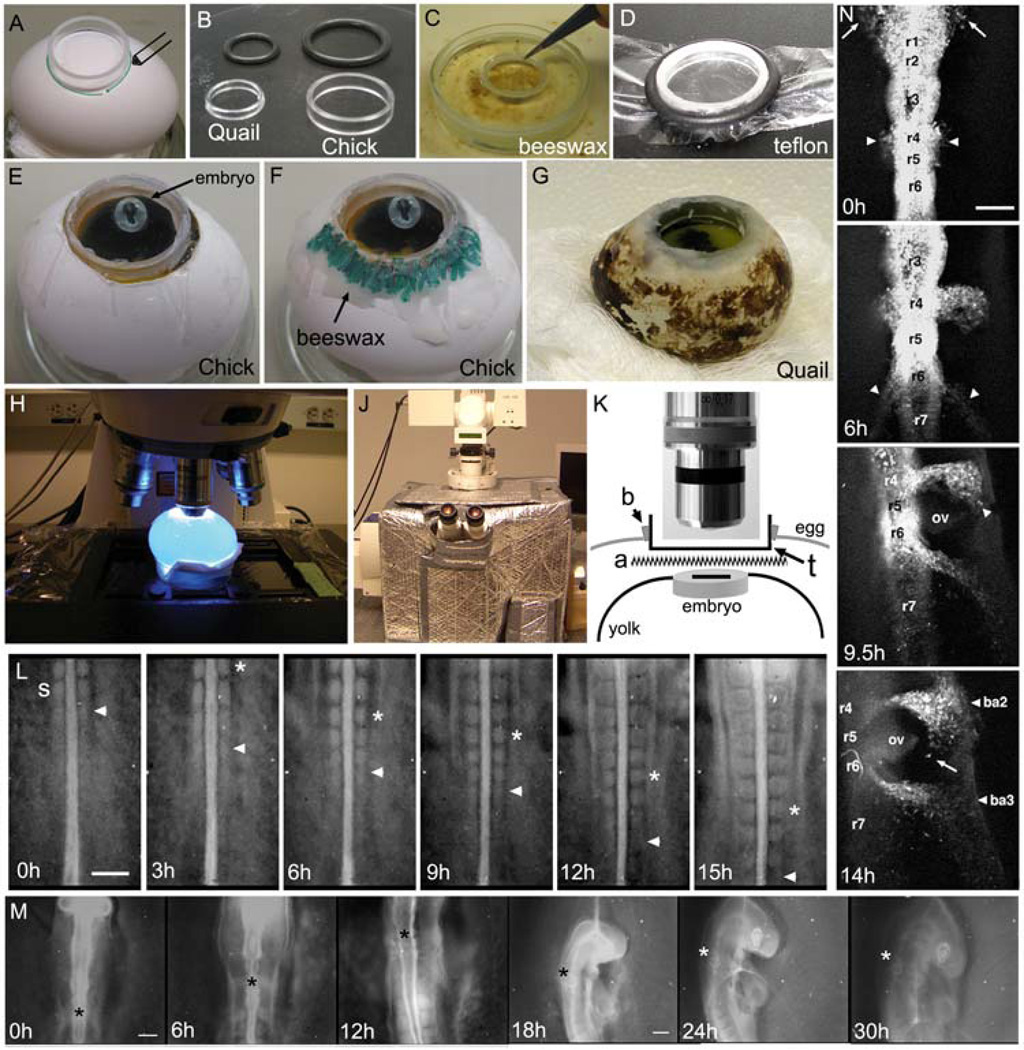
Fig. 1. In ovo culture and imaging of chick embryogenesis.
(A–G) The initial steps of in ovo imaging begin with marking the position of the presumptive window in the eggshell and creation of the teflon membrane that will fit into the window to provide an optical pathway to the embryo. (A) An acrylic ring is placed and marked over the position of the embryo (determined by candling) to create a window into the eggshell. (B) The ring diameter can vary depending on the size of the egg or embryo. We show the quail and chick appropriate rings and rubber o-rings used to stretch the teflon membrane over the ring. (C) The ring is first dipped into melted beeswax before (D) placing the ring onto the Teflon membrane and securing in tightly with the rubber o-ring, around its circumference. (E) After stretching and cutting the teflon to fit tightly over the ring, the ring is placed into the hole in the eggshell and laid over the embryo (the embryo contrast has been adjusted to better visualize its position). (F) Warmed beeswax (highlighted in green) is spread around the ring to seal it into the eggshell. (G) The same method can be applied to eggs of various sizes, including the smaller quail egg. (H) The egg is placed on the microscope stage under the objective (highlighted with blue excitation light), which (J) is surrounded by a heated chamber. (K) The optical pathway through the teflon window includes the teflon membrane (t), laid above the albumen and Ringer’s solution (a) that sits over the embryo on top of the yolk surface. (L) A typical in ovo time-lapse imaging session of somitogenesis shows the consistent addition of somites (2somites/3hrs(h)). The last forming somite is marked by the arrowhead and the 2 somites formed in between each frame is marked by the asterisk. The time-lapse shows individual frames up to 15hrs of a 24hr long session. The embryo was unlabeled and the image was created by opening the shutter of a widefield microscope at 3min intervals. The reflected light image was enhanced by a low light level light source shined onto the teflon surface. (M) A typical in ovo time-lapse of head morphogenesis reveals the tremendous amount of growth and movement (rotation) of the early chick embryo (compare the size of the head at 0h and 30h). The image sequence is shown over 30hrs of a 36hr time-lapse session and the asterisk marks the axial level of rhombomere 4. After 12hrs, the growth of the embryo required the change to a lower magnification objective (5X to 2.5X). The embryo was not labeled and images were collected in the same manner as in (L). (N) A fluorescently labeled (DiI) embryo is followed using in ovo time-lapse imaging and reveals the migration of cranial and post-otic neural crest cells emerging from both sides of rhombomere 1(r1, arrows) and r4 (arrowheads) at 0h. By 6h, neural crest cells emerge from r6 (arrowheads) and (9.5h) as the embryo rotates, neural crest cells begin to reach the peripheral branchial arches near the front of the migratory stream (arrowhead). At 14hrs, the embryo has rotated to reveal the position of the branchial arches (ba2 and ba3) and otic vesicle (ov). Individual migratory neural crest cells are seen moving at the migratory front (arrow). The scalebars are 200um (L,M,and N).
Acknowledgements
We would like to thank Dan Kiehart (Duke University) for the initial ideas of using teflon membranes in embryo culture for intravital imaging and Scott Fraser (Caltech) for insights into adapting the teflon membrane technique to avian embryo culture. P.M.K. would like to thank the National Institutes of Health (1R01HD057922) and the Stowers Institute for Medical Research for funding.
References
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morph. 1951;88:49–92. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows interactions during migration to the branchial arches. Dev. 2000;127:1161–1172. doi: 10.1242/dev.127.6.1161. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Kasemeier-Kulesa JC. Construction of a heated incubation chamber around a microscope stage for time-lapse imaging. Cold Spring Harbor, NY: CSH Protocols; 2007. CSH Protocols; 2008. [DOI] [PubMed] [Google Scholar]
- Stark DA, Kasemeier-Kulesa JC, Kulesa PM. Photoactivation cell labeling for cell tracing in avian development. Cold Spring Harbor, NY: CSH Protocols; 2008. CSH Protocols; 2007. [DOI] [PubMed] [Google Scholar]