Abstract
Plants that have been wounded by insects or other herbivores may be more susceptible to infection by adventitious microbes. Wound-induced signal molecules, which serve to induce responses in the plant that retard further feeding, might also act to prepare a plant for possible pathogen attack. We have examined the effect of a wound-generated systemic messenger (systemin) on a pathogen-stimulated defense-response marker, the oxidative burst. We observed that neither systemin nor its inactive analog (A-17) was able to directly induce H2O2 biosynthesis in suspension-cultured tomato (Lycopersicon esculentum L.) cells, regardless of the duration of exposure of the cells to the two peptides. Similarly, neither systemin nor A-17 was capable of modifying an oligogalacturonide-elicited oxidative burst, as long as elicitor addition occurred within minutes of treatment with systemin or A-17. In contrast, preexposure of the cell cultures to systemin (but not to A-17) led to a time-dependent enhancement of the oligogalacturonide-elicited oxidative burst. By 12 h of exposure, the H2O2 biosynthetic capacity of systemin-treated cells exceeded that of the control cells by a factor of 16 ± 2. A similar up-regulation by systemin of a mechanically stimulated oxidative burst was also observed. Because the systemin-induced augmentation in oxidant synthesis is quantitatively prevented by coincubation with 2 μm cycloheximide, and because the oxidative burst of oligogalacturonic acid-elicited control cells (no systemin exposure) is unaffected by preincubation with cycloheximide, we conclude that systemin enhancement of the tomato-cell oxidative burst requires protein synthesis.
Tomato (Lycopersicon esculentum L.) cells respond to wounding/herbivore attack by releasing a highly mobile octadecameric peptide termed systemin (Pearce et al., 1991). Systemin, the first peptide hormone found in the plant kingdom, was originally synthesized as a 200-amino acid precursor protein called prosystemin (McGurl et al., 1992). After its proteolytic activation/release, the oligopeptide is rapidly translocated into unwounded tissues, where it is thought to bind a 50-kD plasma membrane receptor (Pearce et al., 1993; Schaller and Ryan, 1994). Subsequent to receptor activation, the signaling pathway is hypothesized to proceed via stimulation of a phospholipase, resulting in intracellular release of linolenic acid, metabolism of linolenic acid to jasmonate and methyl jasmonate, and transcriptional activation by jasmonate of proteinase inhibitor genes and other resistance mechanisms (Farmer and Ryan, 1992; Mueller et al., 1993).
Plants are also able to mount defense responses against disease-causing microbes. After recognition of the invading pathogen, the responsive plant cell may attempt to limit fungal/bacterial ingress by promoting a variety of disease-resistance strategies, including cell wall stabilization (Bradley et al., 1992), stomatal closure (Hammond-Kossack et al., 1996), phytoalexin biosynthesis (Nicholson, 1992; Davis et al., 1993), expression of pathogenesis-related proteins and other toxic peptides (Bol et al., 1990), induction of localized cell death leading to a hypersensitive response (Doke, 1983a; Greenberg et al., 1994), and generation of active oxygen species, primarily O2−· and H2O2 (Doke et al., 1996; Low and Merida, 1996). The latter process, frequently termed the oxidative burst, may be the most rapid, arising within minutes of elicitor addition and extending for various lengths of time, depending on the compatibility of the host plant-pathogen interaction (Baker et al., 1993; Chandra et al., 1996). Because it is thought to be required for many subsequent defense responses (Doke et al., 1996; Low and Merida, 1996), and because it is probably expressed in most if not all plant species (Doke, 1983b; Baker et al., 1993; Chandra and Low, 1995; Kauss and Jeblick, 1995; Chandra et al., 1996; Fauth et al., 1996), the oxidative burst has often been used as a crude gauge of a plant's ability to recognize and respond to a disease-causing microbe (Doke et al., 1996).
Although these two defense mechanisms are directed against very different enemies, they nevertheless share several common features. Both responses are initiated at the site of attack but eventually involve mobile messengers that communicate a localized defense alert throughout naive regions of the plant (Malamy et al., 1990; Pearce et al., 1991; Ryals et al., 1995). Both responses involve the biosynthesis of toxic compounds such as phytoalexins, which presumably retard the invasion of microbes and macroscopic herbivores (Nicholson, 1992; Davis et al., 1993; Nojiri et al., 1996). Finally, in both responses, similar pathogenesis-related genes are induced that are thought to increase the probability of plant survival against subsequent attempts at invasion/colonization (Wasternack and Parthier, 1997). Thus, it is not surprising that several second messengers such as ethylene and jasmonic acid can participate in both defense responses and possibly even activate shared resistance responses (O'Donnell et al., 1996; Creelman and Mullet, 1997).
In view of the commonalties between pathogen- and herbivore-resistance strategies, the question naturally arises whether successful defense against onslaught from one prepares the plant to resist attack by the other. In an initial attempt to address this issue, we have examined whether pretreatment of tomato cells with systemin affects the cells' ability to respond to pathogen-generated elicitors. We report here that systemin has no immediate effect on the capacity of tomato cells to generate an elicitor-induced oxidative burst. However, after a several-hour pretreatment that involves systemin-induced gene expression, we observed tomato cells responding to elicitation by generating H2O2 at a rate at least 1 order of magnitude greater than cells not previously exposed to systemin. We propose that this cross-talk between herbivore- and pathogen-directed defense strategies may help protect the plant against opportunistic microbes.
MATERIALS AND METHODS
Pyranine was obtained from Molecular Probes (Eugene, OR). Systemin and A-17, an inactive analog of systemin, were synthesized as described elsewhere (Pearce et al., 1993). All other chemicals were obtained from major suppliers.
Tomato Cell-Suspension Cultures
Rio Grande-PtoR (Pto/Pto, Fen/Fen) is a variety of tomato (Lycopersicon esculentum L.) that is both resistant to bacterial speck disease caused by avrPto-expressing Pseudomonas syringae pv tomato and sensitive to the organic pesticide fenthion (Martin et al., 1993). This tomato variety was used to generate cell-suspension cultures that were used in all experiments. Tomato stems were surface sterilized and placed on agar plates (R-3 medium [Schnapp et al., 1991] with 5% agar) to allow callus formation. The calli were fragmented, transferred to liquid R-3 medium, and cultured by rotary shaking at 19°C as described previously (Bressan et al., 1981). The resulting cell cultures were maintained by transferring 3 mL of culture to 25 mL of fresh medium every 14 d. Cells were most responsive to elicitor stimulation up to 18 h after transfer to fresh medium and were used for oxidative-burst evaluations during this period.
Elicitors
An OGA fraction that elicits H2O2 production in a variety of cultured plant species was purified as described previously (Legendre et al., 1993). The OGA stock preparation used in this study contained approximately 500 μg/mL, as determined by the method of Blumenkrantz and Asboe-Hansen (1981).
Spectrofluorimetric Determination of H2O2 Production
H2O2 production by cultured tomato cells was detected by monitoring the oxidative quenching of the fluorescent peroxidase substrate pyranine (8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt; 405 nm excitation/512 nm emission) as described previously (Apostol et al., 1989a; Legendre et al., 1993). Cells (1.5 mL) were mixed with 5 μL of pyranine (0.2 mg/mL stock solution) in a fluorimetric cuvette and maintained in suspension by mild stirring. After addition of elicitor, the lost dye fluorescence was continuously monitored, and the initial slope of the quenching curve was used to determine the rate of H2O2 production.
RESULTS
Systemin Is Not an Elicitor or Immediate Potentiator of the Oxidative Burst
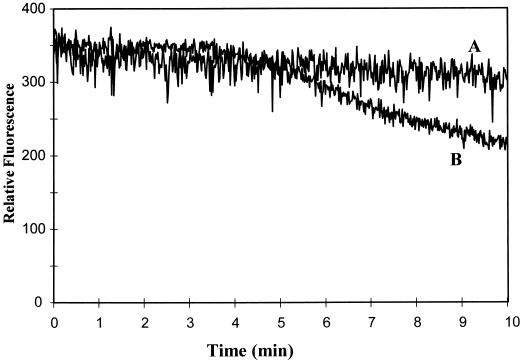
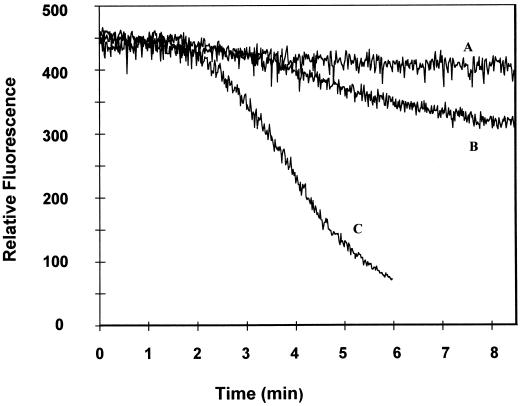
To determine whether systemin might serve as an elicitor of pathogen defense responses, we investigated systemin's ability to directly stimulate or augment an elicitor-stimulated oxidative burst in cultured tomato cells. As shown in Figure 1 (trace A), with the addition of systemin at concentrations (1 μm) above those known to induce maximal wounding responses such as proteinase inhibitor biosynthesis (Pearce et al., 1991), the peptide promoted no detectable H2O2 production in tomato suspension cultures. Furthermore, the addition of systemin directly before elicitor stimulation of the same cells (trace B) caused no further increase in H2O2 biosynthesis compared with the treatment of the cells with OGA elicitor alone. As anticipated, A-17, an inactive analog of systemin differing only by a single Ala substitution at position 17, also had no effect on oxidant biosynthesis, regardless of whether it was introduced alone or in conjunction with OGA. These data indicate that any effect systemin might have on the oxidative burst is not manifested immediately after systemin addition.
Figure 1.
Effect of systemin and A-17 on the generation of H2O2 by unstimulated and OGA-elicited tomato cell-suspension cultures. Twelve-hour-old tomato cell-suspension cultures were treated with buffer (control), 1 μm systemin, or 1 μm A-17, the inactive systemin analog, and assayed directly for H2O2 production (trace A). Alternatively, a separate flask of cells was treated as above and then stimulated with a low concentration of OGA elicitor (20 μL of a 500 μg/mL stock solution added to 1.5 mL of cell suspension) and assayed for H2O2 production (trace B). Because the tomato cells that were treated with buffer alone, systemin, or A-17 generated no measurable H2O2, they are collectively represented by a single trace (A). Similarly, because the buffer-, systemin-, and A-17-treated cells all yielded an identical, slowly developing oxidative burst after OGA elicitation, they are also displayed as a single representative trace (B). Each experiment was conducted at least four times with similar results.
Preincubation with Systemin Enhances Both the OGA-Induced and the Osmotically Induced Oxidative Bursts
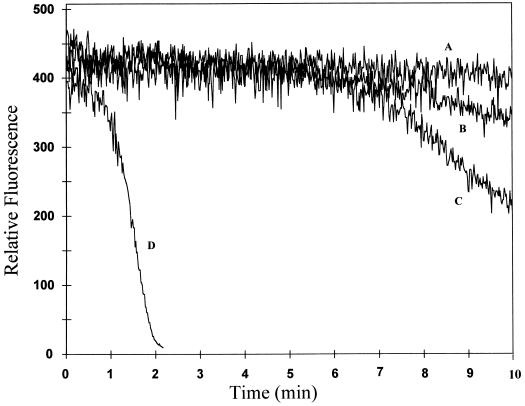
Previous research has established that the systemin-induced accumulation of proteinase inhibitor mRNAs typically begins approximately 2 to 4 h after the addition of systemin to plant leaves and continues to increase for more than 12 h (Farmer and Ryan, 1990; Pearce et al., 1991). Therefore, we investigated whether an induction period might also be required before systemin could independently induce or potentiate an elicitor-induced oxidative burst. As noted above, OGA readily stimulated the synthesis of H2O2 within minutes of its addition to cultured tomato cells (Fig. 1). For the cells used here, which had been transferred to fresh medium 12 h before elicitation, the low concentration of OGA used was observed to promote a mild oxidative burst within approximately 6 min of addition to the suspension culture (Fig. 2, trace B). Importantly, transfer of another aliquot of the same cell suspension to systemin-containing medium 12 h before examination caused no unelicited synthesis of active oxygen species (Fig. 2, trace A), indicating that even extended exposure to systemin induces no autologous generation of an oxidative burst. In contrast, stimulation of the 12-h systemin-pretreated cell suspension with OGA not only shortened the delay before onset of H2O2 production, but also promoted oxidant biosynthesis at approximately 16 times the rate of OGA-stimulated cells that were not pretreated with systemin (Fig. 2, compare traces B and D). In more than 10 replicates of this series of experiments on many different batches of cells, the systemin-promoted enhancement of H2O2 generation ranged from 10 to 20 times that of nonpretreated but OGA-stimulated cells. Because similar pretreatment with A-17 exerted no effect, we conclude that pretreatment with systemin augments the tomato cell's ability to activate H2O2 production in response to elicitor stimulation.
Figure 2.
Effect of preincubation with systemin on H2O2 production by unstimulated and OGA-elicited tomato cells. Suspension-cultured tomato cells were transferred to fresh growth medium containing 1 μm A-17 or no additive (traces B and C), or 1 μm systemin (trace D). Twelve hours later, the cells were stimulated with a low amount (20 μL, traces B and D) or a saturating amount (100 μL, trace C) of OGA, and the rate of H2O2 biosynthesis was monitored by following the quenching of pyranine fluorescence. Alternatively, an aliquot of the A-17-, systemin-, or buffer (control)-treated cell suspension was incubated for 12 h, as above, and then assayed for H2O2 generation in the absence of OGA elicitation (trace A). Because none of the three unelicited cultures (A-17-, systemin-, or buffer-pretreated cells not elicited with OGA) generated detectable H2O2, they are all represented by a common trace (A). Each study was conducted at least five times with similar results.
The effects of systemin and A-17 on oxidant biosynthesis by tomato cells were also assayed at much lower (10−9 m) peptide concentrations. As above, no unelicited production of H2O2 was observed, regardless of the duration of exposure to either peptide (data not shown). Unfortunately, the enhancement of OGA-stimulated H2O2 generation subsequent to the usual 12-h preincubation with systemin was more variable than at higher systemin concentrations. Although we suspect that different rates of systemin breakdown during the 12-h preincubation may be the cause of the observed variability, all further incubations were still conducted at 10−6 m systemin to avoid the variability problem altogether.
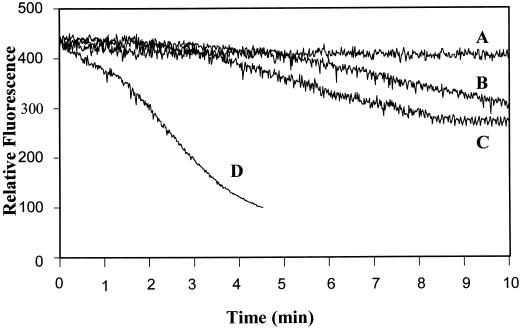
In addition to chemical elicitation, pathogen ingress can generate mechanical signals that are sensed by the plant and used to activate defense responses (Yahraus et al., 1995). However, because mechanical stimuli may be perceived very differently by a host plant than chemical stimuli, it seemed important to also explore whether pretreatment with systemin might enhance a mechanically induced oxidative burst. As described previously (Yahraus et al., 1995), mechanical initiation of the burst reaction can be accomplished in suspension-cultured cells either by direct physical pressure or by a decrease in osmotic pressure. Figure 3 shows that 12-h-old tomato cells produced a mild oxidative burst after dilution with an equal volume of water (trace B). A similar response was also measured in cells exposed for the entire 12-h incubation period to A-17, the inactive analog of systemin (trace B). In contrast, tomato cells cultured for the same 12-h period with 1 μm systemin generated a measurably (>10 times) enhanced oxidative burst after the same 1:1 dilution with water (trace D). As with elicitor stimulation, the lag period between application of the mechanical signal and generation of reactive oxidants was also measurably shortened in the systemin-pretreated cells (compare traces B and D).
Figure 3.
Effect of preincubation with systemin on H2O2 production by unstimulated and osmotically stressed tomato cells. Suspension-cultured cells were transferred to fresh growth medium containing 1 μm A-17 or no additive (traces B and C), or 1 μm systemin (trace D), and assayed 12 h later for H2O2 production after 1:1 dilution (B and D) or 1:2 dilution (C) with water. Concurrently, the unstimulated rate of H2O2 biosynthesis in each of the three samples (A-17, systemin, and buffer [control]-treated suspensions) was measured at the same 12-h time point. Because none of the three unstimulated cell cultures generated any H2O2, they are collectively represented by a common curve (trace A). Similarly, because A-17 addition had no effect on the osmotically stimulated oxidative burst, it is shown together with a nonpretreated but osmotically stimulated control in trace B. Each study was conducted at least three times with similar results.
Time Course of Systemin-Induced Enhancement of the OGA-Stimulated Burst
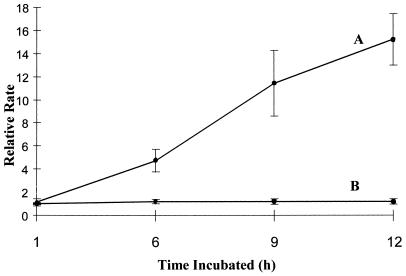
To more accurately evaluate the time required for systemin to exert its effects on the OGA-stimulated oxidative burst, we preincubated suspension-cultured cells with 1 μm systemin for varying periods of time for up to 12 h and then determined the magnitude of the OGA-activated burst. As shown in Figure 4, systemin enhancement of oxidant biosynthesis was seen as early as 6 h after its addition, and this augmentation of the burst continued to increase with longer incubation times. By 12 h after addition, the average rate enhancement reached approximately 16-fold. Unfortunately, the experiment could not generally be continued beyond 12 h because the untreated cells gradually entered the usual elicitor-refractory period (Apostol et al., 1989b) that prohibited further analyses. However, it should be noted that the systemin-treated cells invariably continued to be responsive to elicitors such as OGA many hours beyond the time when untreated cells became inactive. This prolongation of sensitivity to elicitors adds support to the hypothesis that systemin promotes a fundamental change in the tomato cells that renders them more capable of elicited oxidant production. Figure 4 also shows that A-17-pretreated cells were elicited by OGA similarly to control cells, confirming that the effect of systemin was sequence dependent and was not a consequence of nonspecific effects.
Figure 4.
Effect of preincubation time on the magnitude of systemin enhancement of the OGA-stimulated oxidative burst. Tomato cells were transferred to fresh medium containing 1 μm systemin (A), 1 μm A-17 (B), or no additive. At various times after transfer, cells were stimulated with OGA and the rate of H2O2 biosynthesis was assayed, as described in Methods. The relative rate plotted on the y axis refers to the rate of oxidant generation of the systemin-/A-17-pretreated sample divided by the rate of oxidant production of the nonpretreated sample, each stimulated by OGA at the same time point. The data represent the average ± sd, where n = 3.
Systemin Enhancement of the OGA-Induced Oxidative Burst Is Dependent on Protein Synthesis
Systemin-mediated activation of the octadecanoid pathway culminates in the transcription of proteinase inhibitor genes that are thought to contribute to herbivore resistance (Farmer and Ryan, 1992; Mueller et al., 1993; Schaller and Ryan, 1995). Consequently, it seemed prudent to investigate whether systemin-mediated enhancement of the oxidative burst might also require de novo protein synthesis. Cycloheximide, a well-known protein translation inhibitor, was administered to cells concurrently with systemin (i.e. at the time of transfer to fresh medium) and its effects were monitored 12 h later. As seen in Figure 5, cycloheximide abolished the systemin-induced enhancement of the OGA burst. In contrast, cycloheximide had little or no influence on the OGA-stimulated burst in nonpretreated or A-17-pretreated cells, indicating that cycloheximide had no effect on the oxidase complex itself but, rather, prevented its enhancement by systemin. Taken together, these data suggest that the major mechanism by which systemin enhances elicitation of the oxidative burst pathway involves the translation of new polypeptides.
Figure 5.
Effect of cycloheximide on systemin-induced enhancement of the OGA-elicited oxidative burst. Tomato cells were transferred into fresh medium containing or lacking the following components: 1 μm systemin, 1 μm A-17, and/or 2 μm cycloheximide. After 12 h of incubation, the cell suspensions were examined for basal (trace A) and OGA-elicited (traces B and C) H2O2 production. Because many of the responses were similar, they are grouped into the following representative traces: A, nonpretreated and unelicited cells, systemin-pretreated cells (no OGA), A-17-pretreated cells (no OGA), and cycloheximide-pretreated cells (no OGA); B, nonpretreated cells elicited with OGA, A-17-pretreated cells elicited with OGA, A-17- plus cycloheximide-pretreated cells elicited with OGA, systemin- plus cycloheximide-pretreated cells elicited with OGA, and cycloheximide-pretreated cells elicited with OGA; and C, systemin-induced cells elicited with OGA. Each study was repeated at least three times with similar results.
DISCUSSION
We have demonstrated that pretreatment of tomato cells with a wounding-inducible peptide hormone, systemin, strongly augments both an elicitor-induced and an osmotically induced oxidative burst. Not only is the delay between stimulation and appearance of the burst greatly shortened after prolonged exposure to systemin, but the rate of oxidant production is enhanced, and the length of time during which cell cultures remain responsive to elicitation is significantly extended. Because A-17, an inactive peptide analog that differs from systemin in only 1 of 18 amino acids, has no significant effect, we suggest that systemin exerts its influence on the oxidative burst through its normal cell-surface receptor.
Based on functional characteristics, the oxidative burst is thought to serve a defensive function directed primarily at disease-causing microbes (Chandra et al., 1996; Doke et al., 1996; Low and Merida, 1996). The currently acknowledged properties of systemin, in contrast, indicate that it is generated during herbivore feeding and acts to induce systemic protection against herbivores (Farmer and Ryan, 1990; Schaller and Ryan, 1995). Therefore, augmentation of the oxidative burst by pretreatment with systemin implies that a wounded tomato may have evolved strategies to prophylactically counteract opportunistic pathogens. Although no constitutive biosynthesis of H2O2 can be detected in systemin-pretreated cells, once properly initiated by an elicitor, the rate of oxidant production is enhanced by a factor of 16. It would seem, therefore, that an insect-damaged plant not only undertakes to prevent further feeding by insects, but may simultaneously mobilize to thwart adventitious pathogen attack.
The effects of systemin on the tomato-cell oxidative burst were not immediate. Only several hours after its addition were the changes promoted by systemin clearly measurable. That this extended incubation period probably involves the synthesis of new proteins was shown by the inhibition of enhancement of oxidant biosynthesis by cycloheximide. Although no information was obtained regarding the identities of the newly synthesized polypeptides, it is tempting to speculate that more than one biochemical complex might be involved. Thus, not only were the timing of initiation and the rate of oxidant synthesis altered, but the duration of the period during which the cells remained responsive to elicitation was also extended. It will be important to identify the genes whose products are involved in these regulatory events, because they likely encode proteins that control the oxidant-generating capacity of the cell.
Abbreviation:
- OGA
oligogalacturonic acid
Footnotes
This work was supported by National Science Foundation grant no. MCB-9303929.
LITERATURE CITED
- Apostol I, Heinstein PF, Low PS. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiol. 1989;90:109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol I, Low PS, Heinstein PF. Effect of age of cell suspension cultures on susceptibility to a fungal elicitor. Plant Cell Rep. 1989b;7:692–695. doi: 10.1007/BF00272063. [DOI] [PubMed] [Google Scholar]
- Baker CJ, Orlandi EW, Mock NM. Harpin, an elicitor of the hypersensitive response in tobacco caused by Erwinia amylovora, elicits active oxygen production in suspension cells. Plant Physiol. 1993;102:1341–1344. doi: 10.1104/pp.102.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen A. New method for quantitative determination of uronic acids. Anal Biochem. 1981;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bol JF, Linthorst JJM, Cornelissen BJC. Plant pathogenesis-related proteins induced by virus infection. Annu Rev Phytopathol. 1990;28:113–138. [Google Scholar]
- Bradley DJ, Kjellbun P, Lamb CJ. Elicitor-induced and wound-induced oxidative cross-linking of a proline-rich plant-cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Bressan RA, Hasegawa PM, Handa A. Resistance of cultured higher plant cells to polyethylene glycol-induced water stress. Plant Sci Lett. 1981;21:23–30. [Google Scholar]
- Chandra S, Low PS. Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Prod Natl Acad Sci USA. 1995;92:4120–4123. doi: 10.1073/pnas.92.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Martin GB, Low PS. The Pto kinase mediates a signaling pathway leading to the oxidative burst in tomato. Proc Natl Acad Sci USA. 1996;93:13393–13397. doi: 10.1073/pnas.93.23.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Davis D, Merida J, Legendre L, Low PS, Heinstein P. Independent elicitation of the oxidative burst and phytoalexin formation in cultured plant-cells. Phytochemistry. 1993;32:607–611. [Google Scholar]
- Doke N. Generation of superoxide anion by potato tuber protoplasts during the hypersensitive response to hyphal wall components of Phytophthora infestans and specific inhibition of the reaction by suppressors of hypersensitivity. Plant Pathol. 1983a;23:359–367. [Google Scholar]
- Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol. 1983b;23:345–357. [Google Scholar]
- Doke N, Miura Y, Sanchez LM, Park H-J, Noritake T, Yoshioka H, Kawawita K. The oxidative burst protects plants against pathogen attack: mechanism and role as an emergency signal for plant bio-defence. A review. Gene. 1996;179:45–51. doi: 10.1016/s0378-1119(96)00423-4. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth M, Merten A, Hahn MG, Jeblick W, Kauss H. Competence for elicitation of H2O2 in hypocotyls of cucumber is induced by breaching the cuticle and is enhanced by salicylic acid. Plant Physiol. 1996;110:347–354. doi: 10.1104/pp.110.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JT, Guo A, Klessig DF, Ausubel FM. Programmed cell-death in plants: a pathogen-triggered response activated coordinately with multiple defense functions. Cell. 1994;77:551–563. doi: 10.1016/0092-8674(94)90217-8. [DOI] [PubMed] [Google Scholar]
- Hammond-Kossack KE, Silverman P, Raskin I. Race-specific elicitors of Cladosporium fulvum induce changes in cell morphology and the synthesis of ethylene and salicylic acid in tomato plants carrying the corresponding Cf disease resistance gene. Plant Physiol. 1996;110:1381–1394. doi: 10.1104/pp.110.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Jeblick W. Pretreatment of parsley suspension cultures with salicylic acid enhances spontaneous and elicited production of H2O2. Plant Physiol. 1995;108:1171–1178. doi: 10.1104/pp.108.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L, Reuter S, Heinstein PF, Low PS. Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol. 1993;102:233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PS, Merida JR. The oxidative burst in plant defense: function and signal transduction. Physiol Plant. 1996;96:533–542. [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Martin GB, Brommonschenkel SH, Chunwongse J, Frary A, Ganal MW, Spivey R, Wu T, Earle ED, Tanksley SD. Map based cloning of a protein kinase gene conferring disease resistance in tomato. Science. 1993;262:1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- McGurl B, Pearce G, Orozco-Cardenas M, Ryan CA. Structure, expression, and antisense inhibition of the systemin precursor gene. Science. 1992;255:1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- Mueller MJ, Brodschelm W, Spannagl E, Zenk MH. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci USA. 1993;90:7490–7494. doi: 10.1073/pnas.90.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RL. Phenolic-compounds and their role in disease resistance. Annu Rev Phytopathol. 1992;30:369–389. [Google Scholar]
- Nojiri H, Sugimuri M, Hisakazu Y. Involvement of jasmonic acid in elicitor-induced phytoalexin production in suspension cultured rice cells. Plant Physiol. 1996;110:387–392. doi: 10.1104/pp.110.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- Pearce G, Johnson S, Ryan CA. Structure-activity of deleted and substituted systemin, an 18-amino acid polypeptide inducer of plant defensive genes. J Biol Chem. 1993;268:212–216. [PubMed] [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA. A polypeptide from tomato leaves induces wound-inducible proteinase-inhibitor proteins. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Ryals J, Lawton KA, Delaney TP, Friedrich L, Kessman H, Neuenschwander U, Uknes S, Vernooij B, Weymann K. Signal transduction in systemic acquired resistance. Proc Natl Acad Sci USA. 1995;92:4202–4205. doi: 10.1073/pnas.92.10.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller A, Ryan CA. Identification of a 50-kda systemin-binding protein in tomato plasma-membranes having kex2p-like properties. Proc Natl Acad Sci USA. 1994;91:11802–11806. doi: 10.1073/pnas.91.25.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller A, Ryan CA. Systemin: a polypeptide defense signal in plants. BioEssays. 1995;18:27–33. doi: 10.1002/bies.950180108. [DOI] [PubMed] [Google Scholar]
- Schnapp SR, Curtis WR, Bressan RA, Hasegawa PM. Estimation of growth yield and maintenance coefficient of plant cell suspensions. Biotechnol Bioeng. 1991;38:1131–1136. doi: 10.1002/bit.260381003. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Parthier B. Jasmonate signalled plant gene expression. Trends Plant Sci. 1997;2:302–307. [Google Scholar]
- Yahraus T, Chandra S, Legendre L, Low PS. Evidence for a mechanically induced oxidative burst. Plant Physiol. 1995;109:1259–1266. doi: 10.1104/pp.109.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]