Abstract
A 70-year-old woman developed progressive visual loss with compromised visual acuity and visual fields, cells in the anterior chamber and vitreous, attenuated retinal arterioles, and macular edema. She had undergone right oophorectomy and partial salpingectomy nearly 50 years earlier. Full-field and multifocal electroretinography showed waveforms of markedly attenuated amplitudes, findings consistent with cancer-associated retinopathy (CAR). Positron emission tomography revealed a nodule in the anterior wall of a right hydrosalpinx. Total laparoscopic hysterectomy yielded a neuroendocrine fallopian tube malignancy. She underwent partial treatment with paclitaxel and carboplatin that was aborted because of the development of herpes zoster infection. At 15 months following diagnosis, her ophthalmic status was stable. This is the first report of CAR in neuroendocrine carcinoma of the fallopian tube.
Although paraneoplastic syndromes affecting vision, including cancer-associated retinopathy (CAR), are well documented in patients with small cell carcinomas, fallopian tube neuroendocrine carcinoma causing CAR has not been reported. We report such a case.
CASE REPORT
A 70-year-old woman reported progressive worsening of vision in both eyes (left eye more than right eye) for approximately 18 months. The patient had undergone bilateral cataract surgery 18 months earlier with a temporary improvement in vision. She described intermittent diarrhea for 2 years for which evaluation had been unrevealing. Nearly 50 years earlier, she had been diagnosed with an ovarian cyst and had undergone right oophorectomy and partial salpingectomy in conjunction with an incidental appendectomy. Although the cyst was benign, it had been necessary to remove part of the adjoining fallopian tube for complete excision of the ovarian cyst. She also had a history of hypothyroidism and excision of basal cell carcinoma of the nose.
Three months before she presented to us, she had been evaluated by a neuro-ophthalmologist who had recorded visual acuities of 20/20 in the right eye and 20/25 in the left eye. Color vision had been severely impaired, but no afferent pupillary defect had been found. Posterior segment examination had revealed vitreous cells, mild retinal arteriolar attenuation, and normal optic discs. Intraocular pressures had been 32 mm Hg in the right eye and 19 mm Hg in the left eye.
Brain MRI had disclosed mild white matter signal abnormalities. Full-field electroretinography had shown extinguished or attenuated responses in nearly all photopic and scotopic conditions (Fig. 1). Negative tests had included serology for paraneoplastic neuronal and antiretinal antibodies, fluorescent treponemal antibody absorption test, rapid plasma reagin, angiotensin-converting enzyme, antineutrophil cytoplasmic antibody, Borrelia burgdorferi antibody, and spinal fluid examination. The patient had been treated with bimatoprost eyedrops to lower intraocular pressure but was distressed by deteriorating vision and glare.
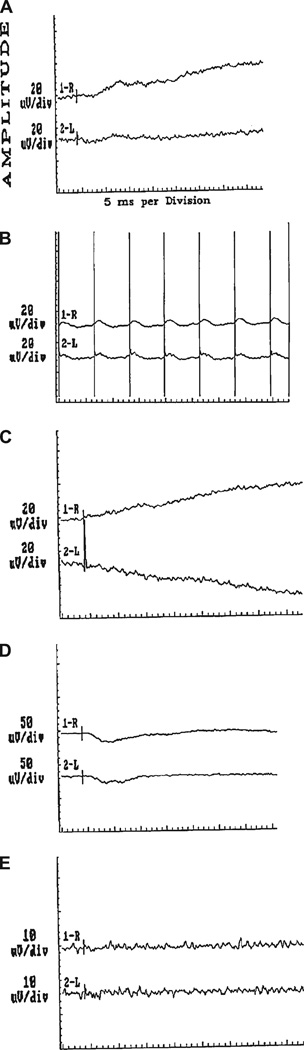
FIG. 1.
Full-field electroretinography performed 2 months before our first consultation shows attenuated or extinguished responses in photopic single flash (A), photopic 30 Hz flicker (B), scotopic potential (C), photopic and scotopic potentials (D), and oscillatory potential (E).
Two months before consulting us, a second neuroophthalmologist recorded that visual acuities had deteriorated to 20/40 in the right eye and 20/50 in the left eye. Color vision was reduced in both eyes, and vitreous cells and slight pallor of both optic nerves were observed. Visual field examination showed enlargement of the blind spots with constricted isopters along the horizontal meridian. A retinal fluorescein angiogram was suggestive of vasculitis. Optical coherence tomography showed subfoveal fluid bilaterally. Multifocal electroretinography showed bilateral near flattening of the waveforms. The patient was diagnosed with bilateral chronic posterior uveitis, retinal vasculitis, and macular edema. No treatment was instituted.
On our examination, best-corrected visual acuities were 20/25 in the right eye and 20/60 in the left eye. She was able to identify 4/14 Ishihara color plates with the right eye and 0/14 with the left eye. There was a left afferent pupillary defect of 0.6–0.9 log units. There were 2+ cells in the anterior chamber and 3+ cells in the vitreous in both eyes. Optic discs appeared normal, but macular edema was seen in the left eye. Amsler grid testing showed a dense ring scotoma with minimal central preservation in both eyes. With automated visual fields (Fig. 2), a dense ring scotoma was present in the right eye and severe constriction in the left eye.
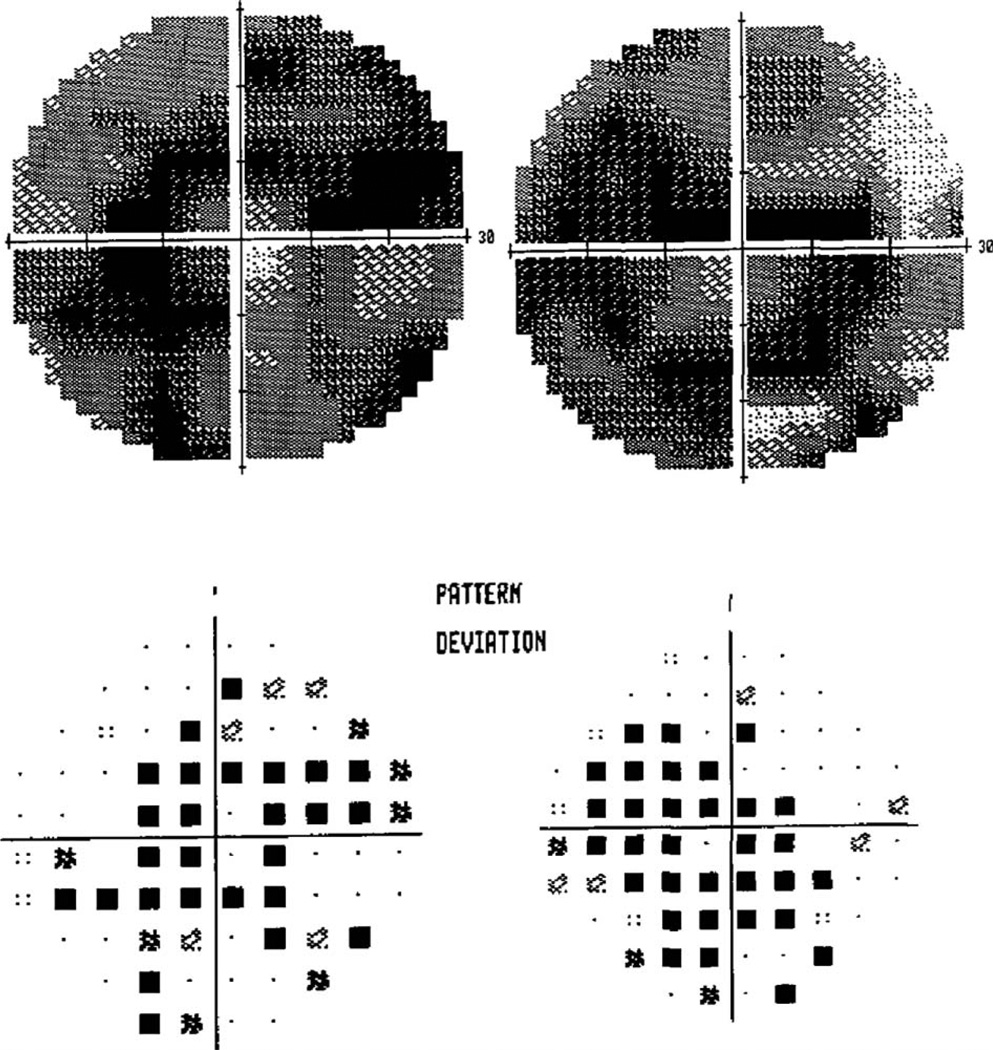
FIG. 2.
Visual fields show bilateral ring-like scotomas with small areas of central preservation. Mean deviations were −21.5 dB in the left eye and −21.32 dB in the right eye.
Further investigations revealed a negative collapsing response-mediating protein 5 antibody but positive serum anti-retinal antibodies against carbonic anhydrase II, alphaenolase, and a 97 kDa protein. Immunohistochemistry, performed at the Ocular Immunology Laboratory at Oregon Health and Science University, Portland, Oregon (G.A.), showed strong cytoplasmic staining of the outer nuclear layer and diffuse staining of the ganglion cell layer and neuronal fibers of human retina.
The patient underwent investigations to exclude lymphoma and other malignancies. Gastrointestinal system evaluation to exclude Whipple disease and neoplasia with endoscopy and colonoscopy showed only diverticular disease. A whole-body fluorodeoxyglucose positron emission tomography (with noncontrast CT for attenuation correction) showed a nodular area in the anterior wall of a right hydrosalpinx. This finding was confirmed by ultrasound, which also showed a thickened endometrial stripe suspicious for endometrial malignancy. CA-125 measurement was 12.9 U/mL (within normal limits).
Over the next 4 months, the hydrosalpinx grew, so the patient underwent a total laparoscopic hysterectomy. After an intraoperative frozen section reported a fallopian tube malignancy, a bilateral salpingo-oophorectomy, pelvic lymph node dissection, and omentectomy were completed. Low-power histopathology showed extensive necrosis (Fig. 3A). High-power histopathology (Fig. 3B) showed hyperchromatic nuclei and scanty indistinct cytoplasm, numerous mitoses, and great variation in tumor cell size with focally irregularly shaped nuclei.
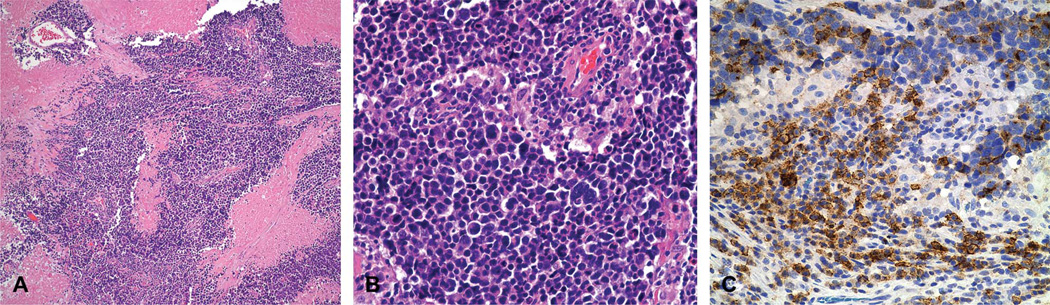
FIG. 3.
Histopathology of the surgical specimen of the fallopian tube. A. Low-power view of tumor shows extensive necrosis (hematoxylin and eosin ×100). B. High-power view shows cells with hyperchromatic irregularly shaped nuclei and scant indistinct cytoplasm, numerous mitotic figures, and great variation in tumor cell size (hematoxylin and eosin ×400). C. Immunohistochemical staining of neoplastic cells is positive for epithelial membrane antigen. In addition, these neoplastic cells are also positive for cytokeratin, synaptophysin, and neurofilament and negative for leukocyte common antigen (not shown).
Immunohistochemical staining was positive for epithelial membrane antigen (Fig. 3C), cytokeratin, chromogranin, synaptophysin, and neurofilament and negative for leukocyte common antigen, placental alkaline phosphatase, and alpha-fetoprotein. The morphology and immunohistochemical profiles supported a diagnosis of high-grade neuroendocrine carcinoma with small cell features. No tumor was found in the ovaries, endometrium, lung, or gastrointestinal tract.
The patient did not receive any immunosuppressive medication, but completed 2 of 6 planned cycles of paclitaxel and carboplatin. She failed to complete chemotherapy due to an outbreak of herpes zoster in the right lower back.
She has no evidence of malignancy at 15 months following surgical treatment. At the most recent ophthalmological evaluation, visual acuities were 20/30 in the right eye and 20/40 in the left eye. Previous binocular vitrectomies had revealed no evidence of malignancy or infection. She continues to have mild panuveitis for which she has been receiving periocular corticosteroid injections. Secondary to the corticosteroid injections, she has developed uncontrolled glaucoma.
DISCUSSION
We believe this to be the first reported case of CAR in the context of a neuro-endocrine carcinoma of the fallopian tube. Such cancers account for only 1% of all gynecologic malignancies (1). Neuroendocrine carcinoma—which is more common in the lung, gastrointestinal tract, and genitourinary tract—is rare in the fallopian tube, where adenocarcinoma is much more likely (2). The first primary neuroendocrine tumor of the fallopian tube was reported in 2004 by Dursun et al (2).
Neuroendocrine carcinomas of the gynecologic tract are associated with poor prognosis (3). However, the patient with fallopian tube neuroendocrine carcinoma reported by Dursun et al (2) was alive at 16 months with no evidence of disease. Our patient is also doing well at 15 months after treatment.
REFERENCES
- 1.Rosenblatt KA, Weiss NS, Schwartz SM. Incidence of malignant fallopian tube tumors. Incidence Gynecol Oncol. 1989;35:236–239. doi: 10.1016/0090-8258(89)90051-6. [DOI] [PubMed] [Google Scholar]
- 2.Dursun P, Salman MC, Taskiran C, Usubutun A, Ayhan A. Primary neuroendocrine carcinoma of the fallopian tube: a case report. Am J Obstet Gynecol. 2004;190:568–571. doi: 10.1016/j.ajog.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez A, Vighi S, Garcia A, Sardi J. Neuroendocrine cervical carcinoma: a diagnostic and therapeutic challenge. Gynecol Oncol. 2001;82:32–39. doi: 10.1006/gyno.2001.6201. [DOI] [PubMed] [Google Scholar]