Abstract
Glutamate-induced neuronal damage is mainly caused by overactivation of N-methyl-D-aspartate (NMDA) receptors. Conversely, normal physiological brain function and neuronal survival require adequate activation of NMDA receptors. Studies have revealed that NMDA receptor-induced neuronal death or survival is mediated through distinct subset of NMDA receptors triggering different intracellular signaling pathways. Here we discuss recent advances in the characterization of NMDA receptors in neuronal protection, emphasizing subunit-specific role, which contributes to temporal-spatial distribution, subcellular localization and diverse channel properties of NMDA receptors.
Keywords: NMDA receptors, glutamate, excitotoxicity, ischemia, neuroprotection
Introduction
NMDA receptors (NMDARs) are ionotropic glutamate receptors that are pivotal in controlling synaptic plasticity and memory function (Collingridge et al., 2004; Nakazawa et al., 2004). They are both voltage-dependent and ligand-gated. Activation of NMDARs requires membrane depolarization to remove the Mg2+ block, and binding of two agonists-glutamate and glycine, which induces Na+, K+ and Ca2+ cation currents. Among these ions, Ca2+ signaling accounts for most, if not all, of the NMDAR-mediated functions. Functional NMDARs are heterotetramers, which consist of two obligatory NR1 subunits and two regulatory subunits from either the NR2 (NR2A, NR2B, NR2C and NR2D) or less commonly, the NR3 (NR3A and NR3B) subunits. While splicing variants of NR1 subunit could alter NMDAR properties (Zukin and Bennett, 1995), the two regulatory subunits primarily diversify and determine properties of NMDARs (Cull-Candy et al., 2001). For example, the decay of evoked currents in diheteromeric NMDARs ranged from tens of milliseconds (ms) to several seconds, with NR2A the fastest and NR2D the slowest, indicating differences in NMDAR-mediated excitatory postsynaptic currents (EPSCs) and glutamate binding affinities (Cull-Candy et al., 2001). Single channel studies revealed that NR1/NR2A or NR1/NR2B forms high-conductance channels with a high sensitivity to extracellular Mg2+, while NR1/NR2C or NR1/ NR2D generate low-conductance openings with a low sensitivity to Mg2+ (Cull-Candy et al., 2001).
Each NMDAR subunit is a modular structure consisting of four distinct domains: an extracellular N-terminal domain, an extracellular ligand binding domain, a transmembrane domain and an intracellular C-terminal domain of variable length (Dingledine et al., 1999; Traynelis et al., 2010). The transmembrane domain, containing three membrane-spanning helices and a membrane re-entrant loop, contributes to the channel pore formation. The cytoplasmic domain is required for protein-protein interactions. It is thought that trafficking and localization of NMDARs depend on intracellular protein-protein interactions. Indeed, the first yeast two-hybrid screen using the NR2B C terminus as bait identified an interaction with PSD-95 (Niethammer et al., 1996), an abundant scaffolding protein present in the postsynaptic density (PSD). This interaction is essential for targeting NMDARs to specific membrane compartments. In addition, the intracellular domain of NMDAR subunits is subject to extensive posttranslational modifications such as phosphorylation, which is essential to regulate trafficking and channel properties of receptors (Chen and Roche, 2007).
The NMDAR subunits are differentially expressed throughout the central nervous system with localization patterns that change during development (Akazawa et al., 1994; Monyer et al., 1994). In the adult brain, NR1 and NR2A are expressed ubiquitously, while expression of NR2B is confined to the forebrain and striatum. NR2C is predominantly expressed in cerebellum. NR2D is mainly expressed early in development and is restricted to brainstem, midbrain and thalamus (Monyer et al., 1994). The NR3A subunit is widely distributed early in development and is localized predominantly in the nucleus of the lateral olfactory tract in the mature rodent brain. It was thought that expression of NR3B is restricted primarily to adult motor neurons. However, a recent study reported immunostaining of NR3B in wider brain regions including the forebrain, cerebellum and spinal cord (Wee et al., 2008).
NMDAR-dependent neuronal cell death and neuroprotective signaling
Glutamate-induced excitotoxicity is neuronal injury and death caused by overactivation of glutamate receptors through excessive glutamate stimulation. It was first reported that high dosage of glutamate caused lesions in the retina of postnatal mice two to ten days of age (P2 to P10) (Lucas and Newhouse, 1957). Similar observation linking excessive glutamate to brain damage was later reported in monkey (Olney and Sharpe, 1969). Choi revealed that excitotoxicity was caused by large amount of calcium (Ca2+) influx induced by high levels of glutamate (Choi, 1987). Subsequent studies suggest that overactivation of NMDARs is necessary and suficient to induce glutamate-mediated calcium toxicity in neurons (Choi et al., 1987; Choi et al., 1988). Under physiological conditions, glutamate can be quickly released to the synaptic cleft with a concentration up to 1 mM, followed by a rapid decrease (Clements et al., 1992). However, during brain trauma or ischemia stroke, reduced blood flow leads to oxygen and glucose shortage, which gradually depletes ATP available for neurons and glial cells to control the extracellular levels of glutamate. Increased accumulation of glutamate in extracellular fluid results in excessive Ca2+ influx, which triggers multiple intracellular cascades and causes damage to neuronal cells in the brain. There is growing evidence linking abnormal NMDAR activity to excitotoxicity and brain damage (discussed in NR2A and NR2B section) (Aarts et al., 2002; von Engelhardt et al, 2007; Liu et al., 2007; Tu et al., 2010). In contrast to their role in neuronal death signaling through overactivation, adequate NMDARs are required for neuroprotection. For example, transient blockade of NMDARs in perinatal rat triggered extensive apoptosis in many brain regions (Ikonomidou et al., 1999). Moreover, the in vitro survival rate of cerebellar granule cells decreased when rat pups were treated with NMDAR antagonist (Ciani et al., 1997).
Given that the functions of NMDARs often correlate with their subcellular localizations, Hardingham and Bading hypothesized that activation of extrasynatic NMDARs results in cell death, while the activity of synaptic NMDARs promotes neuronal survival (Hardingham and Bading, 2010). The differences in signaling between synaptic and extrasynaptic NMDARs could be due to three factors: the NMDAR signaling complex, receptor subunit composition, and trans-synaptic (synaptic) versus chronic (extrasynaptic) activation of NMDARs (Hardingham and Bading, 2010). This review focuses on the subunit-specific function of NMDARs in neuronal damage and protection.
NR2A and NR2B
NR2A and NR2B are the major NR2 subunits expressed in cortex and hippocampus. Expression of NR2A and NR2B is developmentally regulated. At nascent hippocampal synapses in culture, majority of NMDARs are located at extrasynaptic sites and mainly composed of NR1/NR2B (Tovar and Westbrook, 1999). During development, the expression level of NR2A is gradually increased, which leads to a switch from NR2B- to primarily NR2A-containing NMDARs (Cull-Candy et al., 2001). This subunit composition change correlates with NMDAR-mediated functions during development, including synaptic plasticity and neuronal survival. In NR2A null mice, the NMDAR channel current and long-term potentiation at the hippocampal CA1 synapses are significantly reduced, and a moderate deficiency in spatial learning is also observed (Sakimura et al., 1995). The NR2B knockout mice shows impairment of suckling response and die shortly after birth (Kutsuwada et al., 1996). Studies have shown that, in mature neurons, NR2A-containing receptors are enriched at synapses, while NR2B is largely localized at extrasynaptic sites (Steigerwald et al., 2000; Groc et al., 2006; Martel et al., 2009). However, synaptic NR2B-containing receptors and extrasynaptic NR2A-containing receptors have also been observed (Tovar and Westbrook, 1999; Thomas et al., 2006). Using subtype-specific antagonists to selectively block NR1/NR2A or NR1/NR2B diheteromeric NMDARs, it has been proposed that NR2A- and NR2B-containing NMDARs promote neuronal survival and death, respectively (Liu et al., 2007). However, there has been much debate on the selectivity of NR2A-specific antagonists (Neyton and Paoletti, 2006). Further complexity comes from the existence of triheteromeric (NR1/NR2A/NR2B) NMDARs, as there is no effective antagonist available. New drugs that selectively block NMDAR subtypes would be critical in defining roles of NR2A and NR2B in cell survival and death.
The extensive intracellular C-terminal domains of NMDARs interact with a network of cytosolic regulatory proteins, which couple receptors to various intracellular signaling pathways. For example, activation of NR2A-containing NMDARs has been linked to survival signaling through anti-apoptotic effects of phosphatidyl inositol 3-kinase (PI3K) dependent pathway (Lee et al., 2002). In contrast, disrupting the interaction of NR2B-containing NMDARs with PSD-95 has been shown to interrupt downstream signaling that leads to neuronal death (Aarts et al., 2002). Consistently, NMDA-induced apoptosis was significantly reduced in mouse cortical neurons cultured from NR2B, but not NR2A, homozygous knockout embryos (Liu et al., 2007). Conceivably, differential roles of NR2A and NR2B in neuronal survival are likely due to their diversified C-terminal domains, which allow distinct signal transductions triggered by calcium influx.
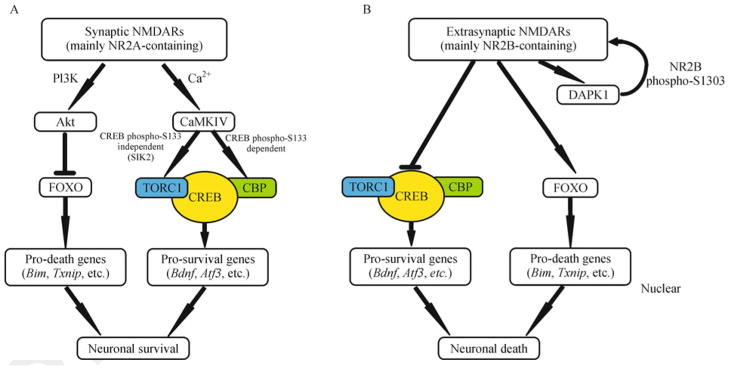
Several signaling pathways are involved in promoting neuronal survival or death. Perhaps the best understood example is the Ca2+/calmodulin-dependent protein (CaM) kinase—cAMP response element binding protein(CREB) signaling pathway. Calcium signals via synaptic NMDARs, mainly NR2A-containing receptors, activate the nuclear CaMK IV and increases phosphorylation of the transcription factor CREB on its crucial regulatory residue serine 133 (Ser133) (Sasaki et al., 2011; Hardingham et al., 2002) (Fig. 1). This phosphorylation of CREB then recruits the CREB coactivator CREB binding protein (CBP) to stabilize the preinitiation complex and increase CRE promoter activity (Mayr and Montminy, 2001). CREB, which is thought to be involved in long-term memory formation, also has a critical role in promoting neuronal survival. Activation of CREB induces the expression of brain-derived neurotrophic factor (BDNF) and potentially other activity-regulated inhibitors of death genes, which protects neurons from NMDAR blockade-induced neuronal death (Hardingham et al., 2002). Interestingly, CREB can be also activated through transducer of regulated CREB activity (TORC), independent of Ser133 phosphorylation (Fig. 1A). Recent studies revealed that synaptic NMDAR activity triggers phosphorylation of salt-inducible kinase 2 (SIK2) by CaMK I/IV, which would then induce TORC1 phosphorylation and its nuclear translocation (Sasaki et al., 2011). In addition, synaptic NMDAR activity initiates neuroprotective signals by regulating the forkhead box protein O (FOXO) class of transcription factors. FOXO regulates many cellular processes including proliferation, differentiation and apoptosis. It has been shown that activation of FOXO contributes to oxidative stress-induced cell death in cerebellar granule neurons (Lehtinen et al, 2006). Synaptic NMDAR activity suppresses FOXO activity, not only by triggering the nuclear export of FOXO via activation of PI3K-Akt pathway (Brunet et al., 1999; Dick and Bading, 2010), but by reducing the expression of FOXO1 (Al-Mubarak et al., 2009) (Fig. 1A). Suppression of FOXO activity decreases the expression level of pro-death genes, such as Fas ligand, Bcl2-interacting mediator of cell death (Bim) and thioredoxin-interacting protein (Txnip) (Papadia et al., 2008; Al-Mubarak et al., 2009). Taken together, both CREB-activation and FOXO-repression signals are essential in synaptic NMDAR-mediated neuronal protection.
Figure 1.
Distinct signaling pathways of synaptic and extrasynaptic NMDAR in neuron fate. (A) Synaptic NMDAR activity regulates gene expression to promote neuronal survivial. Synaptic NMDAR activity down-regulates the expression of pro-death genes by inhibiting forkhead box protein O (FOXO) activity though PI3K-Akt pathway. Synaptic current can also activate CaMKIV and upregulate a battery of pro-survival genes by activation of cAMP response element binding protein (CREB). (B) Extrasynaptic NMDAR signal has opposing effect on gene expression to induce neuronal death. Extrasynaptic NMDAR activity blocks CREB-dependent pro-survival gene expression. FOXOs are also targets of extrasynaptic NMDAR activity to activate expression of pro-death genes.
In contrast to sustained CREB phosphorylation by synaptic NMDAR activity, CREB is only transiently phosphorylated when extrasynaptic NMDARs are activated. Subsequent studies have shown that transient phosphorylation of CREB may be resulted from a dominant dephosphorylation of CREB by protein phosphatase 1 (PP1) (Hardingham et al., 2002). Ifenprodil, which is a selective antagonist of NR2B-containing NMDARs, can block the rapid decay of CREB phospharylation on Ser133 triggered by activation of extra-synaptic NMD ARs. The decrease of CREB phosphorylation on Ser133 correlates with the inhibition of CREB function and CREB-target gene expression, such as BDNF. These results suggest that NR1/NR2B is the core NMDARs existing at extrasynaptic sites to shut off the CREB-mediated protective signals induced by synaptic NMDAR activation (Hardingham et al., 2002) (Fig. 1B). In addition, in contrast to synaptic NMDAR activity, extra-synaptic NMDAR activity promotes the nuclear import of FOXO transcription factors and subsequent activation of FOXO target genes (Dick and Bading, 2010), which contributes to neuronal death by the excitotoxic insult (Fig. 1B). Death-associated protein kinase 1 (DAPK1) adds another layer of regulation to the death signaling pathway (Fig. 1B). It has been demonstrated that DAPK1 physically and functionally binds to the NR2B subunit at extrasynaptic sites to trigger the ischemic brain damage (Tu et al., 2010). DAPK1 phosphorylates NR2B at serine 1303, which enhances the channel conductance of NR1/NR2B receptor and causes increased Ca2+ influx through NMDAR channels at extrasynaptic sites.
Additional NMDAR-dependent death and survival signaling, exemplified by the extracellular signal-regulated kinases-1/2 (ERK1/2) pathway and the neuronal nitric oxide synthase (nNOs) pathway, are discussed here. ERK1/2 signaling pathway plays an important role in NMDAR-dependent neuronal survival. ERK1/2 is activated by Ca2+ influx through NMDARs. Extrasynaptic NMDAR activity inactivates ERK signaling pathway, while activation of synaptic NMDARs induces sustained ERK activation (Chandler et al., 2001; Ivanov et al., 2006). Consistent with being more abundant at extrasynaptic sites, NR2B has been shown to be selectively associated with synaptic Ras GTPase activating protein (SynGAP) (Kim et al., 2005), which represses ERK signaling. Moreover, nNOS, an enzyme that catalyzes the production of highly neurotoxic molecule nitric oxide (NO), is involved in extrasynaptic NMDAR-mediated neuronal death. nNOS is coupled to NMDARs through its interaction with PSD-95 (Sattler et al., 1999), upon which the nNOS activation by NMDAR-mediated calcium influx is also dependent. Studies have shown that disruption of the NR2B—PSD-95—nNOS signaling complex inhibits NMDAR-mediated NO release and prevents neuronal death (Aarts et al., 2002; Cui et al, 2007; Zhou et al., 2010).
NR2C
NR2C is mostly expressed in cerebellar granule cells, indicating its unique role in cerebellum (Wenzel et al., 1997). Indeed, genetic studies have shown that mice lacking NR2C display an impairment of motor coordination, the central function of cerebellum (Sprengel et al., 1998). In the cerebellum, NR2B-containing NMDARs are predominant early in development, while NR2A- and NR2C-containing receptors prevail at later stages. NR2C is also found in the olfactory bulb and thalamus (Wenzel et al., 1997). Using knock-in mice expressing the β-galactosidase reporter under control of the NR2C promoter, recent studies have shown that NR2C is expressed in retrosplenial cortex, pontine and vestibular nuclei (Karavanova et al., 2007). Unexpectedly, NR2C is also detected in a subset of glial cells, which appears negative for NR1 immunoreactivity (Karavanova et al., 2007), suggesting that NR2C-containing glial cells do not form functional NMDARs.
NR2C confers unique properties on NMDARs producing channels with low conductance openings and exhibiting specific channel kinetics (Stern et al., 1992; Farrant et al., 1994). This is true for both recombinant receptors expressed in heterologous cells and for native NMDARs in cerebellum. The disruption of the NR2C gene eliminates the low conductance channels, indicating that NR2C expression is responsible for the developmental expression of low conductance NMDAR channels (Ebralidze et al., 1996). Whole-cell responses from NR1/NR2C-containing receptors display deactivation kinetics with decay time constants of about 250 ms, which is similar to that of NR2B-containing receptors, but is slow compared with NR2A-containing receptors (Cull-Candy and Leszkiewicz, 2004). The decay time constant of NMDAR-mediated EPSC is markedly reduced in NR2C null mice, reflecting a change in the composition of NMDARs (Ebralidze et al., 1996). Interestingly, the peak amplitudes of NMDAR-EPSC are significantly increased in NR2C null mice, suggesting that NR2C-containing NMDARs have low open probability (Ebralidze et al., 1996). Consistently, single channel analysis reveals that NR1/NR2C receptors have a low open probability (0.011), which is approximately 44-fold and 10-fold less than the peak open probability of NR1/NR2A and NR1/NR2B receptors, respectively (Dravid et al., 2008). In addition, NR2C-containing NMDARs require only modest depolarization to overcome Mg2+ blockade. The voltage-dependent Mg2+ block of the NMDAR is believed to perform a crucial function in synaptic plasticity. This low sensitivity to Mg2+ implies that NMDARs with NR2C can operate at a more negative membrane potential (Cull-Candy et al., 2001).
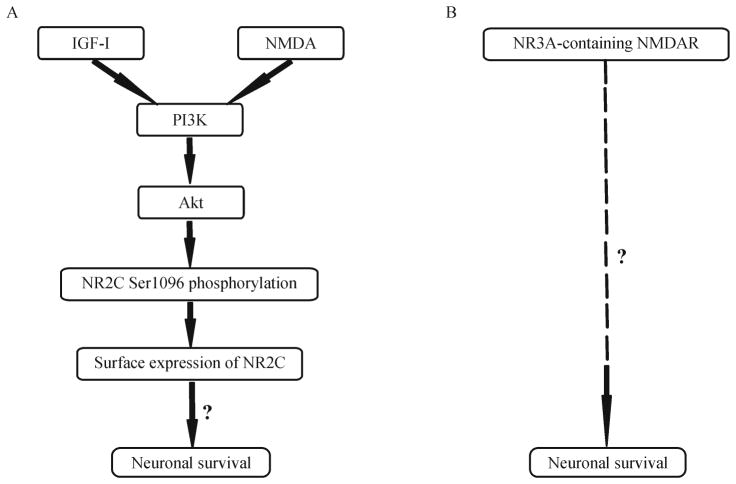
In addition to its dominant role in cerebellar granule cells, several studies have suggested that NR2C may be critical in other regions of the brain. For instance, studies have shown that oligodendrocytes are damaged during ischemia mediated in part by NMDARs. Among NR2 subunits, NR2C is highly expressed on the processes of oligodendrocytes, which are responsible for myelination (Káradóttir et al., 2005; Salter and Fern, 2005; Micu et al., 2006. In addition, expression of NR2C is significantly increased in rat hippocampus following in vitro oxygen-glucose deprivation (an in vitro ischemia model) although NR2C is not normally detected in hippocampus (Small et al., 1997). Consistently, NR2C-immunoreactive positive cells have been observed in rat hippocampus after prolonged neonatal seizures (Ni et al., 2005). Moreover, gene disruption of NR2C in mice protects the brain from ischemic damage resulting from permanent middle cerebral artery occlusion (Kadotani et al., 1998). In contrast, recent studies have suggested a role for NR2C in neuronal survival (Chen and Roche, 2009). Growth factor stimulation and NMDAR activation lead to a robust increase in both PKB phosphorylation of NR2C and surface expression of cerebellar NMDARs (Fig. 2A). Surface expression of NR2C, unlike NR2A and NR2B, protects neurons from NMDA-induced excitotoxicity. Taken together, NR2C may contribute to neuronal death or survival after ischemic damage.
Figure 2.
NR2C and NR3A promote neuronal survival. (A) IGF-1 or NMDA application to neurons phosphorylates serine 1096 of NR2C though PI3K-Akt pathway, which increases the surface expression of NR2C-containing NMDARs and promotes neuronal survival. (B) Overexpression of NR3A-containing NMDARs supports neuronal survival with an unknown mechanism.
NR3A and NR3B
NR3A and NR3B subunits are widely distributed, including cortex, cerebellum, hippocampus in the brain, spinal cord, as well as in glia (Ciabarra et al., 1995; Sucher et al., 1995; Cavara and Hollmann, 2008; Low and Wee, 2010). NR3A is also enriched in the central auditory system. Expression of NR3 subunits is developmentally regulated (Ciabarra et al., 1995; Sucher et al., 1995; Cavara and Hollmann, 2008; Low and Wee, 2010). NR3A expression is largely restricted to a narrow window of postnatal one to two weeks, during which synaptic circuitry is established. Perez-Otano et al. reported that NR3A-containing NMDARs undergo an activity dependent endocytosis during development, implying displacement of NR3 subunit by NR2 at synapses in mature neurons (Pérez-Otaño et al., 2006). Most recently, presynaptic NR3A-containing NMDARs have been found in visual cortical synapses (Larsen et al., 2011). The switch between NR3A and NR2 at presynaptic site regulates glutamate release and spike timing-dependent long term depressiom (LTD) in visual cortex. While level of NR3A is minimal in adolescent, NR3B expression gradually increases and sustains into adult. However, the ultrastructural localization of NR3B within the neuron is not clear.
NR3 subunits bind glycine selectively over glutamate. Compared to the NR1 subunit, NR3 subunits have higher affinity for glycine. Their affinities for other agonists and antagonists are different as well. This is potentially due to differences in hydrogen bonding networks in the ligand binding domains of NR1 and NR3 subunits (Yao et al., 2008). NR3-containing NMDARs exhibit distinct channel properties including reduced current responses, lowered sensitivity to Mg2+, and decreased Ca2+ influx (Das et al., 1998; Nishi et al., 2001; Sasaki et al., 2002). In comparison with NR1/ NR2A, NR1/NR2A/NR3 exhibits a roughly 5-fold reduction in relative Ca2+ permeability (Cull-Candy et al., 2001). In ionotropic glutamate receptors, two flanking residues designated as N and N + 1 positions in the channel pore forming re-entrant loop are critical for Mg2+ sensitivity and Ca2+ permeability. The asparagine and arginine residues occupying these two positions in NR1 and NR2 subunits determine the high Ca2+ permeability property of conventional NMDARs. In NR3 subunits, the amino acid at the N position is changed to glycine, which conceivably attributes to distinct channel property of NR3 (Cavara et al., 2010).
NR3-containing NMDARs in the form of NR1/NR3 or NR1/NR2/NR3 have been reported using the X. laevis oocyte or mammalian cell line heterologous expression systems (Das et al., 1998; Sasaki et al., 2002; Ulbrich and Isacoff, 2008). It is generally considered that NR3A or NR3B interact with NR1 and NR2 subunits to modulate NMDAR activity. Interestingly, the NR1/NR3 composition displays specific glycine evoked currents, indicating the formation of functional channel (Chatterton et al., 2002). A recent study reported the presence of a glycine sensitive and glutamate antagonist insensitive NMDAR in optic nerve myelin, suggesting the existence of a NR1/NR3 NMDAR subtype in vivo (Piña-Crespo et al., 2010). However, whether glycine-gated excitatory channels exist in neuron is not clear.
Functional studies revealed that neurons in NR3A transgenic mice exhibit elevated currents and Ca2+ permeability in response to glutamate and glycine, while NR3A knockout neurons or mice exhibit opposite trend for channel conductance and Ca2+ influx (Ciabarra et al., 1995; Sucher et al., 1995; Tong et al., 2007). This ‘dominant-negative’ effect of NR3 on NMDAR activation is consistent with its distinct channel properties, implying a role of NR3 in neuroprotection upon glutamate-mediated Ca2+ neurotoxicity. Indeed, a recent study reported that cortical neurons with transgenically expressed NR3A exhibited decreased cell death in response to NMDA (Nakanishi et al., 2009) (Fig. 2B). In addition, overexpression of NR3A in a focal cerebral ischemia mice model displaced less damage than wild type mice (Nakanishi et al., 2009). However, it is not known whether the effect is due to direct incorporation of NR3A in synaptic or extrasynaptic of NMDAR.
Prevention of ischemic injury by blocking NMDAR activation induced Ca2+ influx has also been found in CNS myelin. Elevated level of Ca2+ presumably induces degradation of key enzymes or structural proteins in myelin. Micu et al. reported that Ca2+ increase was greatly reduced in a chemical induced ischemia model when a broad-spectrum of NMDAR antagonists MK-801, 7-chlorokynurenic acid and d-AP5 was applied (Micu et al., 2006). Myelin was consequently protected from ischemic injury. Interestingly, reduction of Ca2+ was not observed when NR2A and NR2B specific antagonists were applied. These results and the recent finding of NR1/NR3 NMDAR in myelin (Piña-Crespo et al., 2010) suggest that NR3 as well as NR2C and NR2D might play major roles in neuroprotection in CNS white matter such as myelin.
Conclusions
The various compositions of NMDAR subunits provide an important source of diversity for functional regulation of NMDARs. The NR2 subunits each have unique channel properties and are linked to distinct downstream signal transduction pathways, implying different roles of each subunit in neuronal survival. In this review, we have discussed recent advances in the characterization of NR2 and NR3 subunits in neuronal protection. However, several intriguing questions remain. As many of evidence supports the hypothesis that the location of NMDAR directs signaling toward neuronal death or survival, our understanding will benefit from new information on the subcellular distributions of NMDARs with different subunit compositions. Because NMDARs are involved in multiple neuronal functions, directly blocking all NMDARs can cause adverse side effects. Therefore, it is critical to establish new selective antagonists to distinguish different subtypes of NMDARs or to specifically inhibit downstream signaling that cause excitotoxic neuronal death. Given that NMDARs mediate the Ca2+ influx in synaptic plasticity and glutamate toxicity, the spatial and temporal regulation of the composition of NMDARs clearly has profound implications in regulating neuronal activity and survival.
Acknowledgments
This work was supported by a NINDS Career Transition Award (to B.-S. C.). We apologize to authors whose work could not be cited due to space constraints.
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298(5594):846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Al-Mubarak B, Soriano FX, Hardingham GE. Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene. Channels (Austin) 2009;3(4):233–238. doi: 10.4161/chan.3.4.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347(1):150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Cavara NA, Hollmann M. Shuffling the deck anew: how NR3 tweaks NMDA receptor function. Mol Neurobiol. 2008;38(1):16–26. doi: 10.1007/s12035-008-8029-9. [DOI] [PubMed] [Google Scholar]
- Cavara NA, Orth A, Hicking G, Seebohm G, Hollmann M. Residues at the tip of the pore loop of NR3B-containing NMDA receptors determine Ca2+ permeability and Mg2+ block. BMC Neurosci. 2010;11(1):133. doi: 10.1186/1471-2202-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J Biol Chem. 2001;276(4):2627–2636. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415(6873):793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53(3):362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Growth factor-dependent trafficking of cerebellar NMDA receptors via protein kinase B/Akt phosphorylation of NR2C. Neuron. 2009;62(4):471–478. doi: 10.1016/j.neuron.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Koh JY, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8(1):185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J Neurosci. 1995;15(10):6498–6508. doi: 10.1523/JNEUROSCI.15-10-06498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani E, Rizzi S, Paulsen RE, Contestabile A. Chronic pre-explant blockade of the NMDA receptor affects survival of cerebellar granule cells explanted in vitro. Brain Res Dev Brain Res. 1997;99(1):112–117. doi: 10.1016/s0165-3806(96)00187-3. [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JTR, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5(12):952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Cui H, Hayashi A, Sun HS, Belmares MP, Cobey C, Phan T, Schweizer J, Salter MW, Wang YT, Tasker RA, Garman D, Rabinowitz J, Lu PS, Tymianski M. PDZ protein interactions underlying NMDA receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. J Neurosci. 2007;27(37):9901–9915. doi: 10.1523/JNEUROSCI.1464-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11(3):327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;(255):re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393 (6683):377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- Dick O, Bading H. Synaptic activity and nuclear calcium signaling protect hippocampal neurons from death signal-associated nuclear translocation of FoxO3a induced by extrasynaptic N-methyl-D-aspartate receptors. J Biol Chem. 2010;285(25):19354–19361. doi: 10.1074/jbc.M110.127654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J Physiol. 2008;586(18):4425–4439. doi: 10.1113/jphysiol.2008.158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebralidze AK, Rossi DJ, Tonegawa S, Slater NT. Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene. J Neurosci. 1996;16(16):5014–5025. doi: 10.1523/JNEUROSCI.16-16-05014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Köhr G, Seeburg PH, Monyer H. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 2007;53(1):10–17. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994;368 (6469):335–339. doi: 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci USA. 2006;103(49):18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5(5):405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572(Pt 3):789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadotani H, Namura S, Katsuura G, Terashima T, Kikuchi H. Attenuation of focal cerebral infarct in mice lacking NMDA receptor subunit NR2C. Neuroreport. 1998;9(3):471–475. doi: 10.1097/00001756-199802160-00021. [DOI] [PubMed] [Google Scholar]
- Karavanova I, Vasudevan K, Cheng J, Buonanno A. Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C subunit-beta-galactosidase knock-in mice. Mol Cell Neurosci. 2007;34(3):468–480. doi: 10.1016/j.mcn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438(7071):1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46(5):745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16(2):333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Larsen RS, Corlew RJ, Henson MA, Roberts AC, Mishina M, Watanabe M, Lipton SA, Nakanishi N, Pérez-Otaño I, Weinberg RJ, Philpot BD. NR3A-containing NMDARs promote neuro-transmitter release and spike timing-dependent plasticity. Nat Neurosci. 2011;14(3):338–344. doi: 10.1038/nn.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJS, Xue S, Pei L, Vukusic B, Chéry N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111(2):219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EBE, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125(5):987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27(11):2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low CM, Wee KSL. New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: localization, structure, and function. Mol Pharmacol. 2010;78(1):1–11. doi: 10.1124/mol.110.064006. [DOI] [PubMed] [Google Scholar]
- Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58(2):193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- Martel MA, Wyllie DJ, Hardingham GE. In developing hippocampal neurons, NR2B-containing N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience. 2009;158(1):334–343. doi: 10.1016/j.neuroscience.2008.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2 (8):599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439(7079):988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Tu S, Shin Y, Cui J, Kurokawa T, Zhang D, Chen HSV, Tong G, Lipton SA. Neuroprotection by the NR3A subunit of the NMDA receptor. J Neurosci. 2009;29(16):5260–5265. doi: 10.1523/JNEUROSCI.1067-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5(5):361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26(5):1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Jiang YW, Bo T, Wang JM, Wu XR. c-Fos, N-methyl-d-aspartate receptor 2C, GABA-A-alpha1 immonoreactivity, seizure latency and neuronal injury following single or recurrent neonatal seizures in hippocampus of Wistar rat. Neurosci Lett. 2005;380(1–2):149–154. doi: 10.1016/j.neulet.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16(7):2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y. Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci. 2001;21(23):RC185. doi: 10.1523/JNEUROSCI.21-23-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science. 1969;166(3903):386–388. doi: 10.1126/science.166.3903.386. [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJA, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11(4):476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Otaño I, Luján R, Tavalin SJ, Plomann M, Modregger J, Liu XB, Jones EG, Heinemann SF, Lo DC, Ehlers MD. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci. 2006;9(5):611–621. doi: 10.1038/nn1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piña-Crespo JC, Talantova M, Micu I, States B, Chen HSV, Tu S, Nakanishi N, Tong G, Zhang D, Heinemann SF, Zamponi GW, Stys PK, Lipton SA. Excitatory glycine responses of CNS myelin mediated by NR1/NR3 “NMDA” receptor subunits. J Neurosci. 2010;30 (34):11501–11505. doi: 10.1523/JNEUROSCI.1593-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, Mishina M. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373(6510):151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438(7071):1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Takemori H, Yagita Y, Terasaki Y, Uebi T, Horike N, Takagi H, Susumu T, Teraoka H, Kusano KI, Hatano O, Oyama N, Sugiyama Y, Sakoda S, Kitagawa K. SIK2 is a key regulator for neuronal survival after ischemia via TORC1-CREB. Neuron. 2011;69 (1):106–119. doi: 10.1016/j.neuron.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Sasaki YF, Rothe T, Premkumar LS, Das S, Cui J, Talantova MV, Wong HK, Gong X, Chan SF, Zhang D, Nakanishi N, Sucher NJ, Lipton SA. Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J Neurophysiol. 2002;87(4):2052–2063. doi: 10.1152/jn.00531.2001. [DOI] [PubMed] [Google Scholar]
- Small DL, Poulter MO, Buchan AM, Morley P. Alteration in NMDA receptor subunit mRNA expression in vulnerable and resistant regions of in vitro ischemic rat hippocampal slices. Neurosci Lett. 1997;232(2):87–90. doi: 10.1016/s0304-3940(97)00592-2. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284(5421):1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, Kim JJ, Thompson RF, Sun W, Webster LC, Grant SG, Eilers J, Konnerth A, Li J, McNamara JO, Seeburg PH. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92(2):279–289. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Köhr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20(12):4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P, Béhé P, Schoepfer R, Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc Biol Sci. 1992;250(1329):271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci. 1995;15(10):6509–6520. doi: 10.1523/JNEUROSCI.15-10-06509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95(3):1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tong G, Takahashi H, Tu S, Shin Y, Talantova M, Zago W, Xia P, Nie Z, Goetz T, Zhang D, Lipton SA, Nakanishi N. Modulation of NMDA receptor properties and synaptic transmission by the NR3A subunit in mouse hippocampal and cerebrocortical neurons. J Neurophysiol. 2007;99(1):122–132. doi: 10.1152/jn.01044.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19(10):4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM, Balel C, Wang M, Jia N, Zhang W, Lew F, Chan SL, Chen Y, Lu Y. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140(2):222–234. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich MH, Isacoff EY. Rules of engagement for NMDA receptor subunits. Proc Natl Acad Sci USA. 2008;105(37):14163–14168. doi: 10.1073/pnas.0802075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68(2):469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Wee KS, Zhang Y, Khanna S, Low CM. Immunolocalization of NMDA receptor subunit NR3B in selected structures in the rat forebrain, cerebellum, and lumbar spinal cord. J Comp Neurol. 2008;509 (1):118–135. doi: 10.1002/cne.21747. [DOI] [PubMed] [Google Scholar]
- Yao Y, Harrison CB, Freddolino PL, Schulten K, Mayer ML. Molecular mechanism of ligand recognition by NR3 subtype glutamate receptors. EMBO J. 2008;27(15):2158–2170. doi: 10.1038/emboj.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li F, Xu HB, Luo CX, Wu HY, Zhu MM, Lu W, Ji X, Zhou QG, Zhu DY. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med. 2010;16 (12):1439–1443. doi: 10.1038/nm.2245. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci. 1995;18(7):306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]