Abstract
The inhibitory effects of Chinese leek(Allium tuberosum) on Fusarium oxysporum f. sp. cubense (Foc) and on Fusarium wilt incidence were studied in order to identify a potential efficient way to control the disease. Adopting the rotation system of Chinese leek-banana reduced the Fusarium wilt incidence and disease severity index by 88 %-97 % and 91 %-96 %, respectively, improved the crop value by 36 %-86 %, in an area heavily infested by Foc between 2007 and 2009. As a result of inoculation in the greenhouse, Chinese leek treatment reduced disease incidence and the disease severity index by 58 % and 62 %, respectively in the variety Baxi (AAA) and by 79 % and 81 %, respectively in the variety Guangfen NO.1 (ABB). Crude extracts of Chinese leek completely inhibited the growth of Foc race 4 on Petri dishes, suppressed the proliferation of the spores by 91 % and caused 87 % spore mortality. The findings of this study suggest that Chinese leek has the potential to inhibit Foc growth and Fusarium wilt incidence. This potential may be developed into an environmentally friendly treatment to control Fusarium wilt of banana.
Keywords: Fusarium oxysporum, Panama disease, banana, Allium tuberosum, crop rotation, Biocontrol
Introduction
Fusarium wilt of banana (also called Panama disease), is caused by the soil-borne hyphomycete, Fusarium oxysporum f. sp. cubense (Foc). It is one of the most notorious banana diseases (Ploetz 2000;Stove 1962). Foc race 4 has seriously affected commercial plantations of Cavendish in Taiwan, Northern Territory of Australia, Indonesia, Malaysia, Canary and Madeira Islands, South Africa and Chinese mainland, making their banana export less competitive (Molina et al. 2008; Ploetz, 2006). Mainly due to this disease, the banana production area in Taiwan declined from 50,000 ha in the 1960s to 5,000 ha in the early 1970s (Hwang and Ko 2004). The disease destroyed the banana industries in the main producing areas in Chinese mainland, including Guangdong, Guangxi, Hainan, Fujian and Yunnan (Lian et al. 2009). Currently, the disease incidence in the delta of the Pearl River (a major banana production area in China), ranges between 20 to 40 %, with individual plantations reaching a rate of 90%.
Scientists around the world have investigated resistance breeding (Hwang and Ko 2004 ; Smith et al.2006), chemical control (Nel et al. 2007), biocontrol (Saravanan et al. 2003; Cao et al.2005; Lian et al.2009) and molecular methods (Paul et al. 2011; Yip et al. 2011),in search of methods to control the disease. Some of these studies were found to effectively suppress Foc growth in the laboratory and in greenhouses, but were unable to efficiently control Fusarium wilt in the field.
In China, in Panyu, Guangzhou, an area heavily infested by Foc bananas are rotated with 2-3 years of commercially cultivated Chinese leek (Allium tuberosum)to control Fusarium wilt . This rotation was first identified by chance in 1997 by a farmer whose bananas were heavily infected by Foc with the exception of a section of the plantation where Chinese leeks had been grown for a few years previously. Several farmers have tried this system with great success since.
The objectives of this study were (1): to verify if Fusarium wilt incidence in banana can be decreased by rotation with Chinese leek in the field, and (2) to provide a quantitative estimate of the potential of this method for the control of the Fusarium wilt based on greenhouse and laboratory studies.
Materials and Methods
Site
Panyu area (22°45′-23°05′ N, 113°14′-113°34′) is located in the south of Guangzhou, China. It is characterized by a subtropical marine monsoon climate with a mean annual precipitation of about 1900 mm and a mean annual temperature of 21.6°C. This area, once one of the main production areas of bananas in China, now is heavily infested by Foc, and many banana orchards have been planted with other crops due to the high incidence of Fusarium wilt.
Plant materials and Fungi
The banana cultivars used in the experiment were banana variety Baxi (Musa AAA Cavendish subgroup) and Guangfen No.1 (Musa ABB group) which are highly susceptible to Foc. They were provided by The Banana Tissue Culture Center, Institute of Fruit Tree Research, Guangdong Academy of Agriculture Science. Cupped plantlets of 30mm height were used in the greenhouse and field tests. Fresh Chinese leek (Allium tuberosum cv Xuejiu) was collected from commercial neighbouring fields. Foc race4 isolate CGMCCC 3.12196 was provided by the Key Laboratory of South Subtropical Fruit Biology and Genetic Resource Utilization, Ministry of Agriculture, China.
Field studies
The field trials were carried out with the cooperation of local farmers in Panyu area (Dagang town, Yuwotou town, Dongchong town) between 2007 and 2009. Fields where Chinese leek had just been continuously grown for 3 years were selected as the Chinese leek treatment to evaluate the inhibitory effect of Chinese leek on the incidence of Fusarium wilt. More than 4.5 ha of treated fields were involved in the trial in 2007, 12.8 ha in 2008 and 21.5 ha in 2009, respectively. Neighbouring fields where other vegetables (3 ha in 2007), sugarcane (23 ha in 2008) and rice (1 ha in 2009) had been grown were selected as control fields. All these selected fields were heavily infested by Foc and could not be used to plant banana because of high incidence of Fusarium wilt before cultivating Chinese leek or other crops. In late March and early April each year, after removing the Chinese leek and other crops, three month old 30 mm high Guangfen No.1 (ABB) plantlets were transplanted in the treated and the control fields with a density of 2.0 m × 2.7 m. All plants were managed in the same way and no artificial inoculation was performed. At harvest, the number of infected plants in each plot was recorded each year to evaluate the disease incidence. Per plot, 100 plants were randomly selected and the disease symptoms rated on each plant based on five classes from 0 to 4 modified after Mak (Mak et al.2004): 0 - No streaking or yellowing of leaves, plant appears healthy; 1 - Slight streaking and/or yellowing of lower leaves; 2 - Streaking and/or yellowing of most of the lower leaves; 3 - Extensive streaking and/or yellowing on most or all of the leaves; 4 - Dead plant. Based on the infection counts and the disease symptom assessments the disease incidence and a disease severity index were calculated as follows: Disease incidence (%) =(Number of infected plantlets / Total number of plantlets)×100. Disease severity index =[∑ (Class × Number of plants in that class) / (4 × Total number of assessed plants)] ×100. In addition, yield and crop value were assessed. All trials were replicated three times.
Greenhouse trial
A green house trial was conducted with two banana varieties with and without Chinese leek in peat soil inoculated with Foc , i.e. four treatments: (1) Baxi treatment: Baxi (AAA) +Chinese leek+ Foc; (2)Baxi control: Baxi (AAA) + Foc;(3) Guangfen No.1 treatment: Guangfen No.1 (ABB) +Chinese leek+ Foc;(4)Guangfen No.1 control: Guangfen No.1 (ABB) + Foc.
About 2.0 kg of aerial parts of fresh Chinese leek were cut into 2-5 cm long pieces, and mixed evenly with about 10 kg of peat soil. Soil with Chinese leek was called treated soil and soil without Chinese leek piece was regarded as untreated control. Treated and untreated soils were filled into 40-cm diameter and 50-cm deep pots, respectively, and one plantlet of Baxi (AAA) or Guangfen No.1 (ABB) planted per pot. Per treatment there were 20 pots and the experiment was replicated three times.
One week after planting, all plantlets were watered with a solution of spores of Foc race 4 to a total of 10,000 spores per g of soil. Leaf symptom assessments started ninety days after inoculation when the plantlets showed clear disease symptoms. Symptoms were recorded every 15 d until all the untreated control plantlets showed clear disease symptoms. Disease symptoms were assessed and incidence and severity index calculated as described above. To quantitatively measure the disease resistance, the Area under Disease Progress Curve (AUDPC) was calculated by the trapezoidal integration of the disease severity index from 90 to 180 days after inoculation as follows:
Where X is the disease severity index, n the number of evaluations, and the (ti−1-ti) the time interval (days) between two consecutive evaluations (Das et al.1992)
Preparation of crude extract of Chinese leek
The aerial parts of the Chinese leek were ground into powder with liquid Nitrogen. 1,000 ml of sterilized water were added into 500 g of powder to leach the active ingredients for 24 h. The crude extract was concentrated to 50 ml using a rotary evaporator, and sterilized using a 0.22μm filter.
In vitro studies on solid media
PDA medium was prepared and sterilized by autoclaving at 121° for 15min. Crude extract of Chinese leek (0, 0.5, 1.0, and 2.0 ml, respectively) were added to Petri dishes and gently mixed with 18 ml PDA medium of about 50°. The final volume was supplemented to 20 ml with sterilized water. Immediately after the PDA medium solidified 10 μl of activated Foc race 4 cultures were inoculated onto the middle of the Petri dishes and the dishes were incubated at 28° in the dark. Evaluation of the inhibitory effect of the crude extracts on the mycelium growth of Foc race 4 was assessed by measuring the diameter of the fungal colonies after seven days. Each treatment was replicated six times.
In vitro studies on liquid media
Various amounts (0, 1, 5, and 10 ml) of crude extract of Chinese leek were added to a 100 ml Erlenmeyer flask. Two ml of activated Foc race 4 (106 spores/ml) labeled with green fluorescent protein were added to each flask, and PDA liquid media was added to a total volume of 20ml. The Foc race 4 spores and the crude extract were co-cultured on a shaking table at 150 rpm at 28° for 16h in dark. The growth status of the spores was detected with a Zeiss LSM510 laser scanning confocal microscope (Carl Zeiss, German) under white and fluorescent light. The number of the spores was counted in five visual fields (Plan-Neofluar 40× /1.3 oil DIC objective), the means are reported as the spore numbers in each treatment. The experiment was replicated three times.
Statistical analysis
Data were analyzed using SAS version 8.0 software. All data were checked for homogeneity of variance using “Levene” method before be submitted to one-way ANOVA analysis. The significance of the treatments was determined using Fisher’s protected least significant difference (LSD) test (P≤0.05).
Results
Incidence of Fusarium wilt in the field studies
The banana plants rotated with Chinese leek were generally healthy and few plants showed disease symptoms. In contrast, a great number of plants rotated with other crops were heavily infected and showed obvious yellowing and wilt symptoms (Fig.1). Over all three years, Fusarium wilt incidence and the disease severity index were significantly reduced by adopting the Chinese leek-banana rotation system (P<0.0001) (Table 1). In 2007, 132 of 7360 Chinese leek treated plants were infected and showed typical disease symptoms, while 2325 out of 5390 control plants rotated with Chinese quince or luffa had obvious disease symptoms. In 2008, 653 out of 23214 treated plants and 12469 of 42250 control plants (rotated with sugarcane) showed typical symptoms and in 2009, 1659 out of 39430 treated plants and 540 out of the 1500 control plants (rotated with rice) were infected. In the whole, the disease incidence of the treated plants was reduced by 96.6 % in 2007, 92.4 % in 2008, and 88.4 % in 2009. Moreover, the disease severity indices of the treated plants were reduced by 96.2 %, 94.1%, and 90.9% in 2007-2009, respectively, compared to those of the control plants. The mean yields per ha and the average crop value differed greatly between treated and control fields (Table 1). In the same market price, crop value was increased by 36.2 %-86.0 %.
Figure 1.
Plantations of Guangfen No.1 (ABB) rotated with different crops in Panyu, Guangzhou, China in rotation with Chinese leek (a) rice (b), sugarcane (c) other vegetables (d). Symptoms in (b)-(d) are typical for Fusarium wilt.
Table 1.
The means and corresponding standard error of Fusarium wilt incidence , disease severity index, yield per hectare and crop value of Guangfen No.1(ABB) rotated with Chinese leek and other crops in Panyu, China between2007 and 2009.
| Year | Treatmenty | Disease incidence (%) |
Disease severity index |
yield (kg per ha)y |
Crop value (CNY per ha)z |
|---|---|---|---|---|---|
| 2007 | CL | 1.60±0.42 | 1.25±0.38 | 46125±200 | 276750±1205 |
| CK | 47.03±7.45 | 37.42±6.58 | 24825±3490 | 148955±20941 | |
| P value | <0.0037 | <0.0054 | <0.0037 | <0.0037 | |
| 2008 | CL | 2.15±0.78 | 1.25±0.25 | 45867±367 | 275203±2205 |
| CK | 28.18±2.62 | 21.58±1.69 | 33666±1225 | 201994±7355 | |
| P value | <0.0007 | <0.0003 | <0.0007 | <0.0007 | |
| 2009 | CL | 4.18±0.74 | 2.17±0.30 | 44916±347 | 269494±2086 |
| CK | 35.93±2.33 | 24.58±2.06 | 30033±1093 | 180197±6558 | |
| P value | <0.0002 | <0.0004 | <0.00022 | <0.0002 |
CL means Guangfen No.1 rotated with Chinese leek ( bananas plants grown in the fields where Chinese leek had been grown for three years), CK means bananas rotated with other crops, such as other vegetables(2007), sugarcane(2008) and rice(2009).
Crop values based on market prices of 6.00 CNY(Chinese Yuan) per kg for Guangfen No.1 (ABB).
Greenhouse inoculation experiment
Effects of Chinese leek on Fusarium wilt incidence in potted Baxi (AAA)
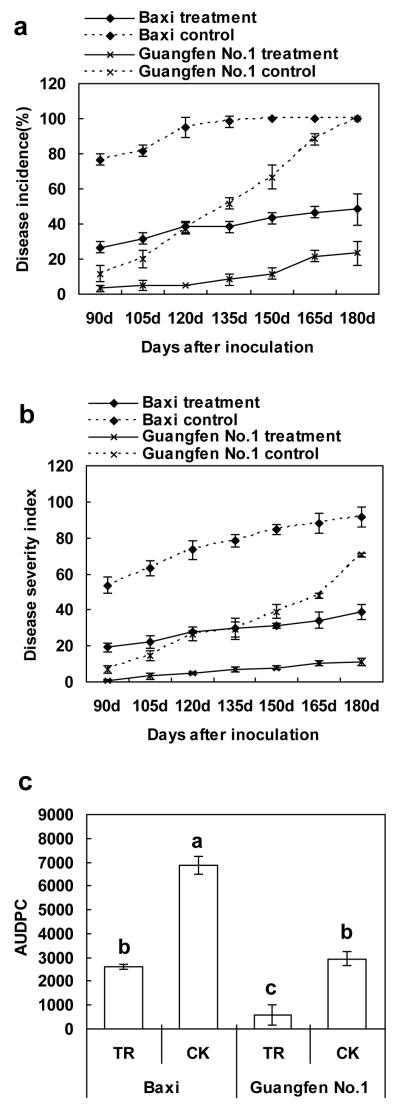
Ninety days after inoculation, the treated plantlets and the untreated controls plantlets clearly differed in their disease symptoms (Fig.2 a, b). Fusarium wilt of the treated plantlets was significantly reduced by 65.2% compared to that of the untreated control plantlets (Fig 3). One hundred and fifty days after the artificial inoculation, 100% of the untreated control plantlets were infected and showed clear disease symptoms, but only 43.3% of the treated plantlets were infected. Disease severity index was 31.25 for the treated plantlets and 84.58 for the untreated control plantlets. Chinese leek reduced the disease severity index by 63.05%. Throughout the experiment, a significant difference existed between the treated plantlets and the untreated control plantlets on the disease incidence (P<0.0001), and the disease severity index (P<0.0001). Disease incidence and the disease severity index of the untreated control plantlets were higher than those of the treated plantlets by 58.06% and 61.82%, respectively (Fig 3). During the whole experiment, the AUDPC of the untreated control plantlets was 2.6 times higher than that of the treated plantlets (P< 0.0001) (Fig.3).
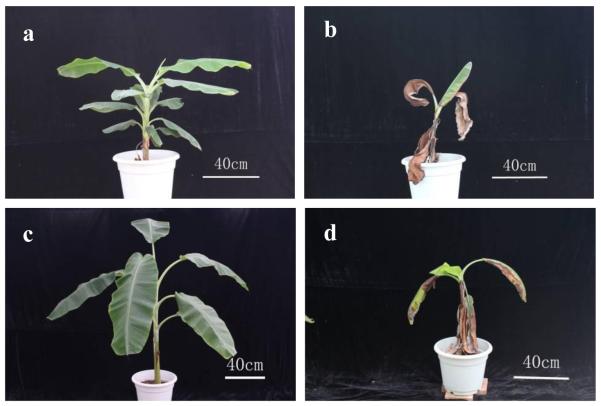
Figure 2.
Potted bananas 180 days after inoculation with Foc race4 in the greenhouse. Banana varieties Baxi (AAA) (a) and Guangfen NO.1 (ABB) (c) plantlets treated with Chinese leek developed in good health. The untreated control plantlets of Baxi (AAA) (b) and Guangfen NO.1 (ABB) (d) showed clear disease symptom.
Figure 3.
Inhibitory effects of Chinese leek on Fusarium wilt incidence (a), disease severity index (b), and AUDPC(c) in potted banana plantlets inoculated with Foc race4. AUDPC was calculated from 90 to 180 days after inoculation(TR: Treatment, CK: Control). Values are the means of three replications with 20 plants in each treatment and vertical bars indicate the standard errors of the means.
Effects of Chinese leek on the Fusarium wilt incidence in potted Guangfen No.1 (ABB) in greenhouse
Guangfen No.1 (ABB) showed disease symptoms later than Baxi (AAA). Ninety days after inoculation (Fig. 2 c,d). Fusarium wilt incidence of treated Guangfen No.1 (ABB) plantlets was significantly reduced by 71.4 % compared with untreated control plantlets (Fig.3). One hundred and eighty days after artificial inoculation, 100% of the untreated control plantlets were infected and showed clear disease symptoms, but only 23.3% of the treated plantlets were infected. Final disease severity index was 10.83 for the treated plantlets and 70.42 for untreated control plantlets, a reduction of 84.6 %. Throughout the experiment, significant difference existed for the disease incidence (P=0.0067) and the disease severity index (P=0.0062) when comparing the treated and the untreated control plantlets. Disease incidence and disease severity index of the untreated control plantlets were higher than those of the treated plantlets by 79.15% and 80.67%, respectively (Fig.3). During the whole experiment, the AUDPC of the untreated control plantlets was 5.0 times higher than that of the treated plantlets (P<0.0001) (Fig.3).
In vitro studies
In solid media the crude extract of Chinese leek at 0.5, 1.0, and 2.0 ml per Petri dish significantly inhibited the mycelial growth of Foc race 4 by 12.1 %, 43.5 %, and 100 %, respectively (Table 2).
Table 2.
The means and corresponding standard error of colony sizes of Foc race 4 on petri dish containing various quantities of the crude extracts of Chinese leek on 7 days after inoculation
| Treatment | Fungi clone size(cm)z |
|---|---|
| 0 uL(Control) | 6.55±0.14a |
| 500uL | 5.75±0.19b |
| 1000uL | 3.70±0.18c |
| 2000uL | 0.00±0.00d |
| P Value | <0.0001 |
The data were the means of six replications. Mean values in the same column followed by the different letter are significantly different by Fisher’s protected least significant difference test (P<0.05).
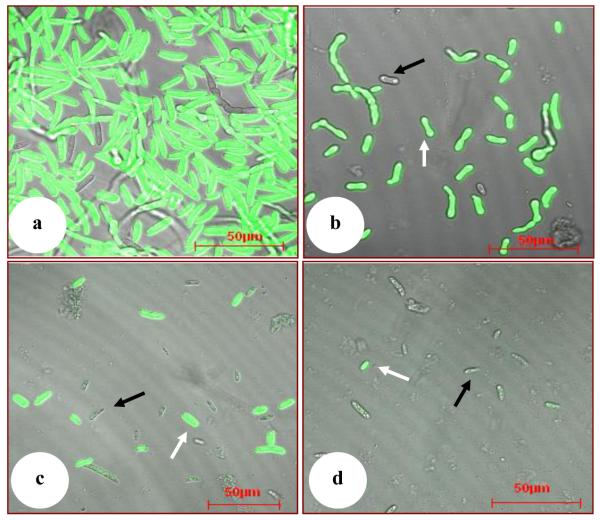
The crude extract of Chinese leek greatly suppressed the proliferation of Foc race 4 (P<0.0001) (Table 3. Fig. 4). Compared to the number of spores in the control under the white light, 1, 5, and 10 ml of Chinese leek crude extract suppressed the proliferation of the fungus by 79.2 %, 84.7 %, and 91.2 %, respectively. Comparing the number of spores under white light and the number of the spores under fluorescent light of the corresponding treatment shows that the crude extract of Chinese leek resulted in mortalities of Foc race 4 of 8, 53, and 87 % at 1, 5, and 10 ml Chinese leek crude extract per flask (Table 3). Differences were statistically significant when 5 ml or 10 ml of the crude extract of Chinese leek were added (P=0.0135 and P=0. 0009, respectively, Table 3).
Table 3.
The means and corresponding standard error of the number of Foc race 4 spores labeled with green fluorescence protein detected with a laser scanning confocal microscope at 16h after co-culture with various quantities of crude extract of Chinese leek on a shaking table.
| Treatment | The number of spores under white lightz |
The number of spores under fluorescent lightz |
|---|---|---|
| 0ml(Control) | 142.7±6.69 a | 142.7±6.69a |
| 1ml | 29.3±2.19 b | 27.0±2.08b |
| 5ml | 21.7±2.03bc | 10.3±1.76c |
| 10ml | 12.7±1.20c | 1.7±0.33d |
| P Value | P<0.0001 | P<0.0001 |
Values in the same column followed by the different letter are significantly different by Fisher’s protected least significant difference test (P<0.05).
Figure 4.
Effect of crude extract of Chinese leek on the proliferation and fluorescence of spores of Foc race 4. Two ml of spores (106 spores/ml) of Foc race 4 labelled with green fluorescent protein were co-cultured with 0 ml (a), 1 ml (b), 5 ml (c), and 10 ml (d) of crude extract of Chinese leek leaves per 20 ml culture on a shaking table for 16 h, and then were detected with a Zeiss LSM510 laser scanning confocal microscope (Carl Zeiss, German) using a Plan-Neofluar 40× /1.3 oil DIC objective. Black arrows indicate dead spores without fluorescence reaction, white arrows indicate the live spores with fluorescence reaction.
Discussion
Overall, disease reductions in the field experiments were around 90 % and yield increases between 36 and 86 %. The field experiments were in line with farmer’s experiences with the positive effects of Chinese leek in the rotation on Fusarium wilt.
Adoption of the rotation system has greatly reduced the Fusarium wilt incidence and has brought huge economic benefit to the farmers, which attracted more farmers to look for this kind of fields to plant bananas. So the rents of the fields where Chinese leek has been grown for years rose from about 20,000 to about 40,000 CNY (Chinese Yuan) ha−1 in recent years. Due to the shortage of this kind of Chinese leek field in the local area, some experienced farmers have moved to adjacent areas growing Chinese leek. As a result, the Chinese leek-banana rotation system has extended to other areas.
Our current field trials and the local farmers’ experiences for more than ten years since 1997 show that the adoption of a Chinese leek-banana rotation system can be an efficient way to control Fusarium wilt in areas heavily infested with the pathogen. At present, in the Panyu area the following planting mode is very popular: a vegetable farmer rents fields and continuously cultivates Chinese leek for 3 years. Then a banana farmer rents the fields for another 3 years to plant Guangfen No.1 (ABB) for one crop, followed by another crop of Baxi (AAA). Afterwards the fields are transferred to a vegetable farmer again. In this way, Fusarium wilt is kept under control and, more importantly, the primary main banana cultivars can be produced again in this heavily Foc infected area. Thus, Chinese leeks have saved the banana production of the area.
In the laboratory, Chinese leek was highly effective in suppressing the growth and proliferation of Foc with a strong lethal effect. Growing Chinese leek in fields heavily infested by Foc apparently gradually reduces the population of Foc, to a low enough level to allow for successful banana production again. It is well known that some plant crude extracts possess antifungal activity (Ameziane et al. 2007; Bluma et al. 2008; Chaijuckam et al. 2010; Harish et al. 2008; Nguyen et al. 2009). Caraway and peppermint crude extracts cause complete growth inhibition of Sclerotium rolfsii (El-Mougy et al. 2009). Leaf crude extracts of Abrus precatorius and Aegle marmelos strongly inhibit Colletotrichum capsici and Alternaria alternata (Anand et al. 2009). Salvia officinalis crude extract controls grapevine downy mildew (Dagostin et al. 2010).
Antibacterial and antifungal effects of crude extracts of Allium plants have been studied extensively in food science (Najjaa et al.2007 , Mau et al. 2001, Lazarevic et al.2011, Yin et al. 1999) and medicine (Chen et al, 2011 , Seyfi. et al , 2010, Lee et al, 2004). There are also some reports on the effects on plant diseases. The crude extract of Green onion (Allium fistulosum), Garlic (Allium sativum) and Chinese leek (Allium tuberosum) inhibited the germination incidence of Alternaria brassicicola by 100% (Ho et al, 2007). Garlic (Allium sativum) crude extracts reduced disease incidence caused by Pseudomonas syringae pv.tomato (Pst) by 58%, and disease severity by 68 %( Balestra et al, 2009). A novel antifungal compound, fistulosim, isolated from Welsh onion (Allium fistulosum L.) showed high activity against Fusarium oxysporum (Phay et al, 1999. Our study proves again that Allium plants have antifungal effects.
Chinese leek is a common vegetable imposing no harm on humans and on the environment. So we believe that the use of Chinese leek to control Fusarium wilt has the potential to be an efficient, environmentally friendly way of disease management. We encourage the study and the application of this information to banana growers in various areas.
Acknowledgments
The research was supported by a grant from the National Science and Technical Support Project of P.R. China (2008BAD92B06) and the National “948” Project of P.R. China (2008-G1 ). We thank participating farmers for their contributions to this project, and Dr Altus Viljoen from University of Stellenbosch ,South Africa and Dr Uri Lavi from ARO-Volcani Center, Israel for suggesting advice for writing the paper.
References
- Ameziane N, Boubaker H, Boudyach H, Msanda F, Jilal A, Benaoumar AA. Antifungal activity of Moroccan plants against citrus fruit pathogens. Agronomy for Sustainable Development. 2007;27:273–277. [Google Scholar]
- Anand T, Bhaskaran R. Exploitation of plant products and bioagents for ecofriendly management of chili fruit rot disease. Journal of Plant Protection Research. 2009;49:195–203. [Google Scholar]
- Balestra GM, Heydari A, Ceccarelli D, Ovidi E, Quattrucci A. Antibacterial effect of Allium sativum and Ficus carica extracts on tomato bacterial pathogens. Crop Protection. 2009;28:807–811. [Google Scholar]
- Bluma R, Amaiden MR, Daghero J, Etcheverry M. Control of Aspergillus section Flavi growth and aflatoxin accumulation by plant essential oils. Journal of Applied Microbiology. 2008;105:203–214. doi: 10.1111/j.1365-2672.2008.03741.x. [DOI] [PubMed] [Google Scholar]
- Cao LX, Qiu ZQ, You JL, Tan HM, Zhou SN. Isolation and characterization of endophytic streptomycete antagonists of fusarium wilt pathogen from surface-sterilized banana roots. FEMS Microbiology Letters. 2005;247:147–152. doi: 10.1016/j.femsle.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Chaijuckam P, Davis RM. Efficacy of natural plant products on the control of aggregate sheath spot of rice. Plant Disease. 2010;94:986–992. doi: 10.1094/PDIS-94-8-0986. [DOI] [PubMed] [Google Scholar]
- Chen CH, Chou TW, Cheng LH, Ho CW. In vitro anti-adenoviral activity of five Allium plants. Journal of the Taiwan Institute of Chemical Engineers. 2011;42:228–232. [Google Scholar]
- Dagostin S, Formolo T, Giovannini O, Pertot I, Schmitt A. Salvia officinalis extract can protect grapevine against plasmopara viticola. Plant Disease. 2010;94:575–580. doi: 10.1094/PDIS-94-5-0575. [DOI] [PubMed] [Google Scholar]
- Das MK, Rajaram S, Mundt CC, Kronstad WE. Inheritance of slow-rusting resistance to leaf rust in wheat. Crop Sci. 1992;32:1452–1456. [Google Scholar]
- El-Mougy NS, Alhabeb RS. Inhibitory effects of powdered caraway and peppermint extracts on pea root rot under greenhouse condition. Journal of Plant Protection Research. 2009;49:93–96. [Google Scholar]
- Harish S, Saravanakumar D, Radjacommare R, Ebenezar EG, Seetharaman K. Use of plant extracts and biocontrol agents for the management of brown spot disease in rice. BioControl. 2008;53:555–567. [Google Scholar]
- Ho WC, Wu TY, Su HJ, Ko WH. Effect of oriental medicinal plant extracts on spore germination of Alternaria brassicicola and nature of inhibitory substances from speedweed. Plant Disease. 2007;91:1621–1624. doi: 10.1094/PDIS-91-12-1621. [DOI] [PubMed] [Google Scholar]
- Hwang SC, Ko WH. Cavendish banana cultivars resistant to fusarium wilt acquired somaclonal variation in Taiwan. Plant Disease. 2004;88:580–588. doi: 10.1094/PDIS.2004.88.6.580. [DOI] [PubMed] [Google Scholar]
- Lazarevic JS, Dord̄evic AS, Zlatkovic BK, Radulovic NS, Palic RM. Chemical composition and antioxidant and antimicrobial activities of essential oil of Allium sphaerocephalon L. subsp. sphaerocephalon (Liliaceae) inflorescences. Journal of the Science of Food and Agriculture. 2011;91:322–329. doi: 10.1002/jsfa.4189. [DOI] [PubMed] [Google Scholar]
- Lee CF, Han CK, Tsau JL. In vitro inhibitory activity of Chinese leek extract against Campylobacter species. International Journal of Food Microbiology. 2004;94:169–174. doi: 10.1016/j.ijfoodmicro.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Lian J, Wang ZF, Cao LX, Tan HM, Inderbitzin P, Jiang ZD, Zhou SN. Artificial inoculation of banana tissue culture plantlets with indigenous endophytes originally derived from native banana plants. Biological Control. 2009;51:427–434. [Google Scholar]
- Mak C, Mohamed AA, Liew KW, Ho YW. Early screening technique for Fusarium wilt resistance in banana micropropagated plants. Banana Improvement. 2004;18:219–227. [Google Scholar]
- Mau JL, Chen CP, Hsieh PC. Antimicrobial effect of extracts from Chinese chieve, cinnamon and corni fructus. J. Agric. Food Chem. 2001;49:183–188. doi: 10.1021/jf000263c. [DOI] [PubMed] [Google Scholar]
- Molina A, Fabregar E, Sinohin VG, Herradura L, Fourie G, Viljoen A. [Retrieved July 1, 2010];Confirmation of tropical race 4 of Fusarium oxysporum f. sp. Cubense infecting cavendish bananas in the Philippines. Bioversity International. 2008 Jul; from http://bananas.bioversityinternational.org/files/files/pdf/partnerships/aps_poster . pdf.
- Najjaa H, Neffati M, Zouari S, Ammar E. Essential oil composition and antibacterial activity of different extracts of Allium roseum L., a North African endemic species. Comptes Rendus Chimie. 2007;10:820–826. [Google Scholar]
- Nel B, Steinberg C, Labuschagne N, Viljoen A. Evaluation of fungicides and sterilants for potential application in the management of Fusarium wilt of banana. Crop Protection. 2007;26:697–705. [Google Scholar]
- Nguyen VN, Nguyen DMC, Seo DJ, Park RD, Jung WJ. Antimycotic activities of Cinnamon-derived compounds against Rhizoctonia solani in vitro. Biocontrol. 2009;54:697–707. [Google Scholar]
- Paparu P, Dubois T, Coyne D, Viljoen A. Defense-related gene expression in susceptible and tolerant bananas (Musa spp.) following inoculation with non-pathogenic Fusarium oxysporum endophytes and challenge with Radopholus similis. Physiological and Molecular Plant Pathology. 2007;71:149–157. [Google Scholar]
- Paul JY, Becker DK, Dickman MB, Harding RM, Khanna HK, Dale JL. Apoptosis-related genes confer resistance to Fusarium wilt in transgenic ‘Lady Finger’ bananas. Plant Biotechnology Journal. 2011;9:1141–1148. doi: 10.1111/j.1467-7652.2011.00639.x. [DOI] [PubMed] [Google Scholar]
- Phay N, Higashiyama T, Tsuji M, Matsuura H, Fukushi Y, Yokota A, Tomita F. An antifungal compound from roots of Welsh onion. Phytochemisty. 1999;52:271–274. [Google Scholar]
- Ploetz RC. Panama disease: A classic and destructive disease of banana. Plant Health Progress. 2000 DIO:10.1094/PHP-2000-1204-01-HM. [Google Scholar]
- Ploetz RC. Panama disease, an old nemesis rears its ugly head: Part 2, the cavendish era and beyond. Plant Health Progress. 2006. DIO: 10.1094/PHP-2006-0308-01-RV.
- Saravanan T, Muthusamy M, Marimuthu T. Development of integrated approach to manage the fusarial wilts of banana. Crop Protection. 2003;22:1117–1123. [Google Scholar]
- Seyfi P, Mostafaie A, Mansouri K, Arshadi D, Mohammadi-Motlagh HR, Kiani A. In vitro and in vivo anti-angiogenesis effect of shallot (Allium ascalonicum):A heat-stable and flavonoid-rich fraction of shallot extract potently inhibits angiogenesis. Toxicology in Vitro. 2010;24:1655–1661. doi: 10.1016/j.tiv.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Smith MK, Hamill SD, Langdon PW, Giles JE, Doogan VJ, Pegg KG. Towards the development of a Cavendish banana resistant to race 4 of fusarium wilt: gamma irradiation of micropropagated Dwarf Parfitt (Musa spp., AAA group, Cavendish subgroup) Australian Journal of Experimental Agriculture. 2006;46:107–113. [Google Scholar]
- Stover RH. Fusarial wilt (Panama Disease) of bananas and other Musa Species. Commonwealth Mycological Institute; Kew, Surrey, UK: 1962. [Google Scholar]
- Yin MC, Tsao SM. Inhibitory effect of seven Allium plants upon three Aspergillus species. Int. J. Food Microbiol. 1999;49:49–56. doi: 10.1016/s0168-1605(99)00061-6. [DOI] [PubMed] [Google Scholar]
- Yip MK, Lee SW, Su KC, Lin YH, Chen TY, Feng TY. An easy and efficient protocol in the production of pflp transgenic banana against Fusarium wilt. Plant Biotechnol Rep. 2011;5:245–254. [Google Scholar]