Abstract
Neurodegenerative disorders lead to disability and death in a significant proportion of the world’s population. However, many disorders of the nervous system remain with limited effective treatments. Kinase pathways in the nervous system that involve phosphoinositide 3-kinase (PI 3-K), protein kinase B (Akt), and the mammalian target of rapamycin (mTOR) offer exciting prospects for the understanding of neurodegenerative pathways and the development of new avenues of treatment. PI 3-K, Akt, and mTOR pathways are vital cellular components that determine cell fate during acute and chronic disorders, such as Huntington’s disease, Alzheimer’s disease, Parkinson’s disease, epilepsy, stroke, and trauma. Yet, the elaborate relationship among these kinases and the variable control of apoptosis and autophagy can lead to unanticipated biological and clinical outcomes. Crucial for the successful translation of PI 3-K, Akt, and mTOR into robust and safe clinical strategies will be the further elucidation of the complex roles that these kinase pathways hold in the nervous system.
Keywords: Akt, apoptosis, Alzheimer’s disease, autophagy, Huntington’s disease, mammalian target of rapamycin (mTOR), Parkinson’s disease, phosphoinositide 3–kinase (PI 3-K), stroke, trauma
Cellular Kinase Pathways of PI 3-K, Akt, and mTOR
Although numerous kinase pathways control and regulate cellular function, the kinase pathways of phosphoinositide 3-kinase (PI 3-K), protein kinase B (Akt), and the mammalian target of rapamycin (mTOR) play a significant role in the onset and progression of neurodegenerative disorders. PI 3-K represents a family of lipid kinases that phosphorylate the 3′-hydroxyl group of phosphatidylinositide and phosphoinositides. Based on their structure and substrate specificity, the PI 3-K family is grouped into three classes, designated as classes I–III [1, 2]. Class I PI 3-K consists of two subunits, a regulatory subunit (p85α, p85β, and p85γ) and a p110 catalytic subunit (p110α, p110β, p110γ, and p110δ). Class II consists of only a p110 catalytic subunit. In contrast, class III consists of vacuolar protein-sorting defective 34 (Vps34) catalytic subunit. Class I PI 3-K phosphorylates phosphoinositide (PI), phosphatidylinositide 3-phosphate (PI-3-P), and phosphatidylinositide (3,4)-bisphosphate (PI-3, 4-P2), producing PI-3-P, PI-3, 4-P2, and phosphatidylinositide (3,4,5)-triphosphate (PI-3, 4, 5-P3) respectively. Class II PI 3-K phosphorylates PI and PI-3-P, resulting in the production of PI-3-P and PI-3, 4-P2. Class III PI 3-K only functions through PI to produce PI-3-P.
The kinases of PI-3K are vital in the signaling pathways leading to the activation of protein kinase B (PKB, also termed Akt) and mTOR. Akt is considered to be a primary component in a variety of pathways to promote cell survival and prevent cell injury [3]. In mammals, three family members of Akt have been identified, which are termed PKBα or Akt1, PKBβ or Akt2, and PKBγ or Akt3 [3–5]. Akt belongs to the cAMP-dependent kinase/protein kinase G/protein kinase C superfamily of protein kinases and consists of three functional domains. The N-terminal pleckstrin homology (PH) domain provides binding sites for membrane phospholipids, which is involved in the recruitment of Akt to the plasma membrane. The catalytic domain of Akt has specificity for serine or threonine residues of several Akt substrates. The C-terminal hydrophobic motif functions to provide a docking site for the activation of kinases.
mTOR, also known as mechanistic target of rapamycin and FK506-binding protein 12-rapamycin complex-associated protein 1 (FRAP1), belongs to the PI 3-K related kinase family and can be activated through the PI 3-K and Akt signaling pathways to control cell growth and proliferation [6, 7]. mTOR is a 289-kDa serine/threonine protein kinase that can oversee transcription, cytoskeleton organization, cell growth, and cell survival. In response to growth factors, nutrients, mitogens, and hormones, mTOR is activated through phosphorylation of its specific residues [8]. Serine2448 is a target of Akt and p70 ribosomal S6 kinase (p70S6K), threonine2446 is a target of AMP activated protein kinase (AMPK) and p70S6K, and serine2481 is a site that is rapamycin insensitive and an autocatalytic site of mTOR [9–11]. In addition, dual phosphorylation on serine2159 and threonine2164 enhances mTOR activity and promotes autophosphorylation on serine2481 [12].
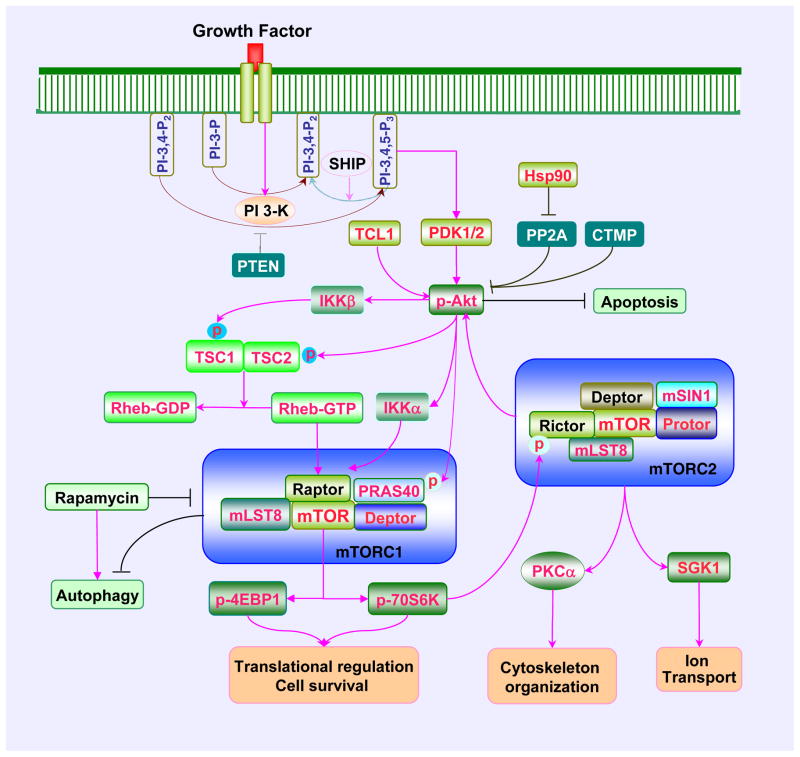
mTOR signaling is dependent upon the protein complexes mTOR Complex 1 (mTORC1) or mTOR Complex 2 (mTORC2) that each contain mTOR [13, 14] (Figure 1). mTORC1 and mTORC2 have been identified based on their components and their sensitivity to rapamycin [15]. Rapamycin is a macrolide antibiotic from Streptomyces hygroscopicus that specifically inhibit mTOR activation [16, 17]. mTORC1 is more sensitive to the inhibitory effect of rapamycin than mTORC2 [18]. Rapamycin inhibits mTORC1 through the binding to immunophilin FK-506-binding protein 12 (FKBP12) [19] that attaches to FKBP12 -rapamycin-binding domain (FRB) at the C-terminal of mTOR to prevent the phosphorylation of mTOR [20]. Prolonged exposure of rapamycin also results in the inhibition of mTORC2 that may involve disrupting the assembly and the integrity of mTORC2 [18].
Figure 1. The PI 3-K/Akt/mTOR signaling cascade.
Growth factors can activate mTOR signaling through phosphoinositide 3 kinase (PI 3-K) and Akt mediated pathways. Growth factors can stimulate PI 3-K activation and promote the production of phosphatidylinositide (3,4)-biphosphate (PI-3, 4-P2) and phosphatidylinositide (3,4,5)-triphosphate (PI-3, 4, 5-P3) resulting in the recruitment of Akt to the plasma membrane and subsequent activation by phosphoinositide dependent kinase 1 (PDK1) and PDK2. Akt activity can be inhibited by several entities that include the phosphatase and tensin homolog deleted from chromosome 10 (PTEN) (specifically dephosphorylates PI-3, 4, 5-P2 and PI-3, 4, 5-P3 at the D3 position), SH2 domain-containing inositol phosphatase (SHIP), and carboxyl-terminal modulator protein (CTMP). In contrast, Akt can be activated by T cell leukemia/lymphoma 1(TCL1) and 90 kDa heat shock protein (Hsp90) which can inhibit protein phosphatase 2A (PP2A). Once active, Akt can result in the activation of mTORC1 through a series of signaling pathway. Akt functions as a key upstream kinase that mediates the phosphorylation of TSC2, resulting in the destabilization of TSC2 and disruption of its interaction with TSC1. Akt also may activate mTORC1 through I-kappaB kinase (IKK). IKKα regulates mTORC1 activity through associating with Raptor and IKKβ can physically interact with and phosphorylate TSC1 resulting in the suppression of TSC1 and the activation of mTORC1. In addition, Akt can directly phosphorylate proline rich Akt substrate 40 kDa (PRAS40) and reduce its binding to regulatory associated protein of mTOR (Raptor) and release mTORC1 from the suppression by PRAS40. Upon activation, mTORC1 phosphorylates its two major downstream targets p70 ribosome S6 kinase (p70S6K) and eukaryotic initiation factor 4E-binding protein 1 (4EBP1) to promote protein synthesis, increase cell survival, and prevent autophagy. This can be reversed by rapamycin. mTORC2 activation also can occur through mTORC1. Activation of p70S6K results in the phosphorylation of Rictor and downregulation of mTORC2 activity. However, TSC1/TSC2 can activate mTORC2 but inhibit mTORC1. mTORC2 regulates actin skeleton organization, apoptosis, cell survival, and ion transport through Akt, protein kinase C alpha (PKCα), and serum- and glucocorticoid-induced protein kinase 1 (SGK1).
mTORC1 contains the mTOR protein that functions as the catalytic multiple domain protein of the complex. The carboxy-terminal kinase domain contains a conserved sequence with homology to the catalytic domain of the PI 3-K family [21]. Raptor is another component of mTORC1 that functions to recruit mTOR substrates to the mTORC1 complex and binds to the N-terminal HEAT (for Huntingtin, Elongation factor 3, A subunit of protein phosphatase-2A, and TOR1) of mTOR [22]. The proline rich Akt substrate 40 kDa (PRAS40) is part of the mTORC1 complex that can competitively inhibit the binding of mTORC1 to Raptor [23, 24]. Mammalian lethal with Sec13 protein 8 (mLST8), also a component of mTORC1, interacts with the kinase domain of mTOR and promotes the stabilization of the association between Raptor and mTOR [25]. Another inhibitor protein of mTORC1 is DEP-domain-containing mTOR-interacting protein (Deptor). Deptor binds to the FAT domain of mTOR (for FKBP associated protein, Ataxia-telengiectasia, and Transactivation/transformation domain-associated protein) and negatively regulates the activity of mTORC1 [26]. mTORC1 plays a key role in the regulation of protein translation. The best two characterized downstream targets of mTORC1 are p70S6K and eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) (Table 1). These proteins are two critical components that regulate translation initiation. The phosphorylation of p70S6K promotes mRNA biogenesis, translation of ribosomal proteins, and cell growth [27, 28]. The phosphorylation of 4EBP1 results in its inactivation. Hypophosphorylated 4EBP1 is active and binds competitively with eukaryotic translation initiation factor 4 gamma (eIF4G) to eukaryotic translation initiation factor 4 epsilon (eIF4E) that regulate translation initiation by interacting with the 5′-mRNA cap structure. The phosphorylation of 4EBP1 by mTORC1 results in its dissociation from eIF4E allowing eIF4G to interact with eIF4E and promote protein translation [29, 30].
Table 1.
Components and Biological Functions of the PI 3-K/Akt/mTOR Signaling Pathway
| Components | Biological Function |
|---|---|
| PI 3-K | Phosphorylates the 3′-hydroxyl group of membrane, phosphatidylinositide and phosphoinositides resulting in the recruitment of PDK1 and Akt to the cell membrane |
| PTEN | Dephosphorylates phosphotidylinositide phosphates and inhibits the activity of PI 3-K |
| SHIP | Dephosphorylates inositides and phosphoinositides and inhibits the activity of PI 3-K |
| CTMP | Binds to the C-terminal of Akt and prevents the phosphorylation of Akt |
| TCL1 | Binds to the PH domain of Akt to increase Akt activity |
| PP2A | Dephosphorylates and inhibits Akt |
| PDK1/2 | Phosphorylates Akt on threonine308 and serine473 |
| Akt | Promotes cell growth and proliferation, phosphorylates TSC2 to inhibit the TSC1/2 complex leading to mTORC1 activation, and functions as downstream target of mTORC2 |
| IKK | Akt activates IKK. IKKα associates with Raptor to increase the activity of mTORC1. IKKβ also phosphorylates TSC1 leading to mTORC1 activation |
| p70S6K | mTORC1 phosphorylates and activates p70S6K to regulate protein synthesis, phosphorylates Rictor to regulate mTORC2, phosphorylates BAD, and increases the expression of Bcl-2/Bcl-xL |
| 4EBP1 | mTORC1 phosphorylates and inactivates 4EBP1 to regulate translation initiation |
| PKCα | mTORC2 activates PKCα to regulate cytoskeleton organization |
| BAD | Akt phosphorylates BAD leading to its dissociation from Bcl-2/Bcl-xL and the inhibition of apoptosis |
| FoxO3a | Akt phosphorylates FoxO3a leading to its cytoplasmic retention with 14-3-3 protein and the inhibition of apoptosis |
| GSK-3β | Akt phosphorylates and inactivates GSK-3β to inhibit apoptosis |
| PRAS40 | Akt phosphorylates PRAS40 leading to its dissociation with Raptor and activation of mTORC1 |
| ULK1 | mTOR phosphorylates and inactivates ULK1 to inhibit autophagy |
| Atg13 | mTOR phosphorylates and inactivates Atg13 to inhibit autophagy |
Note: Atg13: Autophagy related gene 13; CTMP: carboxyl-terminal modulator protein; 4EBP1: eukaryotic initiation factor 4E-binding protein 1; GSK-3β: glycogen synthase kinase-β; IKK: IκB kinase; mTOR: mammalian target of rapamycin; p70S6K: p70 ribosomal S6 kinase; PRAS40: proline-rich Akt substrate 40 kDa; PTEN: the phosphatase and tensin homolog deleted from chromosome 10; Raptor: regulatory-associated protein of mTOR; mTORC1/2: mTOR complex 1/2; PDK: phosphoinositide dependent kinase; PI 3-K: phosphoinositide 3 – kinase; PKCα: protein kinase alpha; PP2A: protein phosphatase 2A; Rictor: rapamycin-insensitive companion of mTOR; SHIP: SH2 domain-containing inositol phosphatase; TCL1: T cell leukemia/lymphoma 1; TSC1/2: tuberous sclerosis complex1/2; ULK1: UNC-51 like kinase 1.
In contrast to mTORC1 that contains the protein Raptor, mTORC2 has the rapamycin-insensitive companion of mTOR known as Rictor [6, 31]. mTORC2 shares several components with mTORC1 that include mTOR, mLST8, and Deptor. Yet, mTORC2 also contains mammalian stress-activated protein kinase interacting protein (mSIN1) and protein observed with Rictor-1 (Protor-1) that are not associated with mTORC1. The regulatory functions of mTORC2 consist of cytoskeleton organization [32], modulation of cell cycle progression [33], and control of cell survival [34]. The primary downstream targets of mTORC2 are Akt, protein kinase C alpha (PKCα), and glucocorticoid-induced protein kinase 1 (SGK1). mTORC2 promotes cell survival through the activation of Akt and uses PKCα for cytoskeleton remodeling [35]. In addition, mTORC2 phosphorylates and activates SGK1 [36], a member of the protein kinase A/protein kinase G/protein kinase C (AGC) family of protein kinases, and is activated by growth factors to control ion transport and growth [37]. P-Rex1 and P-Rex2, and Rho GTPases are also targets of mTORC2 [38]. mTORC2 controls cell migration through activating Rac guanine exchange factors P-Rex1 and P-Rex2 and uses Rho signaling during cell - to – cell contact [39].
Cellular Signaling in the PI 3-K/Akt/mTOR Cascade
The cellular signaling pathways of PI 3-K, Akt, and mTOR are tightly linked and sometimes are targeted for drug discovery as a combined system [40] (Figure 1). Activation of Akt is dependent upon PI 3-K [41, 42]. Receptor tyrosine kinase (RTK) and the G protein-coupled receptor (CPCR) are required for PI 3-K activation. Growth factors or cytokines can stimulate the recruitment of PI 3-K to the plasma membrane [3] (Figure 1). Following activation, PI 3-K phosphorylates membrane lipids and mediates the transition of Akt from the cytosol to the plasma membrane by promoting the binding of Akt to PI-3, 4-P2 and PI-3, 4, 5-P3 through its plectrin homology (PH) domain. As a result, Akt is subsequently phosphorylated on the residues of serine473 and threonine308 by phosphoinositide dependent kinase (PDK) PDK1 and PDK2 (Table 1). PDK1 is responsible for phosphorylating Akt at threonine308 [43]. PDK1 contains a C-terminal PH domain through which PI-3, 4, 5-P3 can recruit PDK1 to the cell membrane and bind to the C-terminal HM domain of Akt. PDK1 cannot directly phosphorylate Akt on serine473, but is important to note that phosphorylation of Akt on serine473 is necessary for the full activation of Akt. In this regard, PDK2 like kinases, such as intergrin-linked kinase, DNA dependent protein kinase, PKCβ, and mTORC2, have been identified to promote Akt phosphorylation on serine473 [44].
The PI 3-K and Akt pathways can be modulated by a variety of cell signals [45–49] (Figure 1). The phosphatase and tensin homolog deleted from chromosome 10 (PTEN), which specifically dephosphorylates PI-3, 4-P2 and PI-3, 4, 5-P3 at the D3 position can block PI 3-K signaling and subsequent Akt activation (Table 1). The src homology 2 (SH2) domain-containing inositol phosphatase (SHIP) is an inositol 5′ phosphatase that dephosphorylates inositides and phosphoinositides on the 5′-position [1]. As a result, PI-3 and 4-P3 are transformed into PI-3, 4-P2 that is less potent than PI-3, 4-P3 to recruit Akt. Both SHIP1 and SHIP2 can negatively regulate the activity of Akt. The SH2 domains containing protein-tyrosine phosphatases SHP1 and SHP2 can also regulate the activity of PI 3-K and Akt. SHP1 associates with the p85 subunit of PI 3-K to negatively regulate the activation of PI 3-K. SHP2 can be necessary for agents that promote cell differentiation to lead to the activation of PI 3-K and Akt [50]. Other cell pathways also determine Akt activity. Carboxyl-terminal modulator protein (CTMP) binds specifically to the carboxyl-terminal regulatory domain of Akt1 at the plasma membrane to prevent Akt1 from phosphorylation. In contrast, the T cell leukemia/lymphoma 1 (TCL1) protein binds to the PH domain of Akt to enhance Akt activity. A 90 kDa heat shock protein (Hsp90) that is involved in modulating oxidative stress in cells [51] also can enhance Akt activity through the inhibition of inhibiting protein phosphatase 2A (PP2A).
Akt is a potent stimulator of mTORC1 in response to growth factors and several Akt mediated pathways can lead to the activation of mTORC1 [15]. Tuberous sclerosis complex (TSC) 1 (hamartin)/TSC2 (tuberin) complex, a negative regulator of mTORC1, is one of the targets of Akt for the regulation of mTORC1 activity (Figure 1). TSC2 functions as a GTPase-activating protein (GAP) converting a small G protein Ras homologue enriched in brain (Rheb-GTP) to the inactive GDP-bound form (Rheb-GDP) [52]. Once active, Rheb-GTP can directly interact with Raptor to activate mTORC1 and also regulate the binding of 4EBP1 to mTORC1 [53]. Akt phosphorylates TSC2 on multiple sites that leads to the destabilization of TSC2 and disruption of its interaction with TSC1. The phosphorylation of TSC2 on the residues of serine939, serine981, and threonine1462 can increase its binding to the anchor protein 14-3-3 and lead to the cellular sequestration by 14-3-3, disruption of the TSC1/TSC2 complex, and subsequent activation of Rheb and mTORC1 [54].
Akt also can activate mTORC1 through I-kappaB kinase (IKK), a downstream target of Akt that can regulate cell survival (Table 1). Within IKK, IKKα and IKKβ are catalytic subunits of IKK that possess serine/threonine kinase activity [55]. IKKγ is a regulatory unit that is essential for IKK function. Akt has been shown to promote the activation of mTORC1 through IKKα, since knockdown of IKKα inhibits mTOR activation in Akt-active cells during inactivation of the negative PI 3-K regulator phosphatase and tensin homolog (PTEN) [56]. IKKα regulates mTOR activity by associating with Raptor that is Akt dependent [56]. In addition, IKKβ can phosphorylate TSC1 on serine487 and serine511 leading to the suppression of TSC1, disruption of TSC1/TSC2 complex, and the activation of mTORC1 [57].
As an Akt proline-rich substrate and a component of mTORC1, PRAS40 is another target of Akt to regulate the activation of mTORC1 (Figure 1). PRAS40 can be phosphorylated on several residues including serine183, serine212, serine221, and threonine246 [58, 59]. The serine sites are targets of mTOR. The residue of threonine246 is the phosphorylation target of Akt. The phosphorylation of PRAS40 leads to its dissociation with Raptor [60] and promotes the binding of PRAS40 to the cytoplasmic docking protein 14-3-3 [61, 62] (Table 1). This process removes PRAS40 from interacting with Raptor and facilitates the activation of mTORC1 [24].
Modulation of Cell Demise by PI 3-K, Akt, and mTOR Signaling
Apoptosis and autophagy are pathways of programmed cell death (PCD) that can impact the course of neurodegenerative disorders [63–65]. Yet, it should be noted that these pathways serve multiple functions. Apoptosis and autophagy can assist with tissue remodeling and regeneration during development and cell injury. Autophagy also can serve to repair and remove non-functioning organelles of a cell [66]. As a result, these pathways serve as important targets when considering therapeutic strategies that involve PI 3-K, Akt, and mTOR signaling. Apoptosis can lead to cell death in neurodegenerative disorders. Apoptotic DNA degradation and caspase 3 in neurons has been reported in the postmortem nigra of Parkinson’s disease (PD) patients, suggesting that apoptosis results in neuronal loss [67]. Apoptotic DNA fragmentation [68] and caspase activation also has been observed [69] in the brains of patients with Alzheimer’s disease (AD). Autophagy also has been suggested as a modulator of disease in the nervous system. During periods of oxidative stress, autophagy can lead to cell death in cerebral astrocytes [70], in cortical neurons [71], and in spinal cord motor neurons [72]. Autophagic can mediate cell death in purkinje neurons [73] and in sympathetic neurons [74]. However, activation of autophagy may sometimes offer cytoprotection during other neurodegenerative disorders [75, 76]. Autophagy has been associated with the processing of the protein α-synuclein in Parkinson’s disease. Activation of autophagy may be necessary to protect against neuronal cell loss and α-synuclein toxicity in Parkinson’s disease [75]. The pathways of autophagy and apoptosis also may function in concert. Methamphatamine leads to cell death not only through apoptosis, but also through autophagy by inhibiting the disassociation of the Bcl-2/Beclin 1 complex [77]. Bcl-2/Bcl-xL is an “anti-apoptotic” protein that blocks autophagy through its inhibitory interaction with Beclin 1 [78]. Autophagy and apoptosis also may have opposing or independent roles. Some studies report that progression of apoptosis may conversely require the inhibition of autophagy [79] or apoptotic neuronal cell may be independent of the progression of autophagy [80].
Apoptosis is considered as a crucial process for tissue remodeling during development and recognized as a central pathway that can lead to cell demise in a variety of tissues [81]. Apoptosis is the result of a series of biochemical and morphological alterations that consist of two distinct components that involve genomic DNA degradation and the loss of plasma membrane lipid asymmetry [64]. The loss of asymmetry of membrane phosphatidylserine (PS) distribution is an early reversible feature of apoptosis. In contrast, the cleavage of genomic DNA into fragments is a typical event that can occur once a cell has been committed to die. Both membrane PS exposure and genomic DNA degradation are considered to be the outcomes of a series of activation of nucleases and proteases that occurs late during apoptosis [82, 83].
Modulation of apoptosis can be controlled through PI 3-K, Akt, and mTOR. The PI 3-K/Akt pathway can prevent apoptosis and increase survival for multiple cell types that include endothelial cells, cardiac cells, neuronal cells, and inflammatory cells [1]. In addition, Akt is a major survival factor that blocks apoptosis progression. The downstream targets of the Akt pathway include BAD, caspase 9, the forkhead transcription factor FoxO3a (FHKRL1), and glycogen synthase kinase-3β (GSK-3β) [84] (Table 1). Akt can phosphorylate FoxO3a, GSK-3b, BAD and caspase 9 to block apoptosis and alter cell longevity pathways [82, 85–87]. Akt preferentially phosphorylates the residue of serine253 of FoxO3a resulting in its export from the nucleus to the cytoplasm and blocking FoxO3a from activating apoptotic genes. Akt also can phosphorylate GSK-3β at serine9 and inactivate this enzyme, thus preventing GSK-3β from initiating an apoptotic pathway. Phosphorylation of Bad at serine136 by Akt can result in the inactivation of Bad and prevent neuronal apoptosis [88, 89]. In contrast, loss of Akt activity prevents mTOR signaling.
mTOR uses Akt to protect endothelial cells against apoptosis [90] and to block forkhead transcription factors, such as FoxO3a [90, 91]. Inflammatory cells also can undergo apoptotic injury during oxidative stress if deprived of Akt and mTOR activation [92, 93]. Apoptotic cell death in dopaminergic neurons can be blocked during application of agents that increase Akt and mTOR activity [94]. Akt also functions to modulate apoptosis with mTOR through the inhibition of PRAS40 [95, 96]. Phosphorylation of PRAS40 by Akt can block the activity of this substrate, lead to its dissociation from mTORC1 to allow mTOR activation, and prevent apoptotic cell injury [97].
mTOR also regulates apoptotic cell death through downstream signaling pathways such as p70S6K and BAD [8, 15, 98]. For example, phosphorylation of BAD leads to the dissociation of this protein from the “anti-apoptotic” protein Bcl-2/Bcl-xL and increases BAD binding to the cytoplasmic docking protein 14-3-3. Activation of p70S6K promotes the phosphorylation of BAD in astrocytes to limit apoptotic cell injury [98]. The activation of mTOR and p70S6K may also decrease apoptosis through pathways that can increase “anti-apoptotic” Bcl-2/Bcl-xL expression [98]. In addition, insulin prevents apoptosis in rat retinal neuronal cells against serum deprivation through the activation of mTOR and p70S6K [99]. Over-expression of wild type p70S6K or of a rapamycin resistant form of the kinase enhances the cytoprotective effect of insulin. In contrast, over-expression of a dominant-negative mutant of p70S6K results in the loss of the ability of insulin to protect neurons [99]. Other growth factors similar to insulin, such as erythropoietin (EPO) [100], have been reported to be dependent upon mTOR activation for cytoprotection against apoptosis [93, 101, 102].
Yet, activation of mTOR signaling pathways may not always prevent apoptosis. During Alzheimer’s disease, post-mitotic neurons that attempt to enter the cell cycle do not replicate, but can result in apoptotic cell death [103, 104]. In studies with amyloid oligomer exposure, neurons can be prevented from entering the cell cycle during the inhibition of mTOR and thus be protected from apoptosis [105]. In addition, during cancer progression, therapeutic strategies may seek to prevent PI 3-K, Akt, or mTOR activity. Prevention of PI 3-K and Akt activation can block medulloblastoma growth [106], reduce colorectal cancer growth [107], enhance radiosensitivity in tumors [108], and limit gynecological malignancies [109].
In contrast to apoptosis in which there is an early preservation of organelles and collapse of the cytoskeleton, during autophagy there is an early destruction of organelles and the preservation of cytoskeleton structures [110]. Under most circumstances, autophagy allows cells to recycle cytoplasmic components and remove defective organelles for tissue remodeling. Autophagy consists of three different categories known as microautophagy, macroautophagy, and chaperone-mediated autophagy [110–112]. Macroautophagy involves the degradation of cytoplasmic material and the sequestration of the cytoplasmic protein and organelles into autophagosomes. Autophagosomes fuse with lysosomes for degradation and reuse for future cellular processes [113]. Microautophagy is the sequestration of cytoplasmic components by invagination of the lysosomal membrane. Vesicles subsequently formed are transferred to the lumen of the lysosomes for digestion. In chaperone mediated autophagy, the cytoplasmic component is delivered by cytosolic chaperones to the receptors on the lysosomal membranes for translocation across lysosomal membranes into the lumen.
Inhibition of PI 3-K, Akt, or mTOR can lead to autophagy (Figure 1). Application of the agent erufosine that blocks Akt activity leads to cell death through autophagy in oral squamous carcinoma cell lines [114]. Inhibition of Akt in ovarian cancer also leads to autophagic cell death [115]. In contrast, over-expression of Akt that also increases mTOR activity prevents autophagy in a non-small cell lung carcinoma cell line [116]. Activation of the PI 3-K/Akt/mTOR axis also has been shown to shift cell death away from autophagy [117]. In regards to neuroprotection, mTOR activation can prevent oxidative stress mediated autophagy in dopamine neurons [94]. Yet, chronic disease, such as during Alzheimer’s disease, may benefit from inhibition of mTOR to allow the progression of autophagy [76]. The degree of mTOR activation may be a significant variable in cases such as this. During the early phases of autophagy, mTOR activity can be inhibited [118]. However, re-activation of mTOR appears to be required to continue with autophagy as long as elevated levels of mTOR activity do not lead to the eventual blockade of autophagy [118]. This modulation of mTOR with autophagy may be a conserved response in multiple cell systems that is governed by nutrient availability [119].
mTOR appears to modulate autophagy through the regulation of autophagic genes (Table 1). mTOR can phosphorylate the mammalian homologue of autophagy related gene 13 (Atg13) and the mammalian Atg1 homologue ULK1 and ULK2 to prevent the progression of autophagy [120]. The focal adhesion kinase family interacting protein of 200 kDa (FIP200) has been identified as a ULK binding protein. FIP200 and Atg13 are critical for the stability and activation of ULK1. Mammalian Atg13 binds to ULK1/2 and FIP200 to activate ULKs and facilitate the phosphorylation of FIP200 by ULKs [120]. As a result, it is suggested that mTOR activation prevents autophagy in mammalian cells through inhibiting the activity of ULK-Atg13-FIP200 complex by phosphorylating Atg13 and ULKs.
The PI 3-K/Akt/mTOR Pathway in Neurological Disorders
Huntington’s disease
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease characterized by the degeneration of striatal GABAergic projecting neurons leading to involuntary movements and cognitive impairment. HD is believed to be the result of intracellular aggregates of huntingtin protein mutations that produce abnormally expanded polyglutamine in the N-terminal region of the huntingtin gene. The intracellular aggregates form in neurons and lead to neuronal degeneration. Autophagy is considered important in this disorder since this cellular mechanism is responsible for the clearing of aggregate-prone proteins [121] (Figure 2). As a result, inhibition of mTOR signaling that can promote autophagy may represent a potential therapeutic strategy for HD. Blockade of mTOR activity with the agent rapamycin has been demonstrated to enhance the autophagic clearance of proteins with long polyglutamines and a polyalanine-expanded protein [122], attenuate huntingtin accumulation and cell death in cell models of HD, and protect against neurodegeneration in a fly model of HD [123]. In addition to rapamycin, some small molecular enhancers of rapamycin promote autophagy with both mTOR dependent and independent mechanisms in mammalian cells and can enhance the clearance of a mutant huntingtin fragment in HD cell models to protect against a mutant huntingtin fragment toxicity in Drosophila [124]. The rapamycin analog CCI-779 also improves behavioral performance and decreases aggregate formation in a mouse model of HD [123]. However, in some experimental models of HD, inhibition of mTOR signaling that involves mTORC1 alone does not affect autophagy or huntingtin accumulation. In contrast, the combined inhibition of mTORC1 and mTORC2 is required for autophagy and reductions in huntingtin accumulation, suggesting that multiple components of the mTOR pathway may modulate the pathology observed in HD [125]. As evidence for this, other studies show that decreased activity of p70S6K protects against early decline in motor performance with beneficial effects on muscle, but mutant huntingtin levels in the brain are not affected [126]. Neuroprotection in the mTOR pathway also may require growth arrest and DNA damage protein 34 (GADD34). GADD34 leads to the dephosphorylation of TSC2 and induction of autophagy in cell models of HD with increased cell survival during GADD34 over-expression [127].
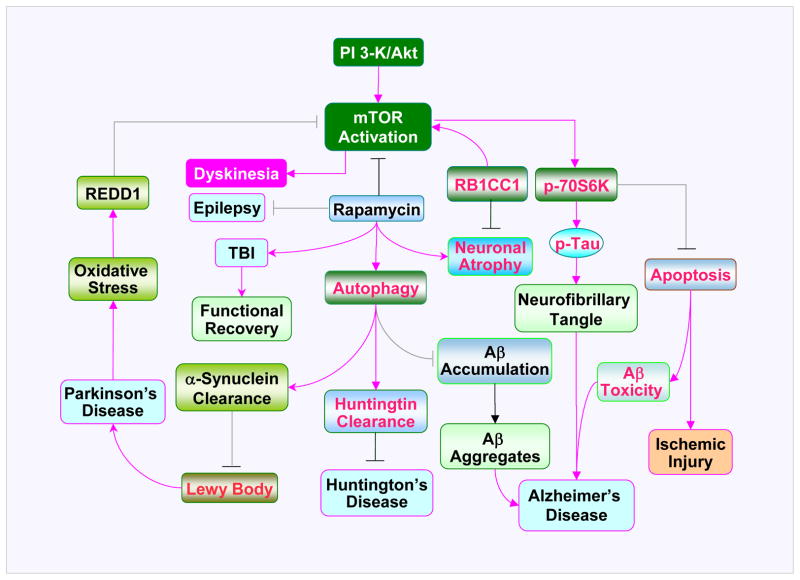
Figure 2. Neurodegenerative diseases in the PI 3-K/Akt/mTOR axis.
Phosphoinositide 3 kinase (PI 3-K)/Akt mediated mTOR activity is involved in pathogenesis of neurodegenerative diseases. Inhibition of mTOR by rapamycin can lead to autophagy under some conditions in neurodegenerative disorders. Autophagy may promote the clearance of aggregate prone proteins, such as huntingtin, α-synuclein, and beta-amyloid (Aβ) that contribute to the development of Huntington’s disease (HD), Parkinson’s disease (PD), and Alzheimer’s disease (AD) respectively. Activation of the downstream target of mTOR, p70 ribosome S6 kinase (p70S6K), through phosphorylation (p) prevents the induction of apoptosis and limits Aβ toxicity and ischemic neuronal injury. Yet, activation of p70S6K can promote the phosphorylation of Tau protein possibly contributing to neurofibrillary tangles. Neuronal atrophy in AD has been attributed to the insufficiency of retinoblastoma tumor suppressor (RB1) inducible Coiled-Coil 1 (RB1CC1), which functions to activate mTOR. Activation of mTOR prevents neurodegeneration of dopaminergic neurons during oxidative stress in models of PD. The stress response protein REDD1 expressed during PD inhibits the activation of mTOR. In some scenarios, activation of mTOR may also lead to dyskinesias. Inhibition of mTOR signaling through rapamycin may reduce the occurrence of epilepsy and improve functional recovery following traumatic brain injury (TBI).
Alzheimer’s disease
Some studies suggest that pathways associated with PI 3-K, Akt, and mTOR may foster memory formation [128]. Inhibition of mTOR activity has been shown to impair memory consolidation [129]. However, the degree of activity for the PI 3-K, Akt, and mTOR pathways that may be necessary to be therapeutic in disorders such as AD has not been determined (Figure 2). An increase in phosphorylated level of Akt substrates, such as mTOR, GSK-3β, and tau protein have been observed in AD, to indicate that these substrates may promote AD progression [130]. p70S6K activation also has been associated with hyperphosphorylated tau formation and potential neurofibrillary accumulation in AD patients [131]. Furthermore, mTOR inhibition that is associated with autophagy in murine models of AD improves memory and reduces amyloid (Aβ) levels [76].
Other investigations support the premise that activation of mTOR to some degree is necessary to prevent pathology during AD. A decrease in mTOR activity in peripheral lymphocytes appears to correlate with the progression of AD [132]. Loss of mTOR signaling has been shown to impair long-term potentiation and synaptic plasticity in models of AD [133]. In addition, Aβ is toxic to cells [93, 134] and can block the activation of mTOR and p70S6K in neuroblastoma cells and in lymphocytes of patients with AD [135]. Yet, activation of mTOR and p70S6K has been shown to prevent cell death during Aβ exposure in microglia [93]. These inflammatory cells are necessary for Aβ sequestration to prevent toxicity of Aβ exposure. Other work also suggests that blockade of mTOR activity may lead to neuronal atrophy in AD. This has been attributed to the insufficiency of retinoblastoma tumor suppressor (RB1) inducible Coiled-Coil 1 (RB1CC1) that has been observed in the brains of AD patients. In these patients, RB1CC1 appears to be necessary for neurite growth and to maintain mTOR signaling, but the reduced expression of RB1CC1 leads to reduced mTOR activity, neuronal apoptosis, and neuronal atrophy [136].
Parkinson’s disease
PD is a movement disorder characterized by resting tremor, rigidity, and bradykinesia due to the degeneration of dopaminergic neurons in the substantia nigra. Decreased mTOR activity may lead to neurodegeneration during PD (Figure 2). The stress response protein REDD1 (RTP801) is up-regulated in dopaminergic neurons in PD patients [137] and can modulate the activity of mTOR. REDD1 is an inhibitor of mTORC1 activity [138] and is highly expressed in several cellular models of PD such as treatment with 6-hydroxydopamine (6-OHDA), MPTP, and rotenone [137]. REDD1 is regarded as a potential contributor to neuronaldegeneration in PD, since gene silencing of REDD1 is neuroprotective against 6-OHDA [139]. REDD1 has been demonstrated to cause neuronal death by dephosphorylating and inactivating Akt that subsequently leads to the loss of mTOR signaling. Other factors involving mTOR may also contribute to neurodegeneration in PD. mTOR activation may be required to prevent cell death during oxidative stress in dopaminergic neurons, since inhibition of mTOR activity can result in autophagic neuronal death during oxidative stress [94]. Loss of mTOR activity and the chronic activation of the mTOR pathway 4EBP1 by leucine-rich repeat kinase 2 (LRRK2), a site for dominant mutations PD, is believed to alter protein translation and lead to the loss of dopaminergic neurons [140]. Yet, activation of 4EBP1 can suppress pathologic experimental phenotypes of PD including degeneration of dopaminergic neurons in Drosophila [141].
Similar to other neurodegenerative disorders with the PI 3-K/Akt/mTOR pathways, excessive activation may lead to disability in PD patients. Treatment with derivatives a dopamine, such as L-DOPA, lead to dopamine D1 receptor-mediated activation of mTORC1 resulting in dyskinesia [142]. Other studies suggest that mTOR inactivation and promotion of autophagy may preserve dopaminergic neurons. In models of PD, treatment with rapamycin preserves neuronal survival that is believed to be dependent upon Akt activation, but is not associated with mTOR signaling [139]. Activation of autophagy also may be required to protect against α-synuclein toxicity in PD [75]. During the inhibition of mTOR and the promotion of autophagy, the accumulation of toxic α-synuclein in transgenic mice is reduced and neurodegeneration is decreased [143].
Epilepsy
Epilepsy is a chronic neurological disorder characterized by recurrent seizures. The mTOR signaling pathway has been reported as an underlying mechanism in tuberous sclerosis (TS), a disorder in which epilepsy occurs in over 80% of patients [144]. Mutations of TSC1 and TSC2 that lead to hyperactive mTOR result in a high incidence of epilepsy in experimental models [145]. Early inhibition of mTOR signaling with rapamycin in animal models of TS can prevent astrogliosis, neuronal disorganization, and seizures, suggesting that the aberrant mTOR activation interferes with normal brain function and leads to epilepsy [146] (Figure 2). mTOR activation has also been linked to acquired epilepsy. Inhibition of mTOR activity during kainate-induced epilepsy decreases neuronal cell death, neurogenesis, mossy fiber sprouting, and the development of spontaneous epilepsy [147]. Chronic hippocampal infusion of rapamycin also limits mossy fiber sprouting in a rat pilocarpine model of temporal lobe epilepsy [148].
Acute Central Nervous System Injury
Recent studies suggest that the PI 3-K/Akt/mTOR pathway may regulate acute nervous system injury and subsequent neurodegeneration. In a model of neonatal hypoxia-ischemia, the promotion of autophagy through mTOR inhibition increased neuronal protection. Yet, prevention of neuronal cell death also required the activation of the PI 3-K and Akt pathways, suggesting that under some circumstances neuronal protection may require inhibition of mTOR to foster autophagy in conjunction with increased activity of the PI 3-K/Akt axis [149]. Other studies also suggest that mTOR inhibition results in increased neuronal and vascular survival. Inhibition of PTEN (phosphatase and tensin homolog deleted on chromosome 10) has been demonstrated to lead to increased cerebral infarction that was associated with increased mTOR phosphorylation and activation [150]. In a canine model of subarachnoid hemorrhage, inhibition of mTOR signaling prevents cerebral vasospasm and preserves endothelial cell function [151]. Inhibition of mTOR and p70S6K activity also improves functional recovery in closed head injury models [152] (Figure 2). Rapamycin treatment that inhibits mTOR has been shown to promote autophagy, inhibit mTOR-mediated inflammation, reduce neural tissue damage, and limit locomotor impairment following spinal cord injury [153].
However, the role of the PI 3-K/Akt/mTOR pathway can be variable and may require activation to promote neuronal survival. Treatments that increased the expression of Raptor were associated with neuroprotection during hypoxia in invertebrate models of stroke [154]. In middle cerebral artery rat stroke models, agents that increase activity of Akt, mTOR, and p70S6K serve to reduce stroke size [155]. mTOR activity is also required in primary cerebral microglia [92] and in neurons [6] to prevent apoptotic cell death during oxygen-glucose deprivation. Activation of mTOR is also necessary for the cytokine EPO to prevent microglial cell death during ischemic insults [102]. Following spinal cord injury, improved spinal cord plasticity through exercise may require an increase in mTOR expression and increased p70S6K activity [156]. Axonal regeneration in the nervous system may require mTOR activation in conjunction with signal transducers and activators of transcription (STAT) pathways [157]. During loss of PTEN or TSC1 that allow for increased mTOR activity, axonal regeneration is increased in adult retinal ganglion cells and in corticospinal neurons after optic nerve injury and spinal cord injury respectively [158, 159]. In addition, ATP administration can significantly increase Akt/mTOR/p70S6K signaling that is accompanied by improved locomotor function following spinal cord injury [160]. Studies with bisperoxovanadium that can enhance the activities of Akt and mTOR have shown to reduce motor neuron death, increase tissue sparing, and reduce cavity formation after spinal cord injury in rats [161].
Conclusion and Future Perspectives
PI 3-K, Akt, and mTOR signaling offer exciting prospects to target neurodegenerative pathways for the development of new therapeutic avenues for disorders of the central nervous system. The PI 3-K/Akt/mTOR cascade is a vital component for determination of cell fate in a variety of acute and chronic disorders that can include HD, AD, PD, epilepsy, stroke, and trauma. Onset and progression of these neurodegenerative disorders can be the consequence of PCD pathways involving apoptosis and autophagy that can be modulated by PI 3-K, Akt, and mTOR signaling.
Currently, the United States Food and Drug Administration has approved several rapamycin (sirolimus) and rapamycin derivative compounds (“rapalogs”) for the treatment of renal cancer (everolimus, temsirolimus), allograft rejection (everolimus, sirolimus), subependymal giant cell astrocytoma associated with tuberous sclerosis (everolimus), neuroendocrine pancreatic tumors (everolimus), and prevention of vascular re-stenosis (sirolimus, zotarolimus, umirolimus) [6, 31]. Preclinical work suggests that modulation of the PI 3-K, Akt, mTOR axis can increase radiosensitivity against tumor cell growth and the vascular supply of tumors [108]. Early clinical trials using everolimus for the treatment of advanced neuroendocrine tumors suggest that progression free survival can be improved [162]. Agents that can regulate the PI 3-K, Akt, mTOR axis are also under investigation in early phase trials to treat hematological malignancies in children and adults [163, 164]. In addition, agents that can target different Akt classes with the alkyl-lysophospholipids and small molecule inhibitors of Akt as well as combined targeting of mTORC1 and mTORC2 with or without PI 3-K inhibition, and strategies that focus upon PDK1 and eIF4E are under consideration for disorders that can involve the central nervous system, breast, hematological tumors, and solid tumors.
Yet, for the effective translation of these cellular targets into robust clinical entities directed against neurodegenerative disorders over the next 5 to 10 years, a number of questions that have arisen from current investigations need to be addressed. For example, what conditions in the nervous system allow apoptosis and autophagy to work in concert or remain as independent pathways? Exposure to oxidative stress in neurons results in apoptotic cell death that can be averted through PI 3-K and Akt pathways, but the targeting of autophagic pathways in primary neurons during oxidative stress may yield limited benefits [80], suggesting that in some cases neurodegenerative pathways may proceed only through one specific PCD pathway. In contrast, other experimental models suggest that apoptosis may directly control and block the onset and progression of autophagy [79]. More complexities arise when one examines whether PCD pathways such as autophagy are beneficial or detrimental during neurodegenerative disorders. Autophagy can lead to cell death in cerebral astrocytes [70], in cortical neurons [71], and in spinal cord motor neurons [72] during oxidant stress. As a result, autophagy should be averted in these conditions. On the flip side, autophagy pathways may be necessary with inhibition of mTOR signaling to clear mutant huntingtin in HD [124] and to improve cognition and limit Aβ levels in AD [76].
Are these variable outcomes with the pathways of PCD intimately tied to the complex relationship among PI 3-K, Akt, and mTOR? Increased phosphorylated substrates of Akt, mTOR, GSK-3β, and tau protein have been linked to the progression of AD progression [130]. Loss of mTOR activity that allows for the induction of autophagy in experimental models of AD has been shown to improve memory and reduce Aβ levels [76]. However, other work suggests that the degree and duration of the presence of the PI 3-K/Akt/mTOR cascade may be a vital factor for cell protection. Inhibition of mTOR can impair long-term potentiation and synaptic plasticity in models of AD [133] and activation of mTOR signaling can block inflammatory cell death during Aβ exposure [93]. Yet, excessive activation of mTOR may lead to dyskinesia in PD patients [142]. Evidence that supports the need for a fine biological control of pathways such as mTOR also can be found in other systems of the body. Acute activation of mTOR can prevent cardiac cell death [165], but long-term mTOR expression and activity can result in vascular injury [166]. To successfully move forward into the clinical realm for neurodegenerative disorders with PI 3-K, Akt, and mTOR, future studies over the next several years must continue to elucidate the complex roles that these kinase pathways hold in the nervous system and during the PCD pathways of apoptosis and autophagy.
Executive Summary.
PI 3-K
Phosphatidylinositide 3-kinase (PI 3-K) is a family of lipid kinases that phosphorylate the 3′-hydroxyl group of phosphatidylinositide and phosphoinositides. PI 3-K phosphorylates membrane lipids to play a key role in Akt activation.
Akt
Activation of protein kinase B (Akt) is dependent upon PI 3-K that can phosphorylate membrane lipids and foster the transition of Akt from the cytosol to the plasma membrane to facilitate Akt phosphorylation at serine473 and threonine308 by phosphoinositide dependent kinase (PDK) 1 and PDK2 respectively.
mTOR
Mammalian target of rapamycin (mTOR), a 289 kDa, serine/threonine protein kinase, belongs to PI 3-K related kinase family and can be activated through PI 3-K and Akt signaling pathway. mTOR functions through two complexes, mTORC1 and mTORC2, that are composed of mTOR and its regulatory proteins.
mTORC1 contains mTOR, regulatory-associated protein of mTOR (Raptor), proline rich Akt substrate 40 kDa (PRAS40), mammalian lethal with Sec13 protein 8 (mLST8), and DEP-domain-containing mTOR-interacting protein (Deptor). mTORC1 is sensitive to rapamycin and plays a key role in protein synthesis.
mTORC2 contains mTOR, mLST8, Deptor, rapamycin-insensitive companion of mTOR (Rictor), mammalian stress-activated protein kinase interacting protein (mSIN1), and protein observed with Rictor-1 (Protor-1). mTORC2 is more resistance to rapamycin and primarily functions to regulate cytoskeleton organization.
Huntington’s disease (HD)
Inhibition of mTOR signaling by rapamycin can promotes the clearance of mutant huntingtin aggregates.
Alzheimer’s disease (AD)
Phosphorylated Akt, mTOR and GSK-3β are increased in AD, suggesting a potential link for these kinases with the progression of AD.
mTOR with increased phosphorylation of p70S6K promotes Tau phosphorylation in neurons during AD.
mTOR may prevent neuronal atrophy and block β-amyloid induced neurotoxicity.
Parkinson’s disease (PD)
Increased Akt and mTOR signaling protects dopaminergic neurons in PD.
Oxidative stress induced mTOR inhibition is associated with dopaminergic degeneration.
Akt and mTOR may potentiate dyskinesia and limit the autophagic clearance of toxic α-synuclein.
Epilepsy
Inhibition of mTOR reduces seizures in tuberous sclerosis and prevents the development of spontaneous recurrent seizure in acquired epilepsy.
Acute Central Nervous System Injury
mTOR prevents apoptosis during ischemia in neurons and astrocytes.
Inhibition of mTOR may be required to prevent vasospasm and preserve endothelial function following subarachnoid hemorrhage
Inhibition of mTOR improves functional recovery following traumatic brain injury.
Conclusions
PI 3-K, Akt, and mTOR signaling offer exciting prospects to target neurodegenerative pathways for the development of new therapeutic avenues for disorders of the central nervous system.
Future studies must continue to elucidate the complex roles that PI 3-K, Akt, and mTOR hold in the nervous system and during apoptosis and autophagy to successfully translate these kinase targets into effective and safe therapeutic strategies for neurodegenerative disease.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Financial and competing interest disclosure: The authors have no financial or competing interests to disclosure.
References
- 1.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22(11):1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Ruiz P, Rodriguez-Ubreva J, Cariaga AE, Cortes MA, Colas B. SHP-1 in cell-cycle regulation. Anticancer Agents Med Chem. 2011;11(1):89–98. doi: 10.2174/187152011794941154. [DOI] [PubMed] [Google Scholar]
- 3.Chong ZZ, Li F, Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20(1):299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Z, White MF. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal. 2011;14(4):649–661. doi: 10.1089/ars.2010.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 6.Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010;3(6):374–391. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X, Zhang H, Yang J, et al. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40(1):193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong ZZ, Maiese K. Mammalian Target of Rapamycin Signaling in Diabetic Cardiovascular Disease. Cardiovasc Diabetol. 2012;11(1):45. doi: 10.1186/1475-2840-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds THT, Bodine SC, Lawrence JC., Jr Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem. 2002;277(20):17657–17662. doi: 10.1074/jbc.M201142200. [DOI] [PubMed] [Google Scholar]
- 10.Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC., Jr Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci U S A. 1998;95(13):7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soliman GA, Acosta-Jaquez HA, Dunlop EA, et al. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem. 2010;285(11):7866–7879. doi: 10.1074/jbc.M109.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekim B, Magnuson B, Acosta-Jaquez HA, Keller JA, Feener EP, Fingar DC. mTOR Kinase Domain Phosphorylation Promotes mTORC1 Signaling, Cell Growth, and Cell Cycle Progression. Mol Cell Biol. 2011;31(14):2787–2801. doi: 10.1128/MCB.05437-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012 doi: 10.1016/j.pneurobio.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loewith R, Jacinto E, Wullschleger S, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10(3):457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 15.Chong ZZ, Shang YC, Maiese K. Cardiovascular Disease and mTOR Signaling. Trends Cardiovasc Med. 2011;21(5):151–155. doi: 10.1016/j.tcm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28(10):727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 17.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 18.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Hara K, Inoue H, et al. Carboxyl-terminal region conserved among phosphoinositide-kinase-related kinases is indispensable for mTOR function in vivo and in vitro. Genes Cells. 2000;5(9):765–775. doi: 10.1046/j.1365-2443.2000.00365.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci U S A. 1995;92(11):4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham RT. mTOR as a positive regulator of tumor cell responses to hypoxia. Current topics in microbiology and immunology. 2004;279:299–319. doi: 10.1007/978-3-642-18930-2_18. [DOI] [PubMed] [Google Scholar]
- 22.Takahara T, Hara K, Yonezawa K, Sorimachi H, Maeda T. Nutrient-dependent multimerization of the mammalian target of rapamycin through the N-terminal HEAT repeat region. J Biol Chem. 2006;281(39):28605–28614. doi: 10.1074/jbc.M606087200. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Zhang Q, Wen Q, et al. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012;24(1):17–24. doi: 10.1016/j.cellsig.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282(27):20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Sarbassov DD, Ali SM, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11(4):895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 26.Peterson TR, Laplante M, Thoreen CC, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137(5):873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24(1):200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25(4):209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 29.Gingras AC, Kennedy SG, O’leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12(4):502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhandari BK, Feliers D, Duraisamy S, et al. Insulin regulation of protein translation repressor 4E-BP1, an eIF4E-binding protein, in renal epithelial cells. Kidney Int. 2001;59(3):866–875. doi: 10.1046/j.1523-1755.2001.059003866.x. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10(11):868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 32.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6(11):1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 33.Rosner M, Fuchs C, Siegel N, Valli A, Hengstschlager M. Functional interaction of mammalian target of rapamycin complexes in regulating mammalian cell size and cell cycle. Hum Mol Genet. 2009;18(17):3298–3310. doi: 10.1093/hmg/ddp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dada S, Demartines N, Dormond O. mTORC2 regulates PGE2-mediated endothelial cell survival and migration. Biochem Biophys Res Commun. 2008;372(4):875–879. doi: 10.1016/j.bbrc.2008.05.154. [DOI] [PubMed] [Google Scholar]
- 35.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 36.Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J. 2011;436(1):169–179. doi: 10.1042/BJ20102103. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416(3):375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 38.Gulhati P, Bowen KA, Liu J, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71(9):3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernandez-Negrete I, Carretero-Ortega J, Rosenfeldt H, et al. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem. 2007;282(32):23708–23715. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- 40.Zou ZQ, Zhang LN, Wang F, Bellenger J, Shen YZ, Zhang XH. The novel dual PI3K/mTOR inhibitor GDC-0941 synergizes with the MEK inhibitor U0126 in non-small cell lung cancer cells. Mol Med Report. 2012;5(2):503–508. doi: 10.3892/mmr.2011.682. [DOI] [PubMed] [Google Scholar]
- 41.Lee G, Goretsky T, Managlia E, et al. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139(3):869–881. 881 e861–869. doi: 10.1053/j.gastro.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maiese K, Chong ZZ, Shang YC, Hou J. Novel Avenues of Drug Discovery and Biomarkers for Diabetes Mellitus. Journal of clinical pharmacology. 2011;51(2):128–152. doi: 10.1177/0091270010362904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Z, Liang J, Li J, et al. Physical association of PDK1 with AKT1 is sufficient for pathway activation independent of membrane localization and phosphatidylinositol 3 kinase. PLoS ONE. 2010;5(3):e9910. doi: 10.1371/journal.pone.0009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glidden EJ, Gray LG, Vemuru S, Li D, Harris TE, Mayo MW. Multiple site acetylation of Rictor stimulates mammalian target of rapamycin complex 2 (mTORC2)-dependent phosphorylation of Akt protein. J Biol Chem. 2012;287(1):581–588. doi: 10.1074/jbc.M111.304337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen JX, Tuo Q, Liao DF, Zeng H. Inhibition of Protein Tyrosine Phosphatase Improves Angiogenesis via Enhancing Ang-1/Tie-2 Signaling in Diabetes. Exp Diabetes Res. 2012;2012:836759. doi: 10.1155/2012/836759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deblon N, Bourgoin L, Veyrat-Durebex C, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012;165(7):2325–2340. doi: 10.1111/j.1476-5381.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7(2):95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14(16):1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saha AK, Xu XJ, Lawson E, et al. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59(10):2426–2434. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koshimizu T, Kawai M, Kondou H, et al. Vinculin functions as regulator of chondrogenesis. J Biol Chem. 2012;287(19):15760–15775. doi: 10.1074/jbc.M111.308072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu MJ, Chen YS, Huang HS, Ma MC. Erythropoietin alleviates post-ischemic injury of rat hearts by attenuating nitrosative stress. Life Sci. 2012;90(19–20):776–784. doi: 10.1016/j.lfs.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4(9):648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 53.Sato T, Nakashima A, Guo L, Tamanoi F. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem. 2009;284(19):12783–12791. doi: 10.1074/jbc.M809207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai SL, Tee AR, Short JD, et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173(2):279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91(2):243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 56.Dan HC, Adli M, Baldwin AS. Regulation of mammalian target of rapamycin activity in PTEN-inactive prostate cancer cells by I kappa B kinase alpha. Cancer Res. 2007;67(13):6263–6269. doi: 10.1158/0008-5472.CAN-07-1232. [DOI] [PubMed] [Google Scholar]
- 57.Lee DF, Kuo HP, Chen CT, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130(3):440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 58.Oshiro N, Takahashi R, Yoshino K, et al. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem. 2007;282(28):20329–20339. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, Harris TE, Lawrence JC., Jr Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem. 2008;283(23):15619–15627. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sancak Y, Thoreen CC, Peterson TR, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25(6):903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Kovacina KS, Park GY, Bae SS, et al. Identification of a proline-rich Akt substrate as a 14–3–3 binding partner. J Biol Chem. 2003;278(12):10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 62.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9(3):316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 63.Maiese K. The Many Facets of Cell Injury: Angiogenesis to Autophagy. Curr Neurovasc Res. 2012;9(2):1–2. doi: 10.2174/156720212800410911. [DOI] [PubMed] [Google Scholar]
- 64.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45(3):217–234. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13(7):805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gumy LF, Tan CL, Fawcett JW. The role of local protein synthesis and degradation in axon regeneration. Exp Neurol. 2010;223(1):28–37. doi: 10.1016/j.expneurol.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tatton NA. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson’s disease. Exp Neurol. 2000;166(1):29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- 68.Broe M, Shepherd CE, Milward EA, Halliday GM. Relationship between DNA fragmentation, morphological changes and neuronal loss in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2001;101(6):616–624. doi: 10.1007/s004010000337. [DOI] [PubMed] [Google Scholar]
- 69.Louneva N, Cohen JW, Han LY, et al. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am J Pathol. 2008;173(5):1488–1495. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin AP, Liu CF, Qin YY, et al. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy. 2010;6(6):738–753. doi: 10.4161/auto.6.6.12573. [DOI] [PubMed] [Google Scholar]
- 71.Wang JY, Xia Q, Chu KT, et al. Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: a widely used inhibitor of autophagy. J Neuropathol Exp Neurol. 2011;70(4):314–322. doi: 10.1097/NEN.0b013e31821352bd. [DOI] [PubMed] [Google Scholar]
- 72.Baba H, Sakurai M, Abe K, Tominaga R. Autophagy-mediated stress response in motor neuron after transient ischemia in rabbits. J Vasc Surg. 2009;50(2):381–387. doi: 10.1016/j.jvs.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 73.Canu N, Tufi R, Serafino AL, Amadoro G, Ciotti MT, Calissano P. Role of the autophagic-lysosomal system on low potassium-induced apoptosis in cultured cerebellar granule cells. J Neurochem. 2005;92(5):1228–1242. doi: 10.1111/j.1471-4159.2004.02956.x. [DOI] [PubMed] [Google Scholar]
- 74.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14(3):180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 75.Spencer B, Potkar R, Trejo M, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29(43):13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nopparat C, Porter JE, Ebadi M, Govitrapong P. The mechanism for the neuroprotective effect of melatonin against methamphetamine-induced autophagy. J Pineal Res. 2010 doi: 10.1111/j.1600-079X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 78.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17(2):268–277. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr Neurovasc Res. 2012;9(2):89–99. doi: 10.2174/156720212800410858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14(9):3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert opinion on therapeutic targets. 2012;16(2):167–178. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Troy CM, Akpan N, Jean YY. Regulation of caspases in the nervous system implications for functions in health and disease. Prog Mol Biol Transl Sci. 2011;99:265–305. doi: 10.1016/B978-0-12-385504-6.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14(5):219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75(3):207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 86.Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52(4):1173–1185. [PMC free article] [PubMed] [Google Scholar]
- 87.Wang S, Chong ZZ, Shang YC, Maiese K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012;9(1):20–31. doi: 10.2174/156720212799297137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res. 2011;8(3):220–235. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koh PO. Nicotinamide attenuates the ischemic brain injury-induced decrease of Akt activation and Bad phosphorylation. Neurosci Lett. 2011;498(2):105–109. doi: 10.1016/j.neulet.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 90.Dormond O, Madsen JC, Briscoe DM. The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem. 2007;282(32):23679–23686. doi: 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011;8(2):103–120. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19(2):263–272. [PMC free article] [PubMed] [Google Scholar]
- 93.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012;4(3):187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi KC, Kim SH, Ha JY, Kim ST, Son JH. A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem. 2010;112(2):366–376. doi: 10.1111/j.1471-4159.2009.06463.x. [DOI] [PubMed] [Google Scholar]
- 95•*.Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 Is an Integral Regulatory Component of Erythropoietin mTOR Signaling and Cytoprotection. PLoS ONE. 2012;7(9):e45456. doi: 10.1371/journal.pone.0045456. The study demonstrates a dominant role for cell survival by the PRAS40 pathway during oxidative stress that invokes apoptotic caspase activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr Neurovasc Res. 2012 doi: 10.2174/156720212803530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thedieck K, Polak P, Kim ML, et al. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One. 2007;2(11):e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pastor MD, Garcia-Yebenes I, Fradejas N, et al. mTOR/S6 kinase pathway contributes to astrocyte survival during ischemia. J Biol Chem. 2009;284(33):22067–22078. doi: 10.1074/jbc.M109.033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu X, Reiter CE, Antonetti DA, Kimball SR, Jefferson LS, Gardner TW. Insulin promotes rat retinal neuronal cell survival in a p70S6K-dependent manner. J Biol Chem. 2004;279:9167–9175. doi: 10.1074/jbc.M312397200. by a. [DOI] [PubMed] [Google Scholar]
- 100.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19(2):145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim J, Jung Y, Sun H, et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113(1):220–228. doi: 10.1002/jcb.23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011;8(4):270–285. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chong ZZ, Li F, Maiese K. Attempted Cell Cycle Induction in Post-Mitotic Neurons Occurs in Early and Late Apoptotic Programs Through Rb, E2F1, and Caspase 3. Curr Neurovasc Res. 2006;3(1):25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu Y, Ren QG, Zhang ZH, et al. Phospho-Rb mediating cell cycle reentry induces early apoptosis following oxygen-glucose deprivation in rat cortical neurons. Neurochem Res. 2012;37(3):503–511. doi: 10.1007/s11064-011-0636-6. [DOI] [PubMed] [Google Scholar]
- 105.Bhaskar K, Miller M, Chludzinski A, Herrup K, Zagorski M, Lamb BT. The PI3K-Akt-mTOR pathway regulates Abeta oligomer induced neuronal cell cycle events. Molecular neurodegeneration. 2009;4:14. doi: 10.1186/1750-1326-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baryawno N, Sveinbjornsson B, Eksborg S, Chen CS, Kogner P, Johnsen JI. Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma growth. Cancer Res. 2010;70(1):266–276. doi: 10.1158/0008-5472.CAN-09-0578. [DOI] [PubMed] [Google Scholar]
- 107.Chung CY, Park YL, Song YA, et al. Knockdown of RON Inhibits AP-1 Activity and Induces Apoptosis and Cell Cycle Arrest Through the Modulation of Akt/FoxO Signaling in Human Colorectal Cancer Cells. Dig Dis Sci. 2012;57(2):371–380. doi: 10.1007/s10620-011-1892-7. [DOI] [PubMed] [Google Scholar]
- 108.Fokas E, Yoshimura M, Prevo R, et al. NVP-BEZ235 and NVP-BGT226, dual phosphatidylinositol 3-kinase/Mammalian target of rapamycin inhibitors, enhance tumor and endothelial cell radiosensitivity. Radiat Oncol. 2012;7(1):48. doi: 10.1186/1748-717X-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Janku F, Wheler JJ, Westin SN, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012;30(8):777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamada E, Singh R. Mapping autophagy on to your metabolic radar. Diabetes. 2012;61(2):272–280. doi: 10.2337/db11-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. [Review] Anat Embryol. 1990;181(3):195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 112.Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120(2):159–162. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 113.Silva DF, Esteves AR, Oliveira CR, Cardoso SM. Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer’s disease. Curr Alzheimer Res. 2011;8(5):563–572. doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- 114.Kapoor V, Zaharieva MM, Das SN, Berger MR. Erufosine simultaneously induces apoptosis and autophagy by modulating the Akt-mTOR signaling pathway in oral squamous cell carcinoma. Cancer Lett. 2012;319(1):39–48. doi: 10.1016/j.canlet.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 115.Le XF, Mao W, Lu Z, Carter BZ, Bast RC., Jr Dasatinib induces autophagic cell death in human ovarian cancer. Cancer. 2010 doi: 10.1002/cncr.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Viola G, Bortolozzi R, Hamel E, et al. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem Pharmacol. 2012;83(1):16–26. doi: 10.1016/j.bcp.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu YT, Tan HL, Huang Q, Ong CN, Shen HM. Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy. 2009;5(6):824–834. doi: 10.4161/auto.9099. [DOI] [PubMed] [Google Scholar]
- 118.Yu L, Mcphee CK, Zheng L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465(7300):942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rong Y, Mcphee CK, Deng S, et al. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc Natl Acad Sci U S A. 2011;108(19):7826–7831. doi: 10.1073/pnas.1013800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jung CH, Jun CB, Ro SH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20(7):1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sarkar S, Ravikumar B, Rubinsztein DC. Autophagic clearance of aggregate-prone proteins associated with neurodegeneration. Methods Enzymol. 2009;453:83–110. doi: 10.1016/S0076-6879(08)04005-6. [DOI] [PubMed] [Google Scholar]
- 122.Berger Z, Ravikumar B, Menzies FM, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15(3):433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 123• *.Ravikumar B, Vacher C, Berger Z, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36(6):585–595. doi: 10.1038/ng1362. The study highlights the potential benefit of autophagy to remove toxic neuronal cellular protein aggregagates. [DOI] [PubMed] [Google Scholar]
- 124.Floto RA, Sarkar S, Perlstein EO, Kampmann B, Schreiber SL, Rubinsztein DC. Small molecule enhancers of rapamycin-induced TOR inhibition promote autophagy, reduce toxicity in Huntington’s disease models and enhance killing of mycobacteria by macrophages. Autophagy. 2007;3(6):620–622. doi: 10.4161/auto.4898. [DOI] [PubMed] [Google Scholar]
- 125.Roscic A, Baldo B, Crochemore C, Marcellin D, Paganetti P. Induction of autophagy with catalytic mTOR inhibitors reduces huntingtin aggregates in a neuronal cell model. J Neurochem. 2011;119(2):398–407. doi: 10.1111/j.1471-4159.2011.07435.x. [DOI] [PubMed] [Google Scholar]
- 126.Fox JH, Connor T, Chopra V, et al. The mTOR kinase inhibitor Everolimus decreases S6 kinase phosphorylation but fails to reduce mutant huntingtin levels in brain and is not neuroprotective in the R6/2 mouse model of Huntington’s disease. Molecular neurodegeneration. 2010;5:26. doi: 10.1186/1750-1326-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hyrskyluoto A, Reijonen S, Kivinen J, Lindholm D, Korhonen L. GADD34 mediates cytoprotective autophagy in mutant huntingtin expressing cells via the mTOR pathway. Exp Cell Res. 2012;318(1):33–42. doi: 10.1016/j.yexcr.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 128.Maiese K, Chong ZZ, Hou J, Shang YC. New strategies for Alzheimer’s disease and cognitive impairment. Oxid Med Cell Longev. 2009;2(5):279–289. doi: 10.4161/oxim.2.5.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4(6):e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Griffin RJ, Moloney A, Kelliher M, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93(1):105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- 131.An WL, Cowburn RF, Li L, et al. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. Am J Pathol. 2003;163(2):591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paccalin M, Pain-Barc S, Pluchon C, et al. Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22(4):320–326. doi: 10.1159/000095562. [DOI] [PubMed] [Google Scholar]
- 133.Ma T, Hoeffer CA, Capetillo-Zarate E, et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee ST, Chu K, Park JE, et al. Erythropoietin improves memory function with reducing endothelial dysfunction and amyloid-beta burden in Alzheimer’s disease models. J Neurochem. 2012;120(1):115–124. doi: 10.1111/j.1471-4159.2011.07534.x. [DOI] [PubMed] [Google Scholar]
- 135• *.Lafay-Chebassier C, Paccalin M, Page G, et al. mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer’s disease. J Neurochem. 2005;94(1):215–225. doi: 10.1111/j.1471-4159.2005.03187.x. The study provides early evidence that dysfunction in mTOR pathways may lead to neurodegeneration. [DOI] [PubMed] [Google Scholar]
- 136.Chano T, Okabe H, Hulette CM. RB1CC1 insufficiency causes neuronal atrophy through mTOR signaling alteration and involved in the pathology of Alzheimer’s diseases. Brain Res. 2007;1168:97–105. doi: 10.1016/j.brainres.2007.06.075. [DOI] [PubMed] [Google Scholar]
- 137.Malagelada C, Ryu EJ, Biswas SC, Jackson-Lewis V, Greene LA. RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson’s disease by a mechanism involving mammalian target of rapamycin inactivation. J Neurosci. 2006;26(39):9996–10005. doi: 10.1523/JNEUROSCI.3292-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Deyoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14–3–3 shuttling. Genes Dev. 2008;22(2):239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci. 2010;30(3):1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Imai Y, Gehrke S, Wang HQ, et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. Embo J. 2008;27(18):2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tain LS, Mortiboys H, Tao RN, Ziviani E, Bandmann O, Whitworth AJ. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat Neurosci. 2009;12(9):1129–1135. doi: 10.1038/nn.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142• *.Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Science signaling. 2009;2(80):ra36. doi: 10.1126/scisignal.2000308. The study illustrates the clinical need for fine biological control of cellular pathways such as mTOR that can be both beneficial as well as detrimental. [DOI] [PubMed] [Google Scholar]
- 143.Crews L, Spencer B, Desplats P, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 2010;5(2):e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Holmes GL, Stafstrom CE. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48(4):617–630. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 145.Waltereit R, Welzl H, Dichgans J, Lipp HP, Schmidt WJ, Weller M. Enhanced episodic-like memory and kindling epilepsy in a rat model of tuberous sclerosis. J Neurochem. 2006;96(2):407–413. doi: 10.1111/j.1471-4159.2005.03538.x. [DOI] [PubMed] [Google Scholar]
- 146.Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63(4):444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29(21):6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29(25):8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Balduini W, Carloni S, Buonocore G. Autophagy in hypoxia-ischemia induced brain injury. J Matern Fetal Neonatal Med. 2012;25 (Suppl 1):30–34. doi: 10.3109/14767058.2012.663176. [DOI] [PubMed] [Google Scholar]
- 150.Shi GD, Ouyang YP, Shi JG, Liu Y, Yuan W, Jia LS. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem Biophys Res Commun. 2011;404(4):941–945. doi: 10.1016/j.bbrc.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 151.Zhang W, Khatibi NH, Yamaguchi-Okada M, et al. Mammalian target of rapamycin (mTOR) inhibition reduces cerebral vasospasm following a subarachnoid hemorrhage injury in canines. Exp Neurol. 2012;233(2):799–806. doi: 10.1016/j.expneurol.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 152.Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26(1):86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 153.Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E. Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma. 2012;29(5):946–956. doi: 10.1089/neu.2011.1919. [DOI] [PubMed] [Google Scholar]
- 154.Sheng B, Liu J, Li GH. Metformin preconditioning protects Daphnia pulex from lethal hypoxic insult involving AMPK, HIF and mTOR signaling. Comp Biochem Physiol B Biochem Mol Biol. 2012 doi: 10.1016/j.cbpb.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 155.Koh PO. Melatonin prevents ischemic brain injury through activation of the mTOR/p70S6 kinase signaling pathway. Neurosci Lett. 2008;444(1):74–78. doi: 10.1016/j.neulet.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 156.Liu G, Detloff MR, Miller KN, Santi L, Houle JD. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol. 2012;233(1):447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun F, Park KK, Belin S, et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480(7377):372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu K, Lu Y, Lee JK, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13(9):1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park KK, Liu K, Hu Y, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hu LY, Sun ZG, Wen YM, et al. ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats. Neuroscience. 2010;169(3):1046–1062. doi: 10.1016/j.neuroscience.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 161.Walker CL, Walker MJ, Liu NK, et al. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7(1):e30012. doi: 10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378(9808):2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 163.Barrett D, Brown VI, Grupp SA, Teachey DT. Targeting the PI3K/AKT/mTOR Signaling Axis in Children with Hematologic Malignancies. Paediatr Drugs. 2012 doi: 10.2165/11594740-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grzybowska-Izydorczyk O, Smolewski P. mTOR kinase inhibitors as a treatment strategy in hematological malignancies. Future Med Chem. 2012;4(4):487–504. doi: 10.4155/fmc.12.14. [DOI] [PubMed] [Google Scholar]
- 165.Hernandez G, Lal H, Fidalgo M, et al. A novel cardioprotective p38-MAPK/mTOR pathway. Exp Cell Res. 2011;317(20):2938–2949. doi: 10.1016/j.yexcr.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sinha SS, Pham MX, Vagelos RH, et al. Effect of rapamycin therapy on coronary artery physiology early after cardiac transplantation. Am Heart J. 2008;155(5):889 e881–886. doi: 10.1016/j.ahj.2008.02.004. [DOI] [PubMed] [Google Scholar]