Abstract
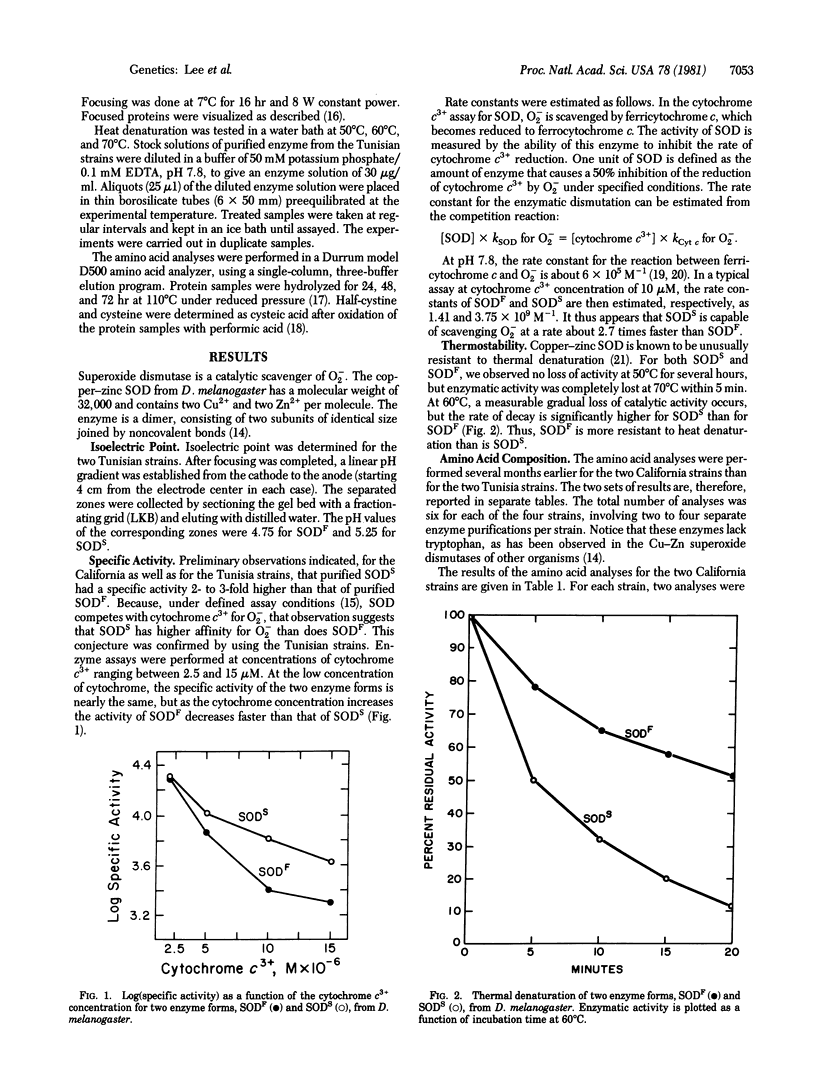
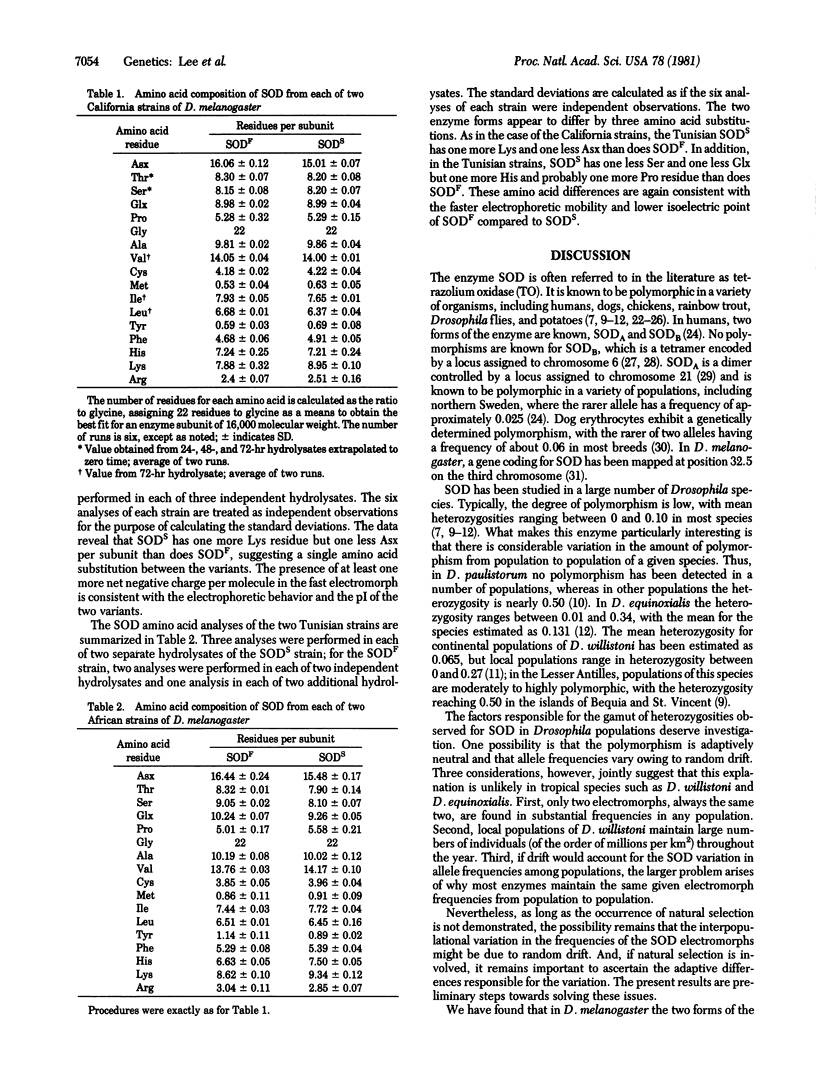
Superoxide dismutase (SOD; superoxide:superoxide oxidoreductase, EC 1.15.1.1) is known to be polymorphic in many organisms; in Drosophila, the degree of polymorphism has a wide range of variation from locality to locality within a given species. We have thoroughly purified from D. melanogaster the two common electromorphs, SODS and SODF. These differ in properties such as isoelectric point, specific activity, rate constant, thermostability, and amino acid composition. The specific activity is three times greater in SODS than in SODF, but the latter is more thermostable. In strains from California, SODS differs from SODF by at least one amino acid substitution: lysine in SODS is replaced by either aspartic acid or asparagine in SODF. This difference is consistent with the electrophoretic mobility and isoelectric points of the two electromorphs. In strains from Africa, SODS and SODF differ by two amino acid substitutions (histidine and proline in SODS vs. serine and either glutamic acid or glutamine in SODF) in addition to the one distinguishing the California strains. Thus the SODF electromorphs from California and from Tunisia, in spite of their identical electrophoretic mobility, differ by at least two amino acid substitutions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C. Aspects of polymorphism in man. Cold Spring Harb Symp Quant Biol. 1955;20:239-51; discussion, 251-5. doi: 10.1101/sqb.1955.020.01.023. [DOI] [PubMed] [Google Scholar]
- Ayala F. J., Powell J. R., Dobzhansky T. Polymorphisms in continental and island populations of Drosophila willistoni. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2480–2483. doi: 10.1073/pnas.68.10.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala F. J., Powell J. R., Tracey M. L. Enzyme variability in the Drosophila Willistoni group. V. Genic variation in natural populations of Drosophila equinoxialis. Genet Res. 1972 Aug;20(1):19–42. doi: 10.1017/s0016672300013562. [DOI] [PubMed] [Google Scholar]
- Ayala F. J., Powell J. R., Tracey M. L., Mourão C. A., Pérez-Salas S. Enzyme variability in the Drosophila willistoni group. IV. Genic variation in natural populations of Drosophila willistoni. Genetics. 1972 Jan;70(1):113–139. doi: 10.1093/genetics/70.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur E. W., Schorr R. T. Genetic polymorphism of tetrazolium oxidase in dogs. Science. 1969 Dec 19;166(3912):1524–1525. doi: 10.1126/science.166.3912.1524. [DOI] [PubMed] [Google Scholar]
- Brewer G. J. Achromatic regions of tetrazolium stained starch gels: inherited electrophoretic variation. Am J Hum Genet. 1967 Sep;19(5):674–680. [PMC free article] [PubMed] [Google Scholar]
- Butler J., Jayson G. G., Swallow A. J. The reaction between the superoxide anion radical and cytochrome c. Biochim Biophys Acta. 1975 Dec 11;408(3):215–222. doi: 10.1016/0005-2728(75)90124-3. [DOI] [PubMed] [Google Scholar]
- Creagan R., Tischfield J., Ricciuti F., Ruddle F. H. Chromosome assignments of genes in man using mouse-human somatic cell hybrids: mitochondrial superoxide dismutase (indophenol oxidase-B, tetrameric) to chromosome 6. Humangenetik. 1973 Dec 10;20(3):203–209. doi: 10.1007/BF00385731. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Fridovich I. On the stability of bovine superoxide dismutase. The effects of metals. J Biol Chem. 1973 Apr 25;248(8):2645–2649. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Friedman M. J., Trager W. The biochemistry of resistance to malaria. Sci Am. 1981 Mar;244(3):154-5, 158-64. doi: 10.1038/scientificamerican0381-154. [DOI] [PubMed] [Google Scholar]
- Harris H., Hopkinson D. A., Robson E. B. The incidence of rare alleles determining electrophoretic variants: data on 43 enzyme loci in man. Ann Hum Genet. 1974 Jan;37(3):237–253. doi: 10.1111/j.1469-1809.1974.tb01832.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McDonald J. F., Ayala F. J. Genetic and biochemical basis of enzyme activity variation in natural populations. I. Alcohol dehydrogenase in Drosophila melanogaster. Genetics. 1978 Jun;89(2):371–388. doi: 10.1093/genetics/89.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo E. Genetic variation in natural populations: patterns and theory. Theor Popul Biol. 1978 Feb;13(1):121–177. doi: 10.1016/0040-5809(78)90039-4. [DOI] [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. II. Preparative isoelectric focusing. Biochim Biophys Acta. 1975 Mar 28;386(1):181–195. doi: 10.1016/0005-2795(75)90258-5. [DOI] [PubMed] [Google Scholar]
- Richmond R. C. Enzyme variability in the Drosophila willistoni group. 3. Amounts of variability in the superspecies, D. paulistorum. Genetics. 1972 Jan;70(1):87–112. doi: 10.1093/genetics/70.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucknagel D. L. The genetics of sickle cell anemia and related syndromes. Arch Intern Med. 1974 Apr;133(4):595–606. [PubMed] [Google Scholar]
- Simic M. G., Taub I. A., Tocci J., Hurwitz P. A. Free radical reduction of ferricytochrome-C. Biochem Biophys Res Commun. 1975 Jan 20;62(2):161–167. doi: 10.1016/s0006-291x(75)80118-5. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Tischfield J., Ruddle F. H. The linkage of genes for the human interferon-induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J Exp Med. 1973 Feb 1;137(2):317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter F. M. Tetrazolium oxidase phenotypes of rainbow trout (Salmo gairdneri) and Pacific salmon (Oncorhynchus spp.). Comp Biochem Physiol B. 1971 Aug 15;39(4):891–895. doi: 10.1016/0305-0491(71)90112-x. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973 Jul 10;248(13):4793–4796. [PubMed] [Google Scholar]
- van Someren H., Westerveld A., Hagemeijer A., Mees J. R., Meera Khan P., Zaalberg O. B. Human antigen and enzyme markers in man-Chinese hamster somatic cell hybrids: evidence for synteny between the HL-A, PGM3, ME1, and IPO-B loci. Proc Natl Acad Sci U S A. 1974 Mar;71(3):962–965. doi: 10.1073/pnas.71.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]



