Abstract
For over 15 years, reproductive toxicologists have explored the physiological outcomes and mechanism of fetal phthalate exposure to determine the risk posed to human male reproductive health. This review examines the fetal male reproductive system response to phthalate exposure across species including rat, mouse, and human, with emphasis on the testis. In the rat, in utero phthalate exposure causes male reproductive tract malformations, in large part, by targeting the testis and inhibiting fetal Leydig cell hormone production. Despite mouse phthalate pharmacokinetics being similar to the rat, inhibition of fetal Leydig cell hormone synthesis is not observed in the mouse. The species-specific differences in testicular response following in utero phthalate exposure and the discordant reaction of the rodent fetal testis when exposed to phthalates ex vivo versus in vivo have made determining risk to humans difficult, yet critically important. The recent use of fetal testis xenotransplants to study phthalate toxicity suggests that the human fetal testis responds like the mouse fetal testis; it appears refractory to phthalate-induced inhibition of testosterone production. Although this result is unfulfilling from the perspective of identifying environmental contributions to human reproductive maldevelopment, it has important implications for phthalate risk assessment.
Key Words: phthalates, endocrine disruptors, testicular dysgenesis syndrome, Leydig, fetal testis
SCOPE OF THE REVIEW
Human male reproductive malformations resulting from improper in utero masculinization are relatively common, and phthalate exposure is a suspected contributor to these malformations (Sharpe and Skakkebaek, 2008; Swan, 2008; Toppari et al., 2010). The recent publication of two reports demonstrating that human fetal testis xenografts are resistant to phthalate-induced endocrine disruption precipitated this review (Heger et al., 2012; Mitchell et al., 2012). Our goals are to first discuss the modes of phthalate fetal testis toxicity using data obtained with the rat model and then explore the conservation of these toxicity outcomes and mechanisms in a species resistant to phthalate endocrine disruption (mouse) and human. Despite a literature on nonreproductive phenotypes associated with phthalate exposure, mechanisms and phenotypes of phthalate toxicity may differ between various organs and even different developmental stages of the same organ. As such, this review is limited to discussing mechanistic toxicology data derived from phthalate exposure of the maternofetal unit or tissue derived from the fetus, with a focus on the fetal testis.
TESTICULAR DYSGENESIS SYNDROME HYPOTHESIS
A human Testicular Dysgenesis Syndrome (TDS) hypothesis has been proposed, suggesting that the postnatal phenotypes of hypospadias, cryptorchidism, testis germ cell cancer, and poor semen quality are manifestations of a single underlying entity: aberrant fetal testis development (Sharpe and Skakkebaek, 2008). According to the TDS hypothesis, a combination of genetic and environmental factors may compromise Leydig cell and seminiferous cord function, leading to altered fetal testis development. Because hormones (insulin-like 3 and testosterone) produced by fetal Leydig cells are crucial for formation and postnatal function of the male reproductive system, the TDS hypothesis emphasizes the central role of the fetal Leydig cell in the syndrome (Sharpe and Skakkebaek, 2008). One animal model used to test the TDS hypothesis is rat exposure to the chemical class known as phthalates. With the exception of testicular germ cell cancer, the hallmarks of human TDS are mimicked in rats exposed in utero to phthalates.
PHTHALATES, HUMAN EXPOSURE, AND MALE REPRODUCTIVE SYSTEM EPIDEMIOLOGY
Phthalates are high production volume chemicals used to impart flexibility, durability, transparency, and longevity to a variety of consumer, industrial, and medical products, including electronics, medical devices, children’s toys, detergents, pharmaceuticals, paints, waxes, personal care products, cosmetics, and food packaging, among others (Heudorf et al., 2007). Because phthalates are not covalently bound in these products, they can leach with product age, use, and ultraviolet light exposure, making them available for biological exposure (Thomas and Thomas, 1984). Human phthalate exposure occurs daily and is ubiquitous, with the major exposure route being ingestion (Fromme et al., 2007). Median phthalate exposure levels across the human population for individual congeners approximate 1–10 μg/kg/day (Latini et al., 2004; Wittassek et al., 2011), although critically ill neonates receiving intensive medical treatment can be exposed to up to 10–20mg/kg/day of a phthalate ester through the use of polyvinyl chloride–based medical devices (Loff et al., 2000).
Formed from phthalic acid derivatives from the oxidation and hydrolysis of naphthalene (Eckerle, 2001), phthalates consist of a six-carbon aromatic ring, containing two adjacent ester groups with alkyl side chains attached to the terminal oxygen. In the rat fetus, not all phthalate congeners are reproductive toxicants; the structure-toxicity relationship centers on alkyl side chain lengths between three and nine carbon atoms, with dipentyl phthalate being the most potent (Hannas et al., 2011b, 2011c). Finally, all reproductively toxic phthalate congeners act via the same mechanism (Gray et al., 2000; Liu et al., 2005).
Following oral exposure, phthalate diesters are quantitatively metabolized in the gut to a monoester form and subsequently oxidized, glucuronidated, and excreted in the urine (Williams and Blanchfield, 1975). Phthalate monoesters may be the ultimate toxic metabolite (Ema and Miyawaki, 2001), although this has not been demonstrated conclusively for fetal testis endocrine disruption. After oral phthalate diester gavage of a pregnant rat, phthalate monoester rapidly crosses the placenta, and maximal fetal plasma concentrations are achieved between 1 and 2h postdosing (Clewell et al., 2008; Fennell et al., 2004). Like its distribution kinetics, the phthalate excretion rate is also rapid; the phthalate monoester half-life in maternal and fetal rat plasma is about 5h (Fennell et al., 2004).
Epidemiology data linking in utero human phthalate exposure to male reproductive tract demasculinization or malformations are limited and somewhat inconsistent; for a review, see Jurewicz and Hanke (2011). These types of studies are difficult to perform because of the need to examine phthalate exposure during the critical window of male reproductive tract masculinization (presumed to be gestational weeks 8–14; Welsh et al., 2008), the relatively low level of human phthalate exposure in pregnant women, and the lack of access to sensitive molecular endpoints during the masculinization window. Because increased male anogenital distance (AGD), testis descent, and the positioning of the urethral opening at the phallus tip require masculinization during the male programming window (van den Driesche et al., 2011; Welsh et al., 2008), these endpoints are the most relevant gross morphology measurements available in the human. To date, one study found a positive association between maternal urine phthalate levels during pregnancy and cryptorchidism (Swan, 2008), but such an association was not seen in another report (Main et al., 2006). Using an occupational exposure model, the risk of hypospadias was correlated with maternal exposure to phthalates in the workplace (Ormond et al., 2009). In two independent studies, phthalate levels in urine from pregnant women have shown significant inverse correlations with AGD of their male offspring (Suzuki et al., 2011; Swan et al., 2005), but this observation was not confirmed by others (Huang et al., 2009). For all of these studies, phthalate exposure measurements were not limited to the putative masculinization programming window. In the study by Swan et al. (Swan, 2008; Swan et al., 2005), a significant contributor to the AGD effect was diethyl phthalate, but this phthalate congener is not active in rat exposure studies (Gray et al., 2000). Exploring the neonatal maternofetal unit, Main et al. (2006) observed an inverse correlation between phthalate levels in human breast milk and serum-free testosterone in their suckling males, as well as a positive correlation between breast milk phthalate levels and serum leutinizing hormone/testosterone ratios. However, these neonatal data may not reflect the biology of the human fetal Leydig cell. Despite their inconsistency, lack of phthalate exposure analysis during the critical developmental window, and correlative nature, available epidemiological data raise some concern about a possible link between human phthalate exposure and male reproductive tract malformations.
PHTHALATE IN UTERO REPRODUCTIVE TOXICITY IN THE RAT
Reproductive Malformations and Proximal Mode of Action
Although the male reproductive toxicity of postnatal phthalate exposure had been known for decades, it was not until 1997 that the more sensitive time period of fetal reproductive development was first appreciated (Wine et al., 1997). Since this initial report, numerous publications from a number of labs have demonstrated in utero phthalate exposure of rats during late gestation produces a phenotype termed the “phthalate syndrome” (Foster, 2006). Hallmarks of fetal male rat phthalate exposure of 500mg/kg/day or greater include poor Wolffian duct differentiation, decreased AGD, retained nipples, cryptorchidism, and hypospadias. These hallmarks result from phthalate-induced inhibition of fetal testis hormone (insulin-like 3 and testosterone) production (Howdeshell et al., 2008; Parks et al., 2000; Wilson et al., 2004) and point to the fetal Leydig cell as the critical cellular target for manifesting gross extratesticular reproductive malformations following in utero phthalate exposure.
Dose Response and Dose Additivity
As mentioned above, reproductively toxic phthalate congeners share a similar mode of action in rats. When pregnant rats are exposed to combinations of reproductively toxic phthalate congeners, the doses are additive in producing reductions in fetal testicular hormone production and reproductive lesions (Howdeshell et al., 2007). Focusing on a dibutyl phthalate (DBP) dose response in Sprague Dawley rats as a case study, significant reductions in gestational day (GD) 19 intratesticular testosterone occur at dose levels ≥ 50mg/kg/day, with a no-observed-effect level of 33mg/kg/day (Lehmann et al., 2004). The DBP dose-response of reproductive malformations in Sprague Dawley rats varies with the endpoint. The most dose-sensitive malformation is nipple retention (≥100mg/kg/day; Mylchreest et al., 2000), and statistically significant induction of other malformations requires dose levels ≥500mg/kg/day (Mylchreest et al., 1998, 2000). Christiansen et al. (2010) reported decreased AGD, mild genital tubercle dysgenesis, and retained nipples in male Wistar rats at a di(2-ethylhexyl) phthalate dose level of 10mg/kg/day. In addition to the lack of dose-response consistency reported by others, caveats with these data are the lack of a linear dose response and the inconsistent observations between the two studies reported by Christiansen et al. (2010). In comparing Wistar and Sprague Dawley rats, Wistar rats show a higher incidence of cryptorchidism and Sprague Dawley a higher incidence of epididymal agenesis (Wilson et al., 2007). However, these strains have similar DBP dose responses for inhibition of fetal testis testosterone production (Hannas et al., 2011c), suggesting the strain-dependent lesion incidences may result from differential androgen signaling requirements in target tissues.
Critical Exposure Window for Reproductive Malformations
To observe phthalate-induced reproductive malformations in the rat, in utero phthalate exposure is required for only a short window. Rat fetal testis testosterone production commences between GD15 and GD16 and reaches a peak at GD18 (Habert and Picon, 1984), and the amount of testosterone produced as a function of fetal body weight remains relatively constant from GD18 until parturition (Welsh et al., 2008). The critical phthalate exposure window for reproductive tract lesion development encompasses approximately GD16–18 (Carruthers and Foster, 2005; Ema et al., 2000). This window was identified by exposing rats in utero, using 2- or 3-day exposure windows, and measuring AGD, nipple retention, cryptorchidism, and Wolffian duct malformations as endpoints. Using the androgen receptor antagonist flutamide, male rat reproductive tract development (increased AGD, Wolffian duct development, testis descent, and urethral closure) was shown to require androgen receptor signaling during a nearly identical window from GD15 to 18 (Welsh et al., 2008). The congruence of the flutamide and DBP data provides compelling evidence for the critical window for phthalate-induced, androgen-dependent reproductive tract malformations being from GD16 to 18. Although there is a critical in utero window for reproductive tract malformations, phthalates inhibit fetal testis testosterone production during the entire fetal period when the testis is highly steroidogenic (Hannas et al., 2011a; Parks et al., 2000; Plummer et al., 2007; Scott et al., 2008; Thompson et al., 2004, 2005). Therefore, the malformation critical window reflects the requirement of peripheral tissues for sufficient testis hormone signaling to guide their development and not susceptibility of the fetal testis to phthalate-induced steroidogenic inhibition.
FETAL TESTIS HISTOPATHOLOGY
Seminiferous Cord Alterations and Multinucleated Germ Cells
In utero phthalate exposure alters seminiferous cord development resulting in focal, dysgenetic cords with intracordal Leydig cells and, more generally, cords with larger diameters harboring a large number of multinucleated germs cells (MNGs). MNGs are of particular interest because of their theoretical potential to undergo mutations (given their abnormal DNA content) and thus the potential to give rise to testis germ cell cancer, one of the hallmark features of TDS.
Most of the rat studies performed to date examining phthalate-induced seminiferous cord effects and MNG formation have involved continuous daily exposure of the pregnant rat, often beginning mid-gestation (Andrade et al., 2006; Barlow and Foster, 2003; Boekelheide et al., 2009; Ferrara et al., 2006; Fisher et al., 2003; Johnson et al., 2008; Kleymenova et al., 2005; Mahood et al., 2007; Mylchreest et al., 2002; Parks et al., 2000; Scott et al., 2007; Shirota et al., 2005; Struve et al., 2009). Overall, the phthalate-exposed fetal testis shows evidence of delayed maturation, being small with less cellular proliferation resulting in fewer cells, and with seminiferous cords of increased diameter containing centrally located germ cells (Boekelheide et al., 2009). The MNGs that are formed typically have 2–4 nuclei, but may have as many as 13 nuclei (Barlow and Foster, 2003), all contained within a common cytoplasm. In the rat, MNG formation increases at DBP dose levels approximating fetal testicular hormone disruption; there is a trend after 20mg/kg/day DBP and a significant increase following 100mg/kg/day exposure (Boekelheide et al., 2009; Mahood et al., 2007). The daily gestational exposure studies have documented a sensitive developmental window for phthalate induction of MNGs with these abnormal cells appearing at approximately GD18 in the rat (Barlow and Foster, 2003; Ferrara et al., 2006; Kleymenova et al., 2005). Induction of MNGs can also be achieved with short-term phthalate exposure during the vulnerable window (Ferrara et al., 2006), which coincides with the time when germ cell proliferation ceases (Boulogne et al., 1999) and intercellular bridges develop between germ cells (Franchi and Mandl, 1964; Nagano and Suzuki, 1978). Theoretically, MNGs could arise either from nuclear division without cytoplasmic division, or from the collapse of intercellular bridges. Given the ability of phthalates to induce MNGs by a single exposure during a time when the germ cells are not proliferating, the most logical conclusion is that MNGs form from the opening of intercellular bridges (Kleymenova et al., 2005). However, this needs to be investigated further. Once MNGs are formed, they persist throughout late gestation and early postnatal life and are then eliminated in a p53-dependent manner from the seminiferous epithelium within 1–2 weeks postnatally (Barlow and Foster, 2003; Fisher et al., 2003).
The seminiferous cord manifestations of delayed maturation require mid-gestation phthalate exposure are present only transiently in late gestation and early postnatal life, and then largely resolve by adulthood (Barlow and Foster, 2003; Boekelheide et al., 2009; Ferrara et al., 2006). There may be persistent later-life abnormalities, depending on the dose of phthalate exposure and the presence of other abnormalities, such as cryptorchidism or epididymal agenesis, that produce secondary effects on the testis. Although peritubular myoid or mesenchymal cells may be the initial phthalate target cells (see below; Johnson et al., 2007), Sertoli cells are the apparent target for phthalate-induced effects on the seminiferous cords, manifesting immaturity and alterations in their apical processes, cytoskeleton, and interactions with germ cells (Fisher et al., 2003; Kleymenova et al., 2005).
After rat in utero phthalate exposure, focal areas of malformed, anastomosing seminiferous tubules are observed in postnatal testes. In normal fetal rat testes, Sertoli cells segregate from interstitial Leydig cells and reside within well-defined seminiferous cords by GD14 (Magre and Jost, 1980). Although this normal cord formation process also occurs in most areas of phthalate-exposed rat fetal testes, a small number of Sertoli cells become intermingled within large, centrally located interstitial Leydig cell aggregates (Hutchison et al., 2008b; Mahood et al., 2005; van den Driesche et al., 2012a). Although not shown formally, peritubular myoid cells may be present as well, and these abnormal aggregates appear to be the antecedent to the dysgenetic seminiferous tubules present in adult testes of animals exposed in utero. Upon formation of seminiferous cords by the aberrantly intermingled cell types in neonatal testes (Hutchison et al., 2008b), Leydig cells become entrapped and persist within the dysgenetic seminiferous cords through adulthood (Mahood et al., 2005).
Cross-species studies of phthalate exposure have been important in interpreting the possible targets and modes of action within the testis. In the mouse, phthalate exposure results in the formation of MNGs and enlarged seminiferous cords without the induction of an antiandrogenic effect (Gaido et al., 2007). This result is neither due to an inability to metabolize phthalate diester to the active phthalate monoester, nor due to an inability to deliver the active monoester to the fetus (Gaido et al., 2007). Although the lack of an antiandrogenic effect of phthalate exposure in mice is discussed in greater detail below, what is important to emphasize here is the common occurrence of seminiferous cord effects, including cord enlargement and the induction of MNGs, in both rat and mouse. The lack of MNG induction in flutamide-exposed rats or mice with defective androgen receptor activity underscores the mechanistic separation of this endpoint from intratesticular testosterone reductions (Scott et al., 2007). These results indicate a shared seminiferous cord target for phthalate effects in mice and rats, presumably in the Sertoli cell, and independence of at least some seminiferous cord effects from changes in Leydig cell function (Gaido et al., 2007; Scott et al., 2007).
A recent study has examined the role of p53 in modulating the in utero mouse response to phthalate exposure (Saffarini et al., 2011). Pregnant p53-deficient mice were exposed to phthalate from GD12 to 20, and then the number of MNGs was quantified at GD19, and on postnatal days 1, 4, 7, and 10. The absence of the proapoptotic gene p53 led to the formation of increased numbers of MNGs, and these cells persisted into adulthood as bizarre abnormal cells in the seminiferous tubules. Ultimately, all of these abnormal cells disappeared, but this was shown to take in excess of 200 days in some animals. The modulating effect of p53 on MNG survival is striking, and a useful tool for exploring the behavior of these unusual cells and their possible role in germ cell cancer.
Sertoli Cell Histopathology
In addition to abnormal interstitial positioning of a Sertoli cell subset, phthalate exposure alters fetal Sertoli cell development at the cellular level. Phthalate exposure (500mg/kg/day) reduces the percentage of proliferating GD21 rat Sertoli cells by ~40% and the absolute number of Sertoli cells per testis by ~50% (Hutchison et al., 2008a; Scott et al., 2008). Because fetal Sertoli cell proliferation depends on Leydig cell testosterone output (Scott et al., 2007), the paucity of Sertoli cell proliferation and numbers is mechanistically downstream of Leydig cell steroidogenic inhibition. Although GD16–18 is the susceptibility window for reproductive tract malformations, the critical phthalate exposure window for Sertoli cell proliferation effects is between GD19 and GD21 (Scott et al., 2008). Coincident with reduced proliferation, Sertoli cells within seminiferous cords exhibit retracted cytoplasmic processes and no longer surround or support centrally located germ cells (Kleymenova et al., 2005). All of these Sertoli cell changes normalize in the postnatal animal after phthalate withdrawal (Auharek et al., 2010; Kleymenova et al., 2005).
Leydig Cell Histopathology
As mentioned above, fetal Leydig cells from phthalate-exposed rats exhibit abnormal aggregation into large clusters located in the central part of the testis. This histological observation, coupled with immunoexpression of a proliferative marker, initially provided evidence to suggest an increased proliferation of Leydig cells (Mylchreest et al., 2002), but stereological quantification showed phthalate exposure has no significant effect on the number of fetal Leydig cells per testis (Mahood et al., 2005; van den Driesche et al., 2012b). As occurs when Leydig cell steroidogenesis is inhibited, phthalate exposure decreases Leydig cell cytoplasmic volume (van den Driesche et al., 2012b). As compared with phthalate exposure within a GD19–20 window, rat Leydig cell aggregation is enhanced by exposure from GD13 to 18 (Hutchison et al., 2008b), and aggregation is observed first on GD17 (Barlow and Foster, 2003).
MODE OF ACTION OF PHTHALATE-INDUCED FETAL LEYDIG CELL STEROIDOGENIC INHIBITION
From a human reproductive health perspective, the most important endpoint of in utero phthalate exposure is a decrease in fetal Leydig cell hormone production. Three main factors contribute to this concern: the ubiquity of human phthalate exposure during pregnancy (Adibi et al., 2008); the ability of rat in utero phthalate exposure to inhibit fetal testis hormone (testosterone and INSL3) production; and the fetal testis hormone production requirement for proper phallus development and testis descent. Our discussion of the phthalate endocrine disruption mode of action will begin with describing data on the phthalate molecular target and expand from there to events culminating in reduced testosterone output.
The phthalate molecular target causing fetal testis toxicity remains an enigma. Data from one study indicate that phthalate monoesters do not readily enter a juvenile mouse Sertoli cell line (Kristensen et al., 2011), suggesting the phthalate molecular target may reside at the plasma membrane or that low intracellular phthalate concentrations are sufficient to cause endocrine disruption. Some phthalate monoesters can bind to intracellular peroxisome proliferator–activated receptors (PPARs) to cause hepatoxicity (Rusyn and Corton, 2011). PPAR activation also has been hypothesized to mediate phthalate reproductive toxicity (Corton and Lapinskas, 2005; Latini et al., 2006), but to date, no data using fetal Leydig cells have been generated to support this hypothesis. In contrast, in utero rat exposure to the PPARα agonist Wy-14,643 or the PPARγ agonist rosiglitazone does not reduce fetal testis steroidogenic gene expression or testosterone production (Boberg et al., 2008; Hannas et al., 2011b). Furthermore, PPARγ mRNA is undetectable in fetal rat testis (Hannas et al., 2011b). Overall, the available information suggests that PPAR activation is not a critical component of fetal testis endocrine disruption following acute phthalate exposure.
In the rat, a cascade of fetal testis gene expression changes has been characterized using microarray-based gene profiling (Thompson et al., 2005). The earliest gene changes, observed approximately 1h after 500mg/kg phthalate exposure, include the increase of a number of mRNAs encoding “immediate early genes” responsive to numerous environmental stressor exposures (Johnson et al., 2007; Thompson et al., 2005). The kinetics of these early gene changes tracks phthalate metabolite levels in the fetus (Thompson et al., 2005; Fennell et al., 2004). Within hours of exposure, multiple fetal testis cell types respond. By in situ hybridization, the earliest expression changes occur in populations of cells located throughout the interstitium and in peritubular myoid cells (Johnson et al., 2007). These data suggest that mesenchymal and peritubular myoid cells may be the initial phthalate target cells and, theoretically, functional changes in these cell populations could lead to functional perturbations of Leydig, Sertoli, and germ cells.
The gene expression response of Leydig cells first appears by 3h of 500mg/kg phthalate exposure (Johnson et al., 2007). Fetal Leydig cells exhibit a high rate of lipid metabolism, which is required to both synthesize and import the testosterone precursor cholesterol. mRNA expression of genes in these pathways are profoundly reduced beginning 3–6h following 500mg/kg DBP exposure (Fig. 1; Johnson et al., 2011; Thompson et al., 2005). This apparently leads to diminished expression of nearly all steroidogenic pathway enzymes and cholesterol-mobilizing proteins, including those controlling the rate-limiting steps of mitochondrial cholesterol importation (STAR) and conversion of cholesterol to pregnenolone (CYP11A1; Thompson et al., 2004). Testis cholesterol and cholesterol-containing lipid droplets in fetal Leydig cells also are reduced after phthalate exposure (Barlow et al., 2003; Johnson et al., 2011; Lehmann et al., 2004). In addition to a reduction in lipid metabolism gene expression pathways, rat phthalate exposure reduces the expression of other fetal Leydig cell-specific genes, including Lhcgr, Gnrhr, and Insl3 (Johnson et al., 2011; Liu et al., 2005; Wilson et al., 2004).
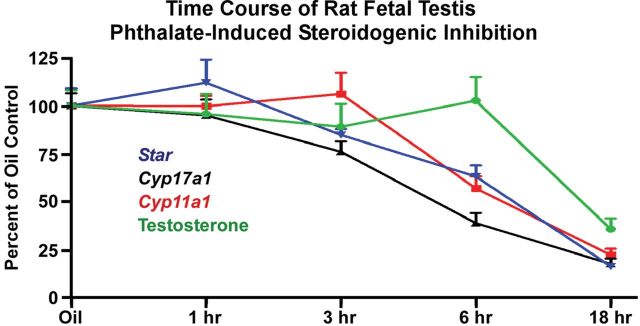
FIG. 1.
Time course of acute phthalate-induced disruption of rat fetal testis steroidogenic gene expression and intratesticular testosterone levels. Sprague Dawley rat dams at GD19 were gavaged with oil vehicle (n = 10) or a single administration of 500mg/kg DBP followed by euthanasia at 1h (n = 5), 3h (n = 5), 6h (n = 5), or 18h (n = 4). Euthanasia for the oil vehicle dams was spread among the 1-, 3-, 6-, and 18-h time points. From fetal testes, steroidogenic gene mRNA levels were quantified by TaqMan-based reverse transcription-polymerase chain reaction relative to Tata binding protein mRNA levels, and intratesticular testosterone levels were measured by radioimmunoassay (Johnson et al., 2011). Shown are the means ± standard errors about the mean. Statistical significance was determined using a one-way analysis of variance and Dunnett’s post hoc test with p < 0.05 considered significant. In this experiment, steroidogenic gene mRNA levels were reduced significantly by 3 (Cyp17a1) or 6 h (Cyp11a1 and Star) postexposure, but significant intratesticular testosterone reductions were not observed until 18h postexposure. Expression of steroidogenic genes remained significantly lower through the 18-h time point. Thus, inhibition of steroidogenic pathway gene expression preceded reductions in intratesticular testosterone levels.
The immediate event precipitating Leydig cell testosterone attenuation appears to be the reduced expression of mRNA and protein within the cholesterol trafficking/biosynthesis and steroidogenic enzymatic pathways. This was discovered soon after fetal rat testis endocrine disruption was appreciated (Shultz et al., 2001) and confirmed in numerous subsequent experiments (Barlow et al., 2003; Borch et al., 2006; Culty et al., 2008; Hannas et al., 2011b; Howdeshell et al., 2007; Johnson et al., 2008; Lin et al., 2008; Liu et al., 2005; Plummer et al., 2007; Thompson et al., 2004, 2005). Not all phthalate congeners are endocrine disruptors or reduce fetal rat Leydig cell steroidogenesis (Gray et al., 2000; Liu et al., 2005), and there is a correlation between congeners that inhibit cholesterol/steroid metabolism gene expression and congeners that inhibit rat testosterone production (Liu et al., 2005). After acute fetal phthalate exposure, fetal testis steroidogenic pathway gene expression decrements occur concomitant with or prior to (Fig. 1) reductions in fetal testis testosterone levels (Thompson et al., 2005). The dose-response relationships overlap between rat fetal testis testosterone production and steroiodogenic/cholesterol metabolism pathways gene expression (Hannas et al., 2011b; Lehmann et al., 2004). Furthermore, the relative potencies of different phthalate congeners for inhibition of steroidogenesis and genes in these pathways mirror each other (Hannas et al., 2011c). From these data, a picture of acute phthalate-induced fetal Leydig cell toxicity emerges in which exposure compromises the basic differentiated phenotype of fetal Leydig cells and manifests as reduced expression of genes encoding cholesterol and steroid metabolism genes and, subsequently, reduced hormone synthesis.
The mechanism of reduced Leydig cell lipid metabolism-related gene and protein expression is becoming clearer, with data pointing to changes in the activity of transcription factors controlling fetal Leydig cell cholesterol and steroid metabolism genes. One transcription factor hypothesized to mediate phthalate effects on fetal Leydig cell gene expression is sterol regulatory element–binding protein (SREBP; Lehmann et al., 2004; Shultz et al., 2001). The two homologs in this family (SREBP1 and SREBP2) are master regulators of lipid metabolism gene expression, including fatty acid, cholesterol and steroid metabolism genes (Brown and Goldstein, 2009; Christenson et al., 2001; Osborne and Espenshade, 2009; Ozbay et al., 2006; Shea-Eaton et al., 2001). Although phthalate exposure does not change fetal rat testis expression of Srebf1 (encoding SREBP1; Lehmann et al., 2004; Johnson et al., 2011), reduced fetal testis levels of Srebf2 mRNA are observed as well as diminished immunoexpression of SREBP2 in fetal rat Leydig cells (Johnson et al., 2011). The decreased expression of genes in pathways controlled by SREBP activity early after phthalate exposure lends additional support to a critical role of SREBP in the phthalate mode of action. CCAAT/enhancer-binding protein-β (CEBPB) regulates steroidogenic pathway gene transcription, and in fetal rat testis, phthalate exposure inhibits binding of CEBPB to the proximal promoters of Cyp11a1, Cyp17a1, and Star (Kuhl et al., 2007). NR5A1 (also known as steroidogenic factor 1; Sf1) transcriptional activity controls the expression of many steroidogenic pathway genes in fetal Leydig cells (Jeyasuria et al., 2004), and reduced NR5A1 expression or activity has been hypothesized as a cause of phthalate-induced endocrine disruption (Borch et al., 2006). Although two studies reported reduced Nr5a1 mRNA levels in phthalate-exposed fetal rat testis (Borch et al., 2006; Plummer et al., 2007), this result has not been observed by others (Liu et al., 2005), and fetal rat testis or Leydig cell NR5A1 protein expression levels are not altered (Kuhl et al., 2007; Plummer et al., 2007). Furthermore, phthalate monoester has no effect on NR5A1 transcriptional activity in a cell-based reporter system (Thompson et al., 2004). In favor of a role for reduced NR5A1 activity, binding of NR5A1 to the Star proximal promoter in fetal rat testis extracts is reduced by in vivo phthalate exposure (Kuhl et al., 2007). A recent publication demonstrated a striking inverse correlation between fetal Leydig cell nuclear immunoexpression of the transcription factor NR2F2 (also known as COUP-TFII) and fetal Leydig cell steroidogenesis (van den Driesche et al., 2012b). In the rat (but not mouse) fetal Leydig cell, phthalate exposure increases nuclear NR2F2 immunoexpression, which correlates in a dose-dependent manner with phthalate-induced steroidogenic inhibition. Because NR2F2 binding sites within steroidogenic gene promoters overlap with NR5A1 binding sites, it is hypothesized that rat phthalate exposure increases NR2F2 binding to steroidogenic gene promoters at the expense of NR5A1 binding, leading to reduced expression of steroidogenic genes (van den Driesche et al., 2012b). The picture coming into focus is that phthalate exposure modifies the constellation of transcription factors bound to steroidogenic gene promoters in fetal Leydig cells, leading to transcriptional repression; however, the mechanism producing altered transcription factor activity is unknown.
Do phthalates change the fate of fetal testis cells? Phthalates do not appear to induce novel gene expression in any testis cell type. Available data demonstrate increased expression of a given gene occurs in the cell type normally producing that transcript (Johnson et al., 2007). The endocrine disrupting effect on the fetal Leydig cell (Thompson et al., 2005) and the inhibition of seminiferous cord formation by fetal somatic cells (Hutchison et al., 2008b) are reversible and occur after phthalate withdrawal. Over developmental time in utero, seminiferous cords elongate and decrease in diameter, and after phthalate exposure, fetal seminiferous cords diameters are increased, suggesting a maturational delay (Barlow and Foster, 2003; Boekelheide et al., 2009; Gaido et al., 2007). Together, these data suggest that the developmental fate of fetal testis cells is not altered; instead, phthalate exposure appears to temporarily perturb the differentiated phenotype of cells resulting in either a delay in development or, as exemplified by rat fetal Leydig cells, a reduction in differentiated function.
IN VIVO PHTHALATE ENDOCRINE DISRUPTION SENSITIVITY AMONG ANIMAL SPECIES
Up to this point in the review, descriptions of the fetal testis endocrine-disrupting phenotype have been limited to experiments involving rat exposures. The other mammalian species examined for male reproductive system effects following in utero phthalate exposure are the rabbit, marmoset, and mouse (Table 1). Phthalate exposure of human fetal testis xenografts will be discussed in a later section. Like the rat, phthalate appears to induce cryptorchidism in rabbits dosed in utero (Higuchi et al., 2003). The marmoset is the only primate species examined for reproductive system effects following in utero phthalate exposure. Using a 500mg/kg/day monobutyl phthalate (MBP) exposure during the expected marmoset fetal masculinization programming window, hypospadias and cryptorchidism were not observed (McKinnell et al., 2009). In this experiment, testosterone levels were examined in neonatal animals and were not altered. However, the testosterone assay was performed well after phthalate exposure ended and, given the rapid recovery of this endpoint after fetal rat exposure (Thompson et al., 2004), fetal testis testosterone production was not analyzed critically. In the rat, endocrine disruption is accompanied by aggregation of Leydig cells, and such histopathology was not observed in the marmoset. After a single 500mg/kg MBP exposure of neonatal marmosets, plasma testosterone levels are reduced (Hallmark et al., 2007), but the relationship of this result to fetal Leydig cell endocrine disruption potential is unclear.
TABLE 1.
Reproductive Phenotype of Mammalian Species After Fetal Phthalate Exposure
| Species | Fetal testis testosterone | Fetal testis steroidogenic genes | Fetal testis Insl3 | Seminiferous cord histopathologya | MNGb | AGDc | Hypospadias | Cryptorchidism |
|---|---|---|---|---|---|---|---|---|
| Rat | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ |
| Moused | ↔ or ↑ | ↑ | ↔ | ↑ | ↑ | ↔e | ↑f | ? |
| Rabbit | ? | ? | ? | ? | ? | ? | ↑g | ↑g |
| Marmoset | ? | ? | ? | ? | ? | ? | ↔ | ↔ |
| Humanh | ? | ? | ? | ? | ? | ↓ | ↑ | ↔ or ↑ |
| Humani | ↔ | ↔ | ↔ | ↔ | ↑ | NA | NA | NA |
Note. ↓, decreased; ↑, increased; ↔, no change; ?, no data available; NA, not applicable.
aIncreased seminiferous cord diameter.
bMultinucleated germ cells.
cAnogenital distance.
dNon-Kunming mouse strains.
eReported as significantly decreased (Liu et al., 2008) but this study used the pup, rather than the dam, as the statistical unit.
fReported as significantly increased (Liu et al., 2008) but was not accompanied by a significant anogenital distance decrease using the dam as the statistical unit.
gOccurred in 1 out of 17 male pups.
hHuman data based on epidemiology.
iHuman data based on human fetal testis xenotransplant experiments.
Despite the potential utility of mouse genetic models for exploring the molecular mechanism of phthalate-induced endocrine disruption, the mouse model of in utero phthalate exposure model has not been widely studied. Early phthalate exposure research employing the mouse used continuous breeding strategies in which male and female mice were exposed to reproductively toxic phthalate congeners throughout breeding. In these studies (Heindel et al., 1989; Lamb et al., 1987), high-dose level phthalate exposure reduced spermatogenesis and fertility in F o males exposed as adults, but the reproductive phenotype of F 1 males exposed in utero to reproductively toxic phthalate congeners was not examined. Another early study of high-dose level mouse gestational exposure from GD0 to 18 observed increased fetal resorption and teratogenicity at GD18, but no reproductive system effects were described (Shiota and Nishimura, 1982).
Recent work indicates that mouse strains widely used in laboratory research are resistant to the fetal testis endocrine-disrupting effects of phthalates. After gestational exposure to phthalate diester or monoester at dose levels causing profound inhibition of rat fetal testis steroidogenesis, mouse fetal intratesticular testosterone content remained at (or above) levels observed in controls (Gaido et al., 2007; van den Driesche et al., 2012b). Corroborating these data, neonatal mouse AGD was unaffected by phthalate exposure during the fetal masculinization programming window (Heger et al., 2012). However, the fetal mouse testis did respond to in utero phthalate exposure: MNGs were induced; seminiferous cord diameters were increased; and the expression of thousands of fetal testis genes was altered (Gaido et al., 2007; Johnson et al., 2011). Although expression of genes governing steroid and cholesterol metabolism were reduced in rats, many of these same genes were unchanged or increased in the mouse (Gaido et al., 2007; Johnson et al., 2011). Because of the histological and molecular changes observed in the mouse fetal testis, a difference in phthalate pharmacokinetics does not seem to explain the species differences. This conclusion is corroborated by data showing similar levels of phthalate monoester in the maternal and fetal serum of rats and mice (Gaido et al., 2007). Some researchers have suggested that in utero mouse fetal testis hormone production and reproductive development are sensitive to the effects of phthalates. Studies from one laboratory reported antiandrogenic effects in C57Bl/6 mice following gestational exposure to phthalates, as evidenced by a dose-dependent decrease in neonatal AGD and an increase in hypospadias (Liu et al., 2008). The AGD data assessed in this study utilized the pup (rather than the litter) as the statistical unit, effectively overestimating group sizes. When the litter is used as the statistical unit, these neonatal mouse AGD data do not reach statistical significance (Heger et al., 2012). Further, the high incidence of reported hypospadias, in consideration of the lack of a true effect on AGD, is difficult to understand and has been questioned (Scott et al., 2009). This group also reported decreases in testosterone and Insl3 mRNA levels in the Kunming mouse strain (Wu et al., 2010). The phylogenetic relationship of this Chinese mouse strain to common western mouse strains (CD1, C57Bl/6, and C3H) is unclear. Although the Kunming mouse may be susceptible to the fetal testis antiandrogenic effects of phthalate exposure, additional research from other laboratories has yet to corroborate these findings. When the available in utero mouse exposure data are considered together, one can conclude that common laboratory mouse strains appear resistant to in vivo phthalate-induced endocrine disruption. In addition, fetal Leydig cell phenotypic differences between mice and rats in utero appear to be due to species-dependent pharmacodynamics, with rat Leydig cell hormone production inhibited and mouse Leydig cell hormone production either unaffected or enhanced.
PHTHALATE EXPOSURE PHENOTYPE OF LEYDIG CELL LINES OR EX VIVO FETAL TESTIS CULTURES
The divergent response observed with in utero phthalate exposure in mice and rats holds particular importance for the extrapolation of risks to humans following phthalate exposure. As in utero experimentation is both costly and labor intensive, in vitro approaches of both human and rodent testis explants and Leydig cell lines potentially offer a straightforward and inexpensive method for studying cellular effects of phthalates. For comparison with in utero experiments, it should be kept in mind that fetal rat testis phthalate monoester concentrations that cause reductions in testosterone production after oral exposure are estimated to be in the low micromolar range (Clewell et al., 2010). Furthermore, in utero phthalate exposure reduces testosterone production within hours of exposure and is accompanied by an overall reduction in steroidogenic pathway gene/protein expression. Most studies involving ex vivo fetal testis culture have utilized a model in which GD14 rat testes are cultured for 3 days with phthalate monoester under either basal or human chorionic gonadotropin (hCG)-stimulated conditions, with media testosterone content measured daily. Using this model, Stroheker et al. (2006) did not observe any dose-related effect of phthalate monoester (MEHP) at concentrations up to 10 μM. A modified GD14 rat testis culture protocol in which only half of the medium was changed each day showed a dose-dependent decrease in CYP17A1 activity and testosterone production (Chauvigne et al., 2009, 2011). Significant effects were seen after 3 days of culture at MEHP concentrations ≥1 μM. In contrast to in utero rat phthalate exposure, ex vivo GD14 rat testis cultures exposed to 10 μM MEHP for 3 days display no reductions in Scarb1, Cyp11a1, or Star gene expression (Chauvigne et al., 2011). In experiments with GD18 rat testes, 48-h exposure to 1mM MBP had no effect on basal testosterone production but reduced hCG-stimulated testosterone production (Hallmark et al., 2007); however, the 1mM MBP concentration is higher than phthalate monoester concentrations observed in rat fetal plasma or fetal testis after 500mg/kg DBP exposure (Clewell et al., 2008, 2010). Finally, culture of GD17 rat testes with 250 μM MBP for 24h did not reduce expression of either steroidogenic genes or other fetal testis genes sensitive to in vivo phthalate exposure (Heger et al., 2012). Notably, MNG formation has not been reported in any ex vivo fetal testis study thus far, despite some reports examining germ cell histopathology (Chauvigne et al., 2009, 2011; Muczynski et al., 2012). For mouse fetal testes, endocrine disruption (both decreased testosterone levels and decreased steroidogenic gene expression) was reported with GD18 testes cultured for 72h with 200 μM MEHP (Lehraiki et al., 2009). Based on these published data, it appears that ex vivo rat and mouse fetal testis cultures do not recapitulate the fetal testis phenotype observed after in vivo exposure. Rat fetal testis studies in which phthalates decreased testosterone production were performed at either very high concentrations (1mM) or did not involve reductions in steroidogenic genes (Star, for example) that are mechanistically important in vivo. Although phthalates have no effect on cultured human fetal testis steroidogenesis (Hallmark et al., 2007; Lambrot et al., 2009), the seemingly poor utility of the ex vivo culture model limits the human risk conclusions that can be drawn from such studies.
In addition to whole organ fetal testis culture, phthalate endocrine disruption research has been conducted using Leydig cell lines derived from postnatal mice. Three different lines have been examined: MA-10 (Anand-Ivell et al., 2009; Clewell et al., 2010; Dees et al., 2001; Fan et al., 2010), MLTC1 (Gunnarsson et al., 2008; Wang et al., 2006, 2007), and BLTK1 (Forgacs et al., 2012). In general, these experiments show phthalate-induced decrements in steroidogenesis, but caveats of such studies are the inconsistent effects on steroidogenic gene expression, the use of transformed cell lines, and the derivation of cell lines from postnatal Leydig cells. Fetal and postnatal Leydig cells are distinct cell types in both origin and function (Huhtaniemi and Pelliniemi, 1992).
HUMAN FETAL TESTIS XENOTRANSPLANTS
Because in vitro studies have thus far been inconsistent both within in vitro models and in comparison to in vivo observations, two different lab groups have developed rodent-host xenograft bioassays to assess the human fetal testis response to phthalates. In one approach (Heger et al., 2012), short-term xenotransplants of fetal mouse (GD15) or rat (GD16) testes were xenografted into the renal subcapsular space of nude rat or mouse hosts, exposed to 250 or 500mg/kg/d di-n-butyl phthalate (DBP) for 1, 2, or 3 days, and harvested 6h after the final dose. In DBP-exposed hosts, an increase in MNG content was observed in both rat and mouse xenografts, with only rat xenografts exhibiting suppressed steroidogenic gene expression and suppressed testosterone secretion, consistent with the intact response. DBP treatment did not affect germ cell content, and host species did not influence histopathology or gene expression endpoints. To explore the human response, a similar exposure paradigm was used, xenografting fetal testes (gestational weeks 10–23) into nude rat hosts. Human fetal testis xenografts exhibited the same MNG induction observed with rat and mouse xenografts. Unlike the rat, but similar to the mouse, human fetal testes were resistant to phthalate-induced suppression of steroidogenic gene expression across a range of phthalate doses (100, 250, and 500mg/kg/day for 2 days). A limitation of this study is the absence of measurements of testosterone production by the human fetal testis xenografts.
In another approach (Mitchell et al., 2012), human fetal testes were grafted into castrated immunodeficient mouse hosts for 6 weeks with or without supplemental hCG. In the presence of supplemental hCG, this model produced sufficient testosterone to increase the host seminal vesicle weight and to maintain normal fetal testis histology (Mitchell et al., 2010). In a subsequent publication (Mitchell et al., 2012), this group showed no inhibition of testosterone (as measured in host serum) or decreased seminal vesicle weight by human testis xenografts following exposure to 500mg/kg DBP or its active monoester metabolite MBP. A limitation of this study was the absence of gene expression analysis of the human xenografts.
ASSESSING THE ENDOCRINE DISRUPTION RISK OF HUMAN IN UTERO PHTHALATE EXPOSURE
Much of the concern over phthalates stems from the demonstrated ability of some phthalate congeners to induce reproductive defects during critical developmental windows in rats. Further, the high production volume, apparent ubiquitous human exposure, high level of exposure in critically ill neonates, and use in children’s toys warrants a closer look at the potential effects of human exposure. In response to public concern, seven phthalate congeners were reviewed in 1999 by an Expert Panel of the Center for the Evaluation of Risks to Human Reproduction of the National Toxicology Program (Shelby, 2002). This group of industry, academic, and government experts analyzed the current data regarding phthalate toxicity and drafted individual assessments detailing the potential for impacts to human reproduction and development. These formal reviews represented the first comprehensive analysis of scientific data on the reproductive effects of phthalates and served to identify gaps in knowledge to aid in prioritizing future research. These reviews identified critically ill neonates as a sensitive subpopulation, with serious concern for adverse effects identified for exposure to di(2-ethylhexyl)phthalate (Kavlock et al., 2006).
Human phthalate exposure levels in the general population are low as compared with the dose levels required to elicit reproductive toxicity in the rat. Although this appears to suggest a negligible human male reproductive system risk, phthalates are only one component of a mixture of chemicals to which humans are exposed. When combined with other endocrine-disrupting compounds in rats, it is clear that phthalates contribute to reproductive toxicity below the no–observed-adverse-effect level of individual phthalate congener exposures (Committee on the Health Risks of Phthalates, 2008). In the face of a multitude of potentially toxic human environmental exposures, the Environmental Protection Agency, in cooperation with the Consumer Products Safety Commission and the Food and Drug Administration agreed to adopt a cumulative assessment plan to examine the effect of multiple phthalates on the exposed organism (http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/phthalates.html). In taking this one step further, the National Research Council of the United States National Academies has recommended focusing on the antiandrogenic endpoints rather than the chemical structure (Committee on the Health Risks of Phthalates, 2008). As the current Environmental Protection Agency practice centers on structurally similar compounds, this approach may underestimate risks from concomitant exposure to other antiandrogenic chemicals which are structurally dissimilar. The National Research Council has suggested this approach, focusing on sensitive pathways of effect, be adopted for risk assessment of other endpoints where diverse chemical classes are implicated.
An important contributor to human risk assessment is the identification of the mechanism of action by which phthalates perturb fetal testis function in model species. For many years, the scientific community has focused on identifying the phthalate mechanism in the rat, and this focus has paid dividends, since it is clear that reduced fetal Leydig cell gene expression and hormone production are the crucial mechanistic steps leading to poor development of accessory reproductive organs. Throwing a wrench into these well-oiled data is the observation that the mouse is resistant to phthalate-induced fetal Leydig cell endocrine disruption. The disparity in species response to phthalates remains a point of contention, particularly with the most recent findings that the in vivo ex situ human fetal testis is resistant to the antiandrogenic effects of phthalates (Heger et al., 2012; Mitchell et al., 2012). There remains a need to better understand the molecular mechanisms responsible for the differences in sensitivity (rats) or resistance (mice) to developmental phthalate exposure. At the same time, insight into the molecular pathways controlling steroidogenesis in the human fetal testis is necessary. In the absence of compelling epidemiological evidence, this molecular mechanistic understanding will be needed for risk assessment to progress beyond the default protective assumption that humans respond similarly to the most sensitive species.
Conclusions
For the past 15 years, much research effort has been expended to describe the reproductive toxicity of in utero phthalate exposure in the rat, a sensitive species. Although still requiring corroboration and further mechanistic exploration, the mouse appears to be resistant to in utero phthalate-induced fetal testis endocrine disruption. The dichotomous response between the mouse and rat correlates with changes in fetal Leydig cell cholesterol and steroid metabolism pathway gene expression and nuclear immunoexpression of the repressive transcription factor NR2F2 (Gaido et al., 2007; Johnson et al., 2011; van den Driesche et al., 2012b). Along with testosterone production, steroidogenic gene expression is a useful biomarker of fetal testis phthalate toxicity. Holding back studies on the phthalate endocrine disruption molecular mechanism is a knowledge gap about the crucial drivers of fetal Leydig cell steroidogenesis and, more generally, the molecular pathways controlling differentiated fetal Leydig cell function (Scott et al., 2009). In the face of poor in vitro models, the use of human fetal testis xenografts opens the door to asking crucial mechanistic questions about the effect of phthalate exposure on human fetal testes. Experiments with xenografts are not without caveats but, along with epidemiology, represent important tools to define the human response to phthalate exposure. Based on the available fetal testis xenograft data, it appears the human fetal testis responds more like a mouse than a rat.
Funding
This work was supported by National Institutes of Health grants R01 ES017272 and P20 RR020173.
ACKNOWLEDGMENTS
We would like to thank Dr Kevin Gaido for critically reading the manuscript and providing valuable comments.
References
- Adibi J. J.,, Whyatt R. M.,, Williams P. L.,, Calafat A. M.,, Camann D.,, Herrick R.,, Nelson H.,, Bhat H. K.,, Perera F. P.,, Silva M. J.,, et al. (2008).Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples.Environ. Health Perspect. 116,467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand-Ivell R.,, Heng K.,, Hafen B.,, Setchell B.,, Ivell R.(2009).Dynamics of INSL3 peptide expression in the rodent testis.Biol. Reprod. 81,480–487 [DOI] [PubMed] [Google Scholar]
- Andrade A. J.,, Grande S. W.,, Talsness C. E.,, Grote K.,, Golombiewski A.,, Sterner-Kock A.,, Chahoud I.(2006).A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)phthalate (DEHP): Effects on androgenic status, developmental landmarks and testicular histology in male offspring rats.Toxicology 225,64–74 [DOI] [PubMed] [Google Scholar]
- Auharek S. A.,, de Franca L. R.,, McKinnell C.,, Jobling M. S.,, Scott H. M.,, Sharpe R. M.(2010).Prenatal plus postnatal exposure to di(n-butyl) phthalate and/or flutamide markedly reduces final Sertoli cell number in the rat.Endocrinology 151,2868–2875 [DOI] [PubMed] [Google Scholar]
- Barlow N. J.,, Foster P. M.(2003).Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to di(n-butyl) phthalate.Toxicol. Pathol. 31,397–410 [DOI] [PubMed] [Google Scholar]
- Barlow N. J.,, Phillips S. L.,, Wallace D. G.,, Sar M.,, Gaido K. W.,, Foster P. M.(2003).Quantitative changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate.Toxicol. Sci. 73,431–441 [DOI] [PubMed] [Google Scholar]
- Boberg J.,, Metzdorff S.,, Wortziger R.,, Axelstad M.,, Brokken L.,, Vinggaard A. M.,, Dalgaard M.,, Nellemann C.(2008).Impact of diisobutyl phthalate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats.Toxicology 250,75–81 [DOI] [PubMed] [Google Scholar]
- Boekelheide K.,, Kleymenova E.,, Liu K.,, Swanson C.,, Gaido K. W.(2009).Dose-dependent effects on cell proliferation, seminiferous tubules, and male germ cells in the fetal rat testis following exposure to di(n-butyl) phthalate.Microsc. Res. Tech. 72,629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borch J.,, Metzdorff S. B.,, Vinggaard A. M.,, Brokken L.,, Dalgaard M.(2006).Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis.Toxicology 223,144–155 [DOI] [PubMed] [Google Scholar]
- Boulogne B.,, Olaso R.,, Levacher C.,, Durand P.,, Habert R.(1999).Apoptosis and mitosis in gonocytes of the rat testis during foetal and neonatal development.Int. J. Androl. 22,356–365 [DOI] [PubMed] [Google Scholar]
- Brown M. S.,, Goldstein J. L.(2009).Cholesterol feedback: From Schoenheimer's bottle to Scap's MELADL.J. Lipid Res. 50 Suppl,S15–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers C. M.,, Foster P. M.(2005).Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate.Birth Defects Res. B Dev. Reprod. Toxicol. 74,277–285 [DOI] [PubMed] [Google Scholar]
- Chauvigne F.,, Menuet A.,, Lesne L.,, Chagnon M.,, Chevrier C.,, Regnier J.,, Angerer J.,, Jegou B.(2009).Time- and dose-related effects of di-(2-ethylhexyl)phthalate and its main metabolites on the function of the rat fetal testis in vitro.Environ. Health Perspect. 117,515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvigne F.,, Plummer S.,, Lesne L.,, Cravedi J. P.,, Dejucq-Rainsford N.,, Fostier A.,, Jegou B.(2011).Mono-(2-ethylhexyl)phthalate directly alters the expression of Leydig cell genes and CYP17 lyase activity in cultured rat fetal testis.PLOS ONE 6,e27172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson L. K.,, Osborne T. F.,, McAllister J. M.,, Strauss J. F.,, 3rd(2001).Conditional response of the human steroidogenic acute regulatory protein gene promoter to sterol regulatory element binding protein-1a.Endocrinology 142,28–36 [DOI] [PubMed] [Google Scholar]
- Christiansen S.,, Boberg J.,, Axelstad M.,, Dalgaard M.,, Vinggaard A. M.,, Metzdorff S. B.,, Hass U.(2010).Low-dose perinatal exposure to di(2-ethylhexyl)phthalate induces anti-androgenic effects in male rats.Reprod. Toxicol. 30,313–321 [DOI] [PubMed] [Google Scholar]
- Clewell R. A.,, Campbell J. L.,, Ross S. M.,, Gaido K. W.,, Clewell H. J.,, 3rd, Andersen M. E.(2010).Assessing the relevance of in vitro measures of phthalate inhibition of steroidogenesis for in vivo response.Toxicol. In Vitro 24,327–334 [DOI] [PubMed] [Google Scholar]
- Clewell R. A.,, Kremer J. J.,, Williams C. C.,, Campbell J. L.,, Jr, Andersen M. E.,, Borghoff S. J.(2008).Tissue exposures to free and glucuronidated monobutylyphthalate in the pregnant and fetal rat following exposure to di-n-butylphthalate: Evaluation with a PBPK model.Toxicol. Sci. 103,241–259 [DOI] [PubMed] [Google Scholar]
- Committee on the Health Risks of Phthalates, N. R. C.(2008).Phthalates and Cumulative Risk Assessment: The Task Ahead The National Academies Press; Washington, D.C. [PubMed] [Google Scholar]
- Corton J. C.,, Lapinskas P. J.(2005).Peroxisome proliferator-activated receptors: Mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol. Sci. 83,4–17 [DOI] [PubMed] [Google Scholar]
- Culty M.,, Thuillier R.,, Li W.,, Wang Y.,, Martinez-Arguelles D. B.,, Benjamin C. G.,, Triantafilou K. M.,, Zirkin B. R.,, Papadopoulos V.(2008). In utero exposure to di-(2-ethylhexyl)phthalate exerts both short-term and long-lasting suppressive effects on testosterone production in the rat.Biol. Reprod. 78,1018–1028 [DOI] [PubMed] [Google Scholar]
- Dees J. H.,, Gazouli M.,, Papadopoulos V.(2001).Effect of mono-ethylhexyl phthalate on MA-10 Leydig tumor cells.Reprod. Toxicol. 15,171–187 [DOI] [PubMed] [Google Scholar]
- Eckerle A.(2001).Ullmann's Encyclopedia of Industrial Chemistry: From print to electronic.Abstr. Papers Am. Chem. Soc. 221,U282–U282 [Google Scholar]
- Ema M.,, Miyawaki E.(2001).Adverse effects on development of the reproductive system in male offspring of rats given monobutyl phthalate, a metabolite of dibutyl phthalate, during late pregnancy.Reprod. Toxicol. 15,189–194 [DOI] [PubMed] [Google Scholar]
- Ema M.,, Miyawaki E.,, Kawashima K.(2000).Critical period for adverse effects on development of reproductive system in male offspring of rats given di-n-butyl phthalate during late pregnancy.Toxicol. Lett. 111,271–278 [DOI] [PubMed] [Google Scholar]
- Fan J.,, Traore K.,, Li W.,, Amri H.,, Huang H.,, Wu C.,, Chen H.,, Zirkin B.,, Papadopoulos V.(2010).Molecular mechanisms mediating the effect of mono-(2-ethylhexyl)phthalate on hormone-stimulated steroidogenesis in MA-10 mouse tumor Leydig cells.Endocrinology 151,3348–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell T. R.,, Krol W. L.,, Sumner S. C.,, Snyder R. W.(2004).Pharmacokinetics of dibutylphthalate in pregnant rats.Toxicol. Sci. 82,407–418 [DOI] [PubMed] [Google Scholar]
- Ferrara D.,, Hallmark N.,, Scott H.,, Brown R.,, McKinnell C.,, Mahood I. K.,, Sharpe R. M.(2006).Acute and long-term effects of in utero exposure of rats to di(n-butyl) phthalate on testicular germ cell development and proliferation.Endocrinology 147,5352–5362 [DOI] [PubMed] [Google Scholar]
- Fisher J. S.,, Macpherson S.,, Marchetti N.,, Sharpe R. M.(2003).Human 'testicular dysgenesis syndrome': A possible model using in-utero exposure of the rat to dibutyl phthalate.Hum. Reprod. 18,1383–1394 [DOI] [PubMed] [Google Scholar]
- Forgacs A. L.,, Ding Q.,, Jaremba R. G.,, Huhtaniemi I. T.,, Rahman N. A.,, Zacharewski T. R.(2012).BLTK1 murine Leydig cells: A novel steroid ogenic model for evaluating the effects of reproductive and developmental toxicants.Toxicol. Sci. 127,391–402 [DOI] [PubMed] [Google Scholar]
- Foster P. M.(2006).Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters.Int. J. Androl. 29,140–147; discussion 181–185 [DOI] [PubMed] [Google Scholar]
- Franchi L. L.,, Mandl A. M.(1964).The ultrastructure of germ cells in foetal and neonatal male rats.J. Embryol. Exp. Morphol. 12,289–308 [PubMed] [Google Scholar]
- Fromme H.,, Gruber L.,, Schlummer M.,, Wolz G.,, Bohmer S.,, Angerer J.,, Mayer R.,, Liebl B.,, Bolte G.(2007).Intake of phthalates and di(2-ethylhexyl)-adipate: Results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data.Environ. Int. 33,1012–1020 [DOI] [PubMed] [Google Scholar]
- Gaido K. W.,, Hensley J. B.,, Liu D.,, Wallace D. G.,, Borghoff S.,, Johnson K. J.,, Hall S. J.,, Boekelheide K.(2007).Fetal mouse phthalate exposure shows that gonocyte multinucleation is not associated with decreased testicular testosterone.Toxicol. Sci. 97,491–503 [DOI] [PubMed] [Google Scholar]
- Gray L. E.,, Jr, Ostby J.,, Furr J.,, Price M.,, Veeramachaneni D. N.,, Parks L.(2000).Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat.Toxicol. Sci. 58,350–365 [DOI] [PubMed] [Google Scholar]
- Gunnarsson D.,, Leffler P.,, Ekwurtzel E.,, Martinsson G.,, Liu K.,, Selstam G.(2008).Mono-(2-ethylhexyl)phthalate stimulates basal steroidogenesis by a cAMP-independent mechanism in mouse gonadal cells of both sexes.Reproduction 135,693–703 [DOI] [PubMed] [Google Scholar]
- Habert R.,, Picon R.(1984).Testosterone, dihydrotestosterone and estradiol-17 beta levels in maternal and fetal plasma and in fetal testes in the rat.J. Steroid Biochem. 21,193–198 [DOI] [PubMed] [Google Scholar]
- Hallmark N.,, Walker M.,, McKinnell C.,, Mahood I. K.,, Scott H.,, Bayne R.,, Coutts S.,, Anderson R. A.,, Greig I.,, Morris K.,, et al. (2007).Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: Comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human.Environ. Health Perspect. 115,390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B. R.,, Furr J.,, Lambright C. S.,, Wilson V. S.,, Foster P. M.,, Gray L. E.,, Jr.(2011a).Dipentyl phthalate dosing during sexual differentiation disrupts fetal testis function and postnatal development of the male Sprague Dawley rat with greater relative potency than other phthalates.Toxicol. Sci. 120,184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B. R.,, Lambright C. S.,, Furr J.,, Evans N.,, Foster P. M.,, Gray E. L.,, Wilson V. S.(2011b).Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: A targeted RT-PCR array approach for defining relative potency.Toxicol. Sci. 125,544–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B. R.,, Lambright C. S.,, Furr J.,, Howdeshell K. L.,, Wilson V. S.,, Gray L. E.,, Jr.(2011c).Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate.Toxicol. Sci. 123,206–216 [DOI] [PubMed] [Google Scholar]
- Heger N. E.,, Hall S. J.,, Sandrof M. A.,, McDonnell E. V.,, Hensley J. B.,, McDowell E. N.,, Martin K. A.,, Gaido K. W.,, Johnson K. J.,, Boekelheide K.(2012).Human fetal testis xenografts are resistant to phthalate-induced endocrine disruption.Environ. Health Perspect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel J. J.,, Gulati D. K.,, Mounce R. C.,, Russell S. R.,, Lamb J. C., 4th.(1989).Reproductive toxicity of three phthalic acid esters in a continuous breeding protocol.Fundam. Appl. Toxicol. 12,508–518 [DOI] [PubMed] [Google Scholar]
- Heudorf U.,, Mersch-Sundermann V.,, Angerer J.(2007).Phthalates: Toxicology and exposure.Int. J. Hyg. Environ. Health 210,623–634 [DOI] [PubMed] [Google Scholar]
- Higuchi T. T.,, Palmer J. S.,, Gray L. E.,, Jr, Veeramachaneni D. N.(2003).Effects of dibutyl phthalate in male rabbits following in utero, adolescent, or postpubertal exposure.Toxicol. Sci. 72,301–313 [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L.,, Furr J.,, Lambright C. R.,, Rider C. V.,, Wilson V. S.,, Gray L. E.,, Jr(2007).Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: Altered fetal steroid hormones and genes.Toxicol. Sci. 99,190–202 [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L.,, Rider C. V.,, Wilson V. S.,, Gray L. E.,, Jr.(2008).Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats.Environ. Res. 108,168–176 [DOI] [PubMed] [Google Scholar]
- Huang P. C.,, Kuo P. L.,, Chou Y. Y.,, Lin S. J.,, Lee C. C.(2009).Association between prenatal exposure to phthalates and the health of newborns.Environ. Int. 35,14–20 [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I.,, Pelliniemi L. J.(1992).Fetal Leydig cells: Cellular origin, morphology, life span, and special functional features.Proc. Soc. Exp. Biol. Med. 201,125–140 [DOI] [PubMed] [Google Scholar]
- Hutchison G. R.,, Scott H. M.,, Walker M.,, McKinnell C.,, Ferrara D.,, Mahood I. K.,, Sharpe R. M.(2008a).Sertoli cell development and function in an animal model of testicular dysgenesis syndrome.Biol. Reprod. 78,352–360 [DOI] [PubMed] [Google Scholar]
- Hutchison G. R.,, Sharpe R. M.,, Mahood I. K.,, Jobling M.,, Walker M.,, McKinnell C.,, Mason J. I.,, Scott H. M.(2008b).The origins and time of appearance of focal testicular dysgenesis in an animal model of testicular dysgenesis syndrome: Evidence for delayed testis development? Int. J. Androl. 31,103–111 [DOI] [PubMed] [Google Scholar]
- Jeyasuria P.,, Ikeda Y.,, Jamin S. P.,, Zhao L.,, De Rooij D. G.,, Themmen A. P.,, Behringer R. R.,, Parker K. L.(2004).Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function.Mol. Endocrinol. 18,1610–1619 [DOI] [PubMed] [Google Scholar]
- Johnson K. J.,, Hensley J. B.,, Kelso M. D.,, Wallace D. G.,, Gaido K. W.(2007).Mapping gene expression changes in the fetal rat testis following acute dibutyl phthalate exposure defines a complex temporal cascade of responding cell types.Biol. Reprod. 77,978–989 [DOI] [PubMed] [Google Scholar]
- Johnson K. J.,, McCahan S. M.,, Si X.,, Campion L.,, Herrmann R.,, Barthold J. S.(2008).The orl rat with inherited cryptorchidism has increased susceptibility to the testicular effects of in utero dibutyl phthalate exposure.Toxicol. Sci. 105,360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J.,, McDowell E. N.,, Viereck M. P.,, Xia J. Q.(2011).Species-specific dibutyl phthalate fetal testis endocrine disruption correlates with inhibition of SREBP2-dependent gene expression pathways.Toxicol. Sci. 120,460–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J.,, Hanke W.(2011).Exposure to phthalates: Reproductive outcome and children health. A review of epidemiological studies.Int. J. Occup. Med. Environ. Health 24,115–141 [DOI] [PubMed] [Google Scholar]
- Kavlock R.,, Barr D.,, Boekelheide K.,, Breslin W.,, Breysse P.,, Chapin R.,, Gaido K.,, Hodgson E.,, Marcus M.,, Shea K.,, et al. (2006).NTP-CERHR expert panel update on the reproductive and developmental toxicity of di(2-ethylhexyl)phthalate.Reprod. Toxicol. 22,291–399 [DOI] [PubMed] [Google Scholar]
- Kleymenova E.,, Swanson C.,, Boekelheide K.,, Gaido K. W.(2005).Exposure in utero to di(n-butyl) phthalate alters the vimentin cytoskeleton of fetal rat Sertoli cells and disrupts Sertoli cell-gonocyte contact.Biol. Reprod. 73,482–490 [DOI] [PubMed] [Google Scholar]
- Kristensen D. M.,, Skalkam M. L.,, Audouze K.,, Lesne L.,, Desdoits-Lethimonier C.,, Frederiksen H.,, Brunak S.,, Skakkebaek N. E.,, Jegou B.,, et al. (2011).Many putative endocrine disruptors inhibit prostaglandin synthesis.Environ. Health Perspect. 119,534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl A. J.,, Ross S. M.,, Gaido K. W.(2007).CCAAT/enhancer binding protein beta, but not steroidogenic factor-1, modulates the phthalate-induced dysregulation of rat fetal testicular steroidogenesis.Endocrinology 148,5851–5864 [DOI] [PubMed] [Google Scholar]
- Lamb J. C. 4th,, Chapin R. E.,, Teague J.,, Lawton A. D.,, Reel J. R.(1987).Reproductive effects of four phthalic acid esters in the mouse.Toxicol. Appl. Pharmacol. 88,255–269 [DOI] [PubMed] [Google Scholar]
- Lambrot R.,, Muczynski V.,, Lecureuil C.,, Angenard G.,, Coffigny H.,, Pairault C.,, Moison D.,, Frydman R.,, Habert R.,, Rouiller-Fabre V.(2009).Phthalates impair germ cell development in the human fetal testis in vitro without change in testosterone production.Environ. Health Perspect. 117,32–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G.,, De Felice C.,, Verrotti A.(2004).Plasticizers, infant nutrition and reproductive health.Reprod. Toxicol. 19,27–33 [DOI] [PubMed] [Google Scholar]
- Latini G.,, Del Vecchio A.,, Massaro M.,, Verrotti A.,, De Felice C.(2006). In utero exposure to phthalates and fetal development.Curr. Med. Chem. 13,2527–2534 [DOI] [PubMed] [Google Scholar]
- Lehmann K. P.,, Phillips S.,, Sar M.,, Foster P. M.,, Gaido K. W.(2004).Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate.Toxicol. Sci. 81,60–68 [DOI] [PubMed] [Google Scholar]
- Lehraiki A.,, Racine C.,, Krust A.,, Habert R.,, Levacher C.(2009).Phthalates impair germ cell number in the mouse fetal testis by an androgen- and estrogen-independent mechanism.Toxicol. Sci. 111,372–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.,, Ge R. S.,, Chen G. R.,, Hu G. X.,, Dong L.,, Lian Q. Q.,, Hardy D. O.,, Sottas C. M.,, Li X. K.,, Hardy M. P.(2008).Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc. Natl. Acad. Sci. U.S.A. 105,7218–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.,, Lehmann K. P.,, Sar M.,, Young S. S.,, Gaido K. W.(2005).Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis.Biol. Reprod. 73,180–192 [DOI] [PubMed] [Google Scholar]
- Liu X.,, He D. W.,, Zhang D. Y.,, Lin T.,, Wei G. H.(2008).Di(2-ethylhexyl) phthalate (DEHP) increases transforming growth factor-beta1 expression in fetal mouse genital tubercles.J. Toxicol. Environ. Health A 71,1289–1294 [DOI] [PubMed] [Google Scholar]
- Loff S.,, Kabs F.,, Witt K.,, Sartoris J.,, Mandl B.,, Niessen K. H.,, Waag K. L.(2000).Polyvinylchloride infusion lines expose infants to large amounts of toxic plasticizers.J. Pediatr. Surg. 35,1775–1781 [DOI] [PubMed] [Google Scholar]
- Magre S.,, Jost A.(1980).The initial phases of testicular organogenesis in the rat. An electron microscopy study.Arch Anat. Microsc. Morphol. Exp. 69,297–318 [PubMed] [Google Scholar]
- Mahood I. K.,, Hallmark N.,, McKinnell C.,, Walker M.,, Fisher J. S.,, Sharpe R. M.(2005).Abnormal Leydig cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis.Endocrinology 146,613–623 [DOI] [PubMed] [Google Scholar]
- Mahood I. K.,, Scott H. M.,, Brown R.,, Hallmark N.,, Walker M.,, Sharpe R. M.(2007).In utero exposure to di(n-butyl) phthalate and testicular dysgenesis: Comparison of fetal and adult end points and their dose sensitivity.Environ. Health Perspect. 115 Suppl 1 55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main K. M.,, Mortensen G. K.,, Kaleva M. M.,, Boisen K. A.,, Damgaard I. N.,, Chellakooty M.,, Schmidt I. M.,, Suomi A. M.,, Virtanen H. E.,, Petersen D. V.,, et al. (2006).Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age.Environ. Health Perspect. 114,270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell C.,, Mitchell R. T.,, Walker M.,, Morris K.,, Kelnar C. J.,, Wallace W. H.,, Sharpe R. M.(2009).Effect of fetal or neonatal exposure to monobutyl phthalate (MBP) on testicular development and function in the marmoset.Hum. Reprod. 24,2244–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. T.,, Childs A. J.,, Anderson R. A.,, van den Driesche S.,, Saunders P. T.,, McKinnell C.,, Wallace W. H.,, Kelnar C. J.,, Sharpe R. M.(2012).Do phthalates affect steroidogenesis by the human fetal testis? Exposure of human fetal testis xenografts to di-n-butyl phthalate.J. Clin. Endocrinol. Metab. 97,E341–348 [DOI] [PubMed] [Google Scholar]
- Mitchell R. T.,, Saunders P. T.,, Childs A. J.,, Cassidy-Kojima C.,, Anderson R. A.,, Wallace W. H.,, Kelnar C. J.,, Sharpe R. M.(2010).Xenografting of human fetal testis tissue: A new approach to study fetal testis development and germ cell differentiation.Hum. Reprod. 25,2405–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muczynski V.,, Cravedi J. P.,, Lehraiki A.,, Levacher C.,, Moison D.,, Lecureuil C.,, Messiaen S.,, Perdu E.,, Frydman R.,, Habert R.,, et al. (2012).Effect of mono-(2-ethylhexyl) phthalate on human and mouse fetal testis: In vitro and in vivo approaches.Toxicol. Appl. Pharmacol. 261,97–104 [DOI] [PubMed] [Google Scholar]
- Mylchreest E.,, Cattley R. C.,, Foster P. M.(1998).Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: An antiandrogenic mechanism? Toxicol. Sci. 43,47–60 [DOI] [PubMed] [Google Scholar]
- Mylchreest E.,, Sar M.,, Wallace D. G.,, Foster P. M.(2002).Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate.Reprod. Toxicol. 16,19–28 [DOI] [PubMed] [Google Scholar]
- Mylchreest E.,, Wallace D. G.,, Cattley R. C.,, Foster P. M.(2000).Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di(n-butyl) phthalate during late gestation.Toxicol. Sci. 55,143–151 [DOI] [PubMed] [Google Scholar]
- Nagano T.,, Suzuki F.(1978).Cell to cell relationships in the seminiferous epithelium in the mouse embryo.Cell Tissue Res. 189,389–401 [DOI] [PubMed] [Google Scholar]
- Ormond G.,, Nieuwenhuijsen M. J.,, Nelson P.,, Toledano M. B.,, Iszatt N.,, Geneletti S.,, Elliott P.(2009).Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: Case-control study.Environ. Health Perspect. 117,303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne T. F.,, Espenshade P. J.(2009).Evolutionary conservation and adaptation in the mechanism that regulates SREBP action: What a long, strange tRIP it's been.Genes Dev. 23,2578–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbay T.,, Rowan A.,, Leon A.,, Patel P.,, Sewer M. B.(2006).Cyclic adenosine 5'-monophosphate-dependent sphingosine-1-phosphate biosynthesis induces human CYP17 gene transcription by activating cleavage of sterol regulatory element binding protein 1.Endocrinology 147,1427–1437 [DOI] [PubMed] [Google Scholar]
- Parks L. G.,, Ostby J. S.,, Lambright C. R.,, Abbott B. D.,, Klinefelter G. R.,, Barlow N. J.,, Gray L. E.,, Jr(2000).The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat.Toxicol. Sci. 58,339–349 [DOI] [PubMed] [Google Scholar]
- Plummer S.,, Sharpe R. M.,, Hallmark N.,, Mahood I. K.,, Elcombe C.(2007).Time-dependent and compartment-specific effects of in utero exposure to di(n-butyl) phthalate on gene/protein expression in the fetal rat testis as revealed by transcription profiling and laser capture microdissection.Toxicol. Sci. 97,520–532 [DOI] [PubMed] [Google Scholar]
- Rusyn I.,, Corton J. C.(2011).Mechanistic considerations for human rel evance of cancer hazard of di(2-ethylhexyl)phthalate.Mutat. Res. 750 141–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffarini C. M.,, Heger N. E.,, Yamasaki H.,, Liu T.,, Hall S. J.,, Boekelheide K.(2011).Induction and persistence of abnormal testicular germ cells following gestational exposure to di-(n-butyl) phthalate in p53-null mice.J. Androl. 33,505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H. M.,, Hutchison G. R.,, Jobling M. S.,, McKinnell C.,, Drake A. J.,, Sharpe R. M.(2008).Relationship between androgen action in the "male programming window, " fetal Sertoli cell number, and adult testis size in the rat.Endocrinology 149,5280–5287 [DOI] [PubMed] [Google Scholar]
- Scott H. M.,, Hutchison G. R.,, Mahood I. K.,, Hallmark N.,, Welsh M.,, De Gendt K.,, Verhoeven G.,, O'Shaughnessy P.,, Sharpe R. M.(2007).Role of androgens in fetal testis development and dysgenesis.Endocrinology 148,2027–2036 [DOI] [PubMed] [Google Scholar]
- Scott H. M.,, Mason J. I.,, Sharpe R. M.(2009).Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds.Endocr. Rev. 30,883–925 [DOI] [PubMed] [Google Scholar]
- Sharpe R. M.,, Skakkebaek N. E.(2008).Testicular dysgenesis syndrome: Mechanistic insights and potential new downstream effects.Fertil. Steril. 89,e33–e38 [DOI] [PubMed] [Google Scholar]
- Shea-Eaton W. K.,, Trinidad M. J.,, Lopez D.,, Nackley A.,, McLean M. P.(2001).Sterol regulatory element binding protein-1a regulation of the steroidogenic acute regulatory protein gene.Endocrinology 142,1525–1533 [DOI] [PubMed] [Google Scholar]
- Shelby M. D.(2002).NTP center for the evaluation of risks to human reproduction phthalates expert panel reports.Reprod. Toxicol. 16,451 [DOI] [PubMed] [Google Scholar]
- Shiota K.,, Nishimura H.(1982).Teratogenicity of di(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) in mice.Environ. Health Perspect. 45,65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota M.,, Saito Y.,, Imai K.,, Horiuchi S.,, Yoshimura S.,, Sato M.,, Nagao T.,, Ono H.,, Katoh M.(2005).Influence of di-(2-ethylhexyl)phthalate on fetal testicular development by oral administration to pregnant rats.J. Toxicol. Sci. 30,175–194 [DOI] [PubMed] [Google Scholar]
- Shultz V. D.,, Phillips S.,, Sar M.,, Foster P. M.,, Gaido K. W.(2001).Altered gene profiles in fetal rat testes after in utero exposure to di(n-butyl) phthalate.Toxicol. Sci. 64,233–242 [DOI] [PubMed] [Google Scholar]
- Stroheker T.,, Regnier J. F.,, Lassurguere J.,, Chagnon M. C.(2006).Effect of in utero exposure to di-(2-ethylhexyl)phthalate: Distribution in the rat fetus and testosterone production by rat fetal testis in culture.Food. Chem. Toxicol. 44,2064–2069 [DOI] [PubMed] [Google Scholar]
- Struve M. F.,, Gaido K. W.,, Hensley J. B.,, Lehmann K. P.,, Ross S. M.,, Sochaski M. A.,, Willson G. A.,, Dorman D. C.(2009).Reproductive toxicity and pharmacokinetics of di-n-butyl phthalate (DBP) following dietary exposure of pregnant rats.Birth Defects Res. B Dev. Reprod. Toxicol. 86,345–354 [DOI] [PubMed] [Google Scholar]
- Suzuki Y.,, Yoshinaga J.,, Mizumoto Y.,, Serizawa S.,, Shiraishi H.(2011).Foetal exposure to phthalate esters and anogenital distance in male newborns.Int. J. Androl. 35, 236–244. [DOI] [PubMed] [Google Scholar]
- Swan S. H.(2008).Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans.Environ. Res. 108,177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S. H.,, Main K. M.,, Liu F.,, Stewart S. L.,, Kruse R. L.,, Calafat A. M.,, Mao C. S.,, Redmon J. B.,, Ternand C. L.,, Sullivan S.,, et al. (2005).Decrease in anogenital distance among male infants with prenatal phthalate exposure.Environ. Health Perspect. 113,1056–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A.,, Thomas M. J.(1984).Biological effects of di-(2-ethylhexyl) phthalate and other phthalic acid esters.Crit. Rev. Toxicol. 13,283–317 [DOI] [PubMed] [Google Scholar]
- Thompson C. J.,, Ross S. M.,, Gaido K. W.(2004).Di(n-butyl) phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism.Endocrinology 145,1227–1237 [DOI] [PubMed] [Google Scholar]
- Thompson C. J.,, Ross S. M.,, Hensley J.,, Liu K.,, Heinze S. C.,, Young S. S.,, Gaido K. W.(2005).Differential steroidogenic gene expression in the fetal adrenal gland versus the testis and rapid and dynamic response of the fetal testis to di(n-butyl) phthalate.Biol. Reprod. 73,908–917 [DOI] [PubMed] [Google Scholar]
- Toppari J.,, Virtanen H. E.,, Main K. M.,, Skakkebaek N. E.(2010).Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): Environmental connection.Birth Defects Res. A Clin. Mol. Teratol. 88,910–919 [DOI] [PubMed] [Google Scholar]
- van den Driesche S.,, Kolovos P.,, Platts S.,, Drake A. J.,, Sharpe R. M.(2012a).Inter-relationship between testicular dysgenesis and Leydig cell function in the masculinization programming window in the rat.PLoS ONE 7,e30111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S.,, Scott H. M.,, MacLeod D. J.,, Fisken M.,, Walker M.,, Sharpe R. M.(2011).Relative importance of prenatal and postnatal androgen action in determining growth of the penis and anogenital distance in the rat before, during and after puberty.Int. J. Androl. 34,e578–586 [DOI] [PubMed] [Google Scholar]
- van den Driesche S.,, Walker M.,, McKinnell C.,, Scott H. M.,, Eddie S. L.,, Mitchell R. T.,, Seckl J. R.,, Drake A. J.,, Smith L. B.,, Anderson R. A.,, Sharpe R. M.(2012b).Proposed role for COUP-TFII in regulating fetal Leydig cell steroidogenesis, perturbation of which leads to masculinization disorders in rodents.PLOS ONE 5,e37064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.,, Song L.,, Hong X.,, Cui L.,, Zhang Z.,, Xiao H.,, Zhou J.,, Wang X.(2006).Low concentrations mono-butyl phthalate stimulates steroidogenesis by facilitating steroidogenic acute regulatory protein expression in mouse Leydig tumor cells (MLTC-1).Chem. Biol. Interact. 164,15––24 [DOI] [PubMed] [Google Scholar]
- Wang Y. B.,, Song L.,, Cui L. B.,, Hong X.,, Zhang Z. D.,, Wang X. R.(2007).Monobutyl phthalate inhibits steroidogenesis by downregulating steroidogenic acute regulatory protein expression in mouse Leydig tumor cells (MLTC-1).J. Toxicol. Environ. Health A 70,947–955 [DOI] [PubMed] [Google Scholar]
- Welsh M.,, Saunders P. T.,, Fisken M.,, Scott H. M.,, Hutchison G. R.,, Smith L. B.,, Sharpe R. M.(2008).Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism.J. Clin. Invest. 118,1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. T.,, Blanchfield B. J.(1975).Retention, distribution, excretion, and metabolism of dibutyl phthalate-7-14C in the rat.J. Agri. Food Chem. 23,854–858 [DOI] [PubMed] [Google Scholar]
- Wilson V. S.,, Howdeshell K. L.,, Lambright C. S.,, Furr J.,, Earl Gray L.,, Jr.(2007).Differential expression of the phthalate syndrome in male Sprague Dawley and Wistar rats after in utero DEHP exposure.Toxicol. Lett. 170,177–184 [DOI] [PubMed] [Google Scholar]
- Wilson V. S.,, Lambright C.,, Furr J.,, Ostby J.,, Wood C.,, Held G.,, Gray L. E.,, Jr.(2004).Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis.Toxicol. Lett. 146,207–215 [DOI] [PubMed] [Google Scholar]
- Wine R. N.,, Li L. H.,, Barnes L. H.,, Gulati D. K.,, Chapin R. E.(1997).Reproductive toxicity of di-n-butylphthalate in a continuous breeding protocol in Sprague Dawley rats.Environ. Health Perspect. 105,102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M.,, Koch H. M.,, Angerer J.,, Bruning T.(2011).Assessing exposure to phthalates – The human biomonitoring approach.Mol. Nutr. Food Res. 55,7–31 [DOI] [PubMed] [Google Scholar]
- Wu S.,, Zhu J.,, Li Y.,, Lin T.,, Gan L.,, Yuan X.,, Xu M.,, Wei G.(2010).Dynamic effect of di-2-(ethylhexyl) phthalate on testicular toxicity: Epigenetic changes and their impact on gene expression.Int. J. Toxicol. 29,193–200 [DOI] [PubMed] [Google Scholar]