Abstract
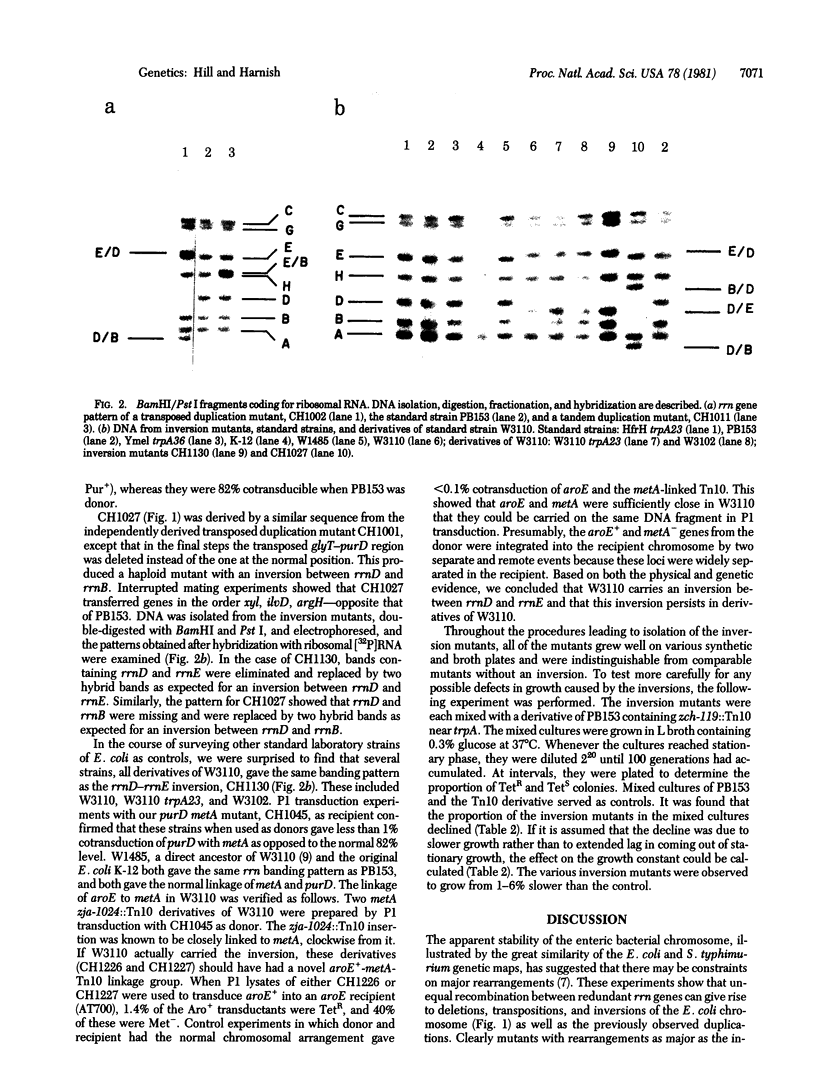
It might be anticipated that the presence of redundant but oppositely oriented sequences in a chromosome could allow inversion of the intervening material through homologous recombination. For example, the ribosomal RNA gene rrnD of Escherichia coli has the opposite orientation fro rrnB and rrnE and is separated from these genes by roughly 20% of the chromosome. Starting with a derivative of Cavalli Hfr, we have constructed mutants that have an inversion of the segment between rrnD and either rrnB or rrnE. These mutants are generally quite viable but do exhibit a slight reduction in growth rate relative to the parental strain. A major line of laboratory E. coli, W3110 and its derivatives, also has an inversion between rrnD and rrnE, probably created directly by a recombinational event between these highly homologous genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Gene duplication in bacteria: alteration of gene dosage by sister-chromosome exchanges. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1083–1087. doi: 10.1101/sqb.1979.043.01.120. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros I., Kiss A., Venetianer P. Physical map of the seven ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1979;6(5):1817–1830. doi: 10.1093/nar/6.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon J., Squires C., Hill C. W. Glycine transfer RNA of Escherichia coli. II. Impaired GGA-recognition in strains containing a genetically altered transfer RNA; reversal by a secondary suppressor mutation. J Mol Biol. 1970 Sep 28;52(3):571–584. doi: 10.1016/0022-2836(70)90420-1. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Combriato G. Genetic duplications induced at very high frequency by ultraviolet irradiation in Escherichia coli. Mol Gen Genet. 1973 Dec 31;127(3):197–214. doi: 10.1007/BF00333760. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Foulds J., Soll L., Berg P. Instability of a missense suppressor resulting from a duplication of genetic material. J Mol Biol. 1969 Feb 14;39(3):563–581. doi: 10.1016/0022-2836(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Grafstrom R. H., Harnish B. W., Hillman B. S. Tandem duplications resulting from recombination between ribosomal RNA genes in Escherichia coli. J Mol Biol. 1977 Nov 5;116(3):407–428. doi: 10.1016/0022-2836(77)90077-8. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Schiffer D., Berg P. Transduction of merodiploidy: induced duplication of recipient genes. J Bacteriol. 1969 Jul;99(1):274–278. doi: 10.1128/jb.99.1.274-278.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P. A ribosomal RNA gene of Escherichia coli (rrnD) on lamnda daro E specialized transducing phages. Mol Gen Genet. 1976 Aug 2;146(3):303–307. doi: 10.1007/BF00701255. [DOI] [PubMed] [Google Scholar]
- Kenerley M. E., Morgan E. A., Post L., Lindahl L., Nomura M. Characterization of hybrid plasmids carrying individual ribosomal ribonucleic acid transcription units of Escherichia coli. J Bacteriol. 1977 Dec;132(3):931–949. doi: 10.1128/jb.132.3.931-949.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A., Sain B., Venetianer P. The number of rRNA genes in Escherichia coli. FEBS Lett. 1977 Jul 1;79(1):77–79. doi: 10.1016/0014-5793(77)80354-2. [DOI] [PubMed] [Google Scholar]
- Konrad E. B. Method for the isolation of Escherichia coli mutants with enhanced recombination between chromosomal duplications. J Bacteriol. 1977 Apr;130(1):167–172. doi: 10.1128/jb.130.1.167-172.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner A. F., Hill C. W. Involvement of ribosomal ribonucleic acid operons in Salmonella typhimurium chromosomal rearrangements. J Bacteriol. 1980 Jul;143(1):492–498. doi: 10.1128/jb.143.1.492-498.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- Schmid M., Roth J. R. Circularization of transduced fragments: a mechanism for adding segments to the bacterial chromosome. Genetics. 1980 Jan;94(1):15–29. doi: 10.1093/genetics/94.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C., Carbon J., Hill C. W. Glycine transfer RNA of Escherichia coli. I. Structural genes for two glycine tRNA species. J Mol Biol. 1970 Sep 28;52(3):557–569. doi: 10.1016/0022-2836(70)90419-5. [DOI] [PubMed] [Google Scholar]
- Vola C., Jarry B., Rosset R. Linkage of 5S RNA and 16S+23S RNA genes on the E. coli chromosome. Mol Gen Genet. 1977 Jun 24;153(3):337–341. doi: 10.1007/BF00431599. [DOI] [PubMed] [Google Scholar]