Abstract
The susceptibility of older adults to the health effects of air pollution is well-recognized. Advanced age may act as a partial surrogate for conditions associated with aging. The authors investigated whether gerontologic frailty (a clinical health status metric) modified the association between ambient level of ozone or particulate matter with an aerodynamic diameter less than 10 µm and lung function in 3,382 older adults using 7 years of follow-up data (1990–1997) from the Cardiovascular Health Study and its Environmental Factors Ancillary Study. Monthly average pollution and annual frailty assessments were related to up to 3 repeated measurements of lung function using cumulative summaries of pollution and frailty histories that accounted for duration as well as concentration. Frailty history was found to modify long-term associations of pollutants with forced vital capacity. For example, the decrease in forced vital capacity associated with a 70-ppb/month greater cumulative sum of monthly average ozone exposure was 12.3 mL (95% confidence interval: 10.4, 14.2) for a woman who had spent the prior 7 years prefrail or frail as compared with 4.7 mL (95% confidence interval: 3.8, 5.6) for a similar woman who was robust during all 7 years (interaction P < 0.001).
Keywords: aging; effect modifier, epidemiologic; environmental exposure; frail elderly; respiratory function tests
A growing body of literature suggests adverse effects of long-term exposure to ambient air pollution on lung function (1). Longitudinal studies with multiple cities or individualized exposures have been primarily conducted in children (2–8), with some conducted in adults (9, 10). In older adults, short- and long-term exposures have been negatively associated with lung function (11–14) and positively associated with respiratory symptoms (15, 16). Older adults are well-recognized as a susceptible subpopulation, and research on these groups is a priority (17–20).
Advanced age alone may not determine susceptibility. Recent evidence suggests that healthy aging may be possible, with morbidity increasingly compressed to the later years of life (21, 22). Susceptibility associated with advanced age may result not from a direct age effect but rather from age acting as an imperfect surrogate for health status. Health status in older adults is complex and multidimensional. One metric, frailty, is generally conceptualized as a syndrome characterized by multisystem decline (23, 24) and has been shown to increase the risk of adverse health outcomes (25, 26).
We hypothesized that frailty status modifies the associations of ambient ozone and particulate matter with an aerodynamic diameter less than 10 µm (PM10) with lung function, as measured by forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC).
MATERIALS AND METHODS
Study population
The Cardiovascular Health Study (CHS) (27, 28) is a longitudinal, population-based prospective study of adults aged 65 years or older that was originally designed to study cardiovascular disease. Between 1989 and 1990, a total of 5,201 study participants (cohort 1) were recruited from 4 US counties through age- and sex-stratified random sampling from Medicare eligibility lists. To be eligible, potential participants could not be institutionalized, unable to give informed consent, in need of a proxy respondent, wheelchair-bound, receiving treatment for cancer, or likely to move away in the next 3 years (27). Between 1992 and 1993, 687 additional African Americans were recruited into the CHS (cohort 2).
Lung function
During 3 clinical examination periods (1989–1990, 1993–1994, and 1996–1997), trained operators administered spirometry pulmonary function tests (PFTs), which have been described in detail elsewhere (29–31). We used maximal reported FVC and FEV1, regardless of assigned quality control grades (A, B, C, D, and F), because many frail participants had maneuvers with low grades.
Frailty
Participants were considered frail if they satisfied at least 3 of 5 criteria: slow walking speed, poor grip strength, exhaustion, unintended weight loss, and low physical activity (25). Information used to construct these criteria was assessed during most clinical examination periods. For periods when the necessary information was not assessed, we singly imputed the missing data (see section 1 of the Web Appendix, which appears on the Journal's website (http://aje.oxfordjournals.org/)). We summarized frailty status using a categorical variable (robust = meeting 0 criteria; prefrail = meeting 1–2 criteria; frail = meeting ≥3 criteria).
Covariates
At the baseline clinical examination (cohort 1: 1989–1990; cohort 2: 1992–1993), extensive information was gathered, including anthropometric, sociodemographic, and behavioral data, cardiovascular (32) and respiratory disease history and status, and other clinical measures. Some repeated and additional information was collected at follow-up clinical examinations.
Air pollution
The CHS Environmental Factors Ancillary Study assigned participants in 3 of the 4 CHS counties (Forsyth County, North Carolina; Sacramento County, California; and Pittsburgh, Pennsylvania) subject-specific monthly average daily ambient PM10, nitrogen dioxide, ozone, sulfur dioxide, and carbon monoxide exposure estimates from 1989 to 2000. Ambient air pollution data were obtained from the Environmental Protection Agency's Aerometric Information Retrieval System and the California Air Resources Board's Ambient Air Quality Data compact disk. Participants' residential address histories were geocoded, and monthly average pollution levels were interpolated to each location using the inverse-distance-weighted average of up to 3 of the nearest air quality monitors within 50 km (see section 2 of the Web Appendix). Here, we used subject-specific monthly averages for 24-hour average PM10 (µg/m3) and 8-hour average daily maximum ozone (ppb). Sacramento County had ozone data year-round. During the nonozone season (November–March), Pittsburgh had limited ozone data and Forsyth County had none.
Exclusion criteria
We excluded participants who, at baseline, had a history of Parkinson's disease (n = 47), adjudicated stroke (n = 249), or Mini-Mental State Examination score <18 (n = 74) or who were taking Sinemet (carbidopa-levodopa; Merck & Company, Inc., Whitehouse Station, New Jersey), Aricept (donepezil hydrochloride; Eisai Company Ltd., Kawashima, Japan), or antidepressants (n = 235), since these participants might have displayed frailty characteristics as a consequence of a single disease (25). We excluded participants with a self-reported race/ethnicity other than white or African-American (n = 31). Missing or unreasonable PFT values (1 observation with FEV1 > 9 L and 4 observations with FEV1 = 0 L) were also excluded. We included the subset of observations with complete information on FEV1, FVC, pollution exposure, frailty status, and adjustment covariates.
Statistical methods
All models were fitted separately by sex because of sex differences in frailty prevalence (27) and pulmonary function (33). The same covariates were included in models for FEV1 and FVC. Data from baseline (cohort 1: 1989–1990; cohort 2: 1992–1993) or the initial PFT (cohort 1: 1989–1990; cohort 2: 1993–1994), if available, were used to develop cross-sectional “base” models (34) for which Akaike's Information Criterion guided inclusion of a set of relevant candidate covariates and interactions from prior analyses in the CHS (31, 35). Exploratory generalized additive models (36) informed the creation of piecewise linear splines with a single sex-specific knot for continuous covariates. The final set of anthropometric, demographic, and behavioral adjustment covariates were: height (knot at 174 cm for women, 189 cm for men), weight (knot at 158 pounds (72 kg) for women, 245 pounds (111 kg) for men), waist circumference (knot at 81 cm for women, 87 cm for men), an indicator for African-American race/ethnicity, pack-years of smoking (knot at 80 years for men and women), years since quitting smoking, smoking status, education, an indicator for CHS community, age, and the interactions of race with age and smoking status. Additional adjustment covariates for cardiovascular and respiratory disease were: use of any beta blockers, a self-report of ever having physician-diagnosed pneumonia, symptoms of dyspnea upon exertion, current asthma diagnosis by a physician, and systolic blood pressure.
Longitudinal models with random intercepts were subsequently developed that included 1) fixed effects for time-constant adjustment covariates and time-varying age and 2) season and its interaction with community, to account for potential confounding. For Yij (FEV1 or FVC) from participant i at observation j, the model was
where Xij represents adjustment covariates, wij summarizes frailty history, and aij summarizes individual-level PM10 or ozone history. We accounted for dependence in unequally spaced repeated measures by including an individual-level random intercept Ui ∼ N (0, τ2) and multivariate normal errors εij with mean 0 and a continuous-time first-order autoregressive correlation structure (37, 38). Longitudinal models were estimated by means of restricted maximum likelihood using lme in the nlme R package (39). P values for interactions were obtained from likelihood ratio tests comparing models—estimated by maximum likelihood—with and without the interaction term(s).
We quantified midterm (subchronic) and long-term (chronic) associations of air pollution with lung function and investigated evidence for modification by frailty history. Midterm exposure was summarized as the average of the current month, the prior month, or the 5 months prior to and including the current month. In these models, we considered modification by current frailty status and included indicators for calendar year to control for potential confounding by long-term trends. For long-term exposure, we used cumulative summaries of pollution (typical pollution months) and frailty (number of years spent frail) motivated by models for change in lung function (see section 3 of the Web Appendix). Similar cumulative summaries have been suggested previously (40) and applied elsewhere (9). Calendar year was a rough surrogate for cumulative exposure, so we excluded it to avoid unstable coefficient estimates. Because of seasonal availability, analyses of midterm ozone associations were performed for an abbreviated ozone season (May–October) to allow for investigation of prior-month associations. Long-term ozone exposure was quantified by typical ozone-season months accumulated only during the ozone season (April–October).
Typical pollution months
Ambient air pollution exposure history for observation j was summarized as the cumulative sum of monthly average exposure from the month after the initial PFT to (and including) the month at observation j. This is similar to the cumulative exposure metric for smoking: pack-years = (cigarettes per day × years smoked)/20, where 20 is the number of cigarettes in a pack. We defined typical air pollution months as
| (2) |
where the normalizing “typical units” were selected on the basis of the data: 30 µg/m3 for 24-hour average PM10 and 70 ppb for 8-hour average daily maximum ozone during ozone season. To translate a typical pollution month's regression coefficient to a 10-μg/m3/month (or 10-ppb/month) scale, multiply by 10/30 (or 10/70).
Number of years spent frail
Frailty history at study year tj was summarized as the number of years the participant had spent frail (or prefrail/frail) since the initial PFT year (t1 = 0):
 |
(3) |
where wi(tj) is binary frailty status (prefrail/frail vs. robust or frail vs. not frail). This assumes that transitions in frailty status occur halfway between equally spaced annual clinical examinations.
RESULTS
At baseline, the 301 participants excluded because of missing covariates were heavier (165.2 pounds (75.0 kg) vs. 159.6 pounds (72.5 kg)) and more likely to be African-American (29.6% vs. 15.3%) than those included, but they were similar in terms of age, sex, and frailty. After exclusions, there were 7,281 observations on 3,382 participants (1,445, 1,009, and 928 participants had 3, 2, and 1 PFT, respectively). Of the 2,993 cohort 1 participants (336 cohort 2 participants) with PFT at the initial assessment, 70.4% (61.6%) also had a PFT at the following assessment. Participants were approximately evenly divided among the 3 communities. More participants were prefrail than frail (prevalences of 50.3% and 8.2% at the initial PFT, respectively). Transitions in frailty status were common. Although 44.9% of cohort 1 participants had the same categorical frailty status at the initial PFT as they had 4 years later at the second PFT, 70.1% of cohort 1 participants underwent a change in frailty status at least once during this interim (44.1% had 2 or more transitions). The number of years spent frail had a right-skewed distribution, while the number of years spent prefrail or frail was more uniformly distributed. Frail participants were older and more likely to be female, to be African-American, to have less education, and to have emphysema, dyspnea upon exertion, asthma, lower FEV1, lower FVC, or a low-quality PFT (grade of D or F) (Table 1).
Table 1.
Characteristics of Participants According to Categorical Frailty Status at the Time of the Initial Pulmonary Function Test, Cardiovascular Health Study, 1989–1997
| Frailty Status |
||||||
|---|---|---|---|---|---|---|
| Robust (n = 1,382) |
Prefrail (n = 1,673) |
Frail (n = 274) |
||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Age, years | 71.2 (4.4) | 73.0 (5.5) | 76.6 (6.3) | |||
| Height, cm | 166.4 (9.3) | 164.7 (9.5) | 162.4 (9.7) | |||
| Weight, poundsa | 158.9 (29.4) | 160.1 (32.0) | 160.5 (37.7) | |||
| Waist circumference, cm | 92.2 (11.8) | 95.3 (12.7) | 98.0 (15.8) | |||
| African-American race/ethnicity | 11.1 | 16.6 | 29.2 | |||
| Male sex | 46.6 | 40.8 | 32.8 | |||
| Education | ||||||
| <8th grade | 5.9 | 11.7 | 20.4 | |||
| Grades 8–11 | 11.0 | 13.7 | 17.2 | |||
| Grade 12 or GED | 28.4 | 29.7 | 26.6 | |||
| ≥1 year of vocational school to 4-year college degree | 40.6 | 34.2 | 28.8 | |||
| Graduate/professional school | 14.0 | 10.7 | 6.9 | |||
| Smoking status | ||||||
| Never smoker | 44.9 | 46.1 | 52.9 | |||
| Former smoker | 45.7 | 41.6 | 33.6 | |||
| Current smoker | 9.3 | 12.3 | 13.5 | |||
| Years since quitting smoking (former smokers) | 20.7 (13.2) | 19.9 (13.1) | 19.9 (14.0) | |||
| Pack-years of smoking (ever smokers) | 34.7 (29.2) | 34.3 (28.5) | 36.9 (30.0) | |||
| Use of any beta blockers | 12.7 | 13.3 | 10.9 | |||
| Systolic blood pressure, mm Hg | 135.7 (21.3) | 137.5 (21.3) | 140.5 (23.6) | |||
| Pneumoniab | 24.5 | 29.0 | 32.8 | |||
| Dyspnea symptoms upon exertion | 3.8 | 14.1 | 30.7 | |||
| Current asthmac | 2.5 | 3.6 | 6.2 | |||
| FEV1, L | 2.2 (0.6) | 2.0 (0.6) | 1.6 (0.6) | |||
| Low-quality FEV1 d | 7.4 | 10.3 | 21.0 | |||
| FVC, L | 3.1 (0.8) | 2.9 (0.9) | 2.4 (0.8) | |||
| Low-quality FVCd | 4.7 | 7.9 | 15.2 | |||
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GED, General Education Diploma; SD, standard deviation.
a 1 pound = 0.45 kg.
b Self-report of ever having physician-diagnosed pneumonia.
c Current asthma diagnosis by a physician.
d Assigned quality control grade of D or F (possible grades: A, B, C, D, and F).
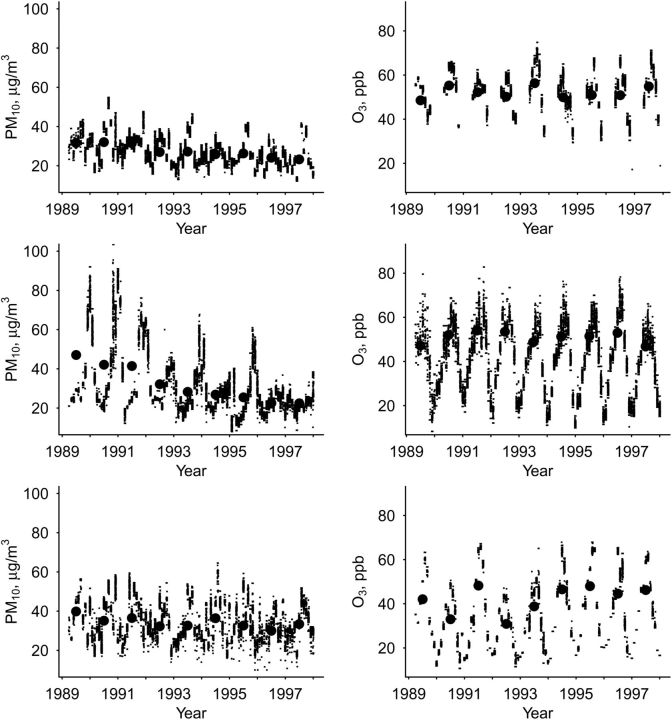
Pollutant summary distributions and within-county correlations are presented in Tables 2 and 3, respectively. PM10 levels declined over the course of the study period, while ozone levels remained relatively stable (Figure 1). The correlation between monthly average ozone and PM10 varied by community (r = 0.53 in Forsyth County, r = 0.36 in Pittsburgh, and r = 0.06 in Sacramento County), so we did not attempt to fit multipollutant models. Typical ozone and PM10 months were strongly correlated (r = −0.86). For cohort 1 participants at the final PFT (when approximately 84 (7 × 12) months or 49 (7 × 7) ozone-season months had passed since the initial PFT), the number of typical pollution months ranged from 66.3 to 107.5 for PM10 and from 37.8 to 69.0 for ozone.
Table 2.
Summary Distributions of Pollutant Levels, Obtained Using Data From All Available Pulmonary Function Test Measurements, Cardiovascular Health Study, 1989–1997
| Pollutant and Summary | Mean (SD) | Minimum | Percentile |
Maximum | ||||
|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | ||||
| PM10 | ||||||||
| Current-month mean | 30.8 (11.2) | 12.2 | 18.8 | 23.0 | 27.6 | 36.3 | 52.8 | 92.0 |
| Prior-month mean | 31.8 (11.4) | 14.0 | 19.0 | 24.0 | 28.9 | 36.8 | 54.8 | 92.0 |
| 5-month mean | 31.5 (8.6) | 14.0 | 20.9 | 25.8 | 29.7 | 35.6 | 46.2 | 74.3 |
| Typical monthsa | 86.0 (7.7) | 66.3 | 73.2 | 79.7 | 87.1 | 92.5 | 96.4 | 107.5 |
| Ozone | ||||||||
| Current-month mean | 39.7 (15.1) | 8.3 | 15.3 | 27.3 | 39.7 | 51.3 | 64.1 | 79.6 |
| Prior-month mean | 23.9 (9.5) | 4.2 | 9.3 | 16.6 | 23.8 | 30.8 | 40.4 | 54.8 |
| Typical monthsa | 49.8 (9.0) | 37.8 | 39.5 | 40.4 | 48.7 | 60.9 | 63.3 | 69.0 |
Abbreviations: PM10, particulate matter with an aerodynamic diameter less than 10 μm; SD, standard deviation.
a Typical-month data are restricted to values obtained from cohort 1 participants at the time of the final pulmonary function test (when approximately 84 (7 × 12) months or 49 (7 × 7) ozone-season months had passed since the initial pulmonary function test), since these cumulative metrics are a function of time.
Table 3.
Within-County Correlations Between Summary Pollutant Levels, Obtained Using Data From All Available Pulmonary Function Test Measurements, Cardiovascular Health Study, 1989–1997
| Pollutant and Summary | PM10 |
Ozone |
|||||
|---|---|---|---|---|---|---|---|
| Current-Month Mean | Prior-Month Mean | 5-Month Mean | Typical Months | Current-Month Mean | Prior-Month Mean | Typical Months | |
| PM10 | |||||||
| Current-month mean | 1.00 | ||||||
| Prior-month mean | 0.56 | 1.00 | |||||
| 5-month mean | 0.59 | 0.79 | 1.00 | ||||
| Typical months | −0.22 | −0.27 | −0.41 | 1.00 | |||
| Ozone | |||||||
| Current-month mean | −0.04 | −0.16 | −0.32 | 0.10 | 1.00 | ||
| Prior-month mean | −0.02 | −0.08 | −0.27 | 0.12 | 0.83 | 1.00 | |
| Typical months | −0.24 | −0.27 | −0.41 | 0.96 | 0.10 | 0.11 | 1.00 |
Abbreviation: PM10, particulate matter with an aerodynamic diameter less than 10 µm.
Figure 1.
Participant-level interpolated monthly mean 24-hour average level of particulate matter with an aerodynamic diameter less than 10 µm (PM10) (left) and 8-hour average daily maximum level of ozone (O3) (right) for Forsythe County, North Carolina (top), Sacramento County, California (middle), and Pittsburgh, Pennsylvania (bottom), Cardiovascular Health Study, 1989–1997. Annual mean values are plotted at the midpoint of each year (for ozone, the mean was calculated for April–October).
Midterm pollution associations
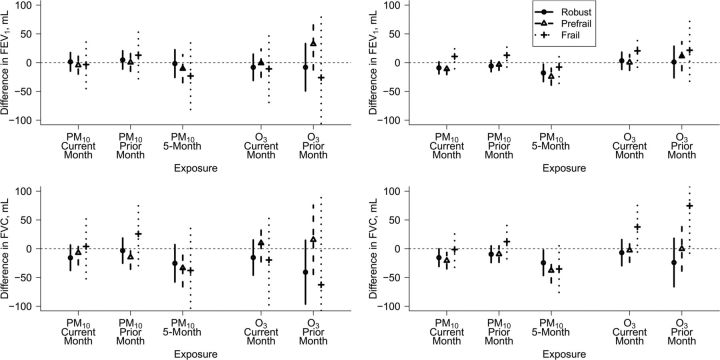
Higher 5-month mean PM10 level was associated with decreased FEV1 and FVC after adjustment for anthropometric, demographic, and behavioral covariates (Table 4), and the magnitude of estimated decreases was larger for the prefrail than for the robust (Figure 2). For example, pooling prefrail and frail men, a 10-μg/m3 increase in 5-month mean PM10 was associated with a difference in FVC of −34.3 mL (95% confidence interval (CI): −66.0, −2.5) as compared with −26.2 mL (95% CI: −58.7, 6.3) in robust men. For other midterm pollutant summaries, patterns in the associations by current frailty status were less consistent. None of the interactions between a midterm pollutant summary and frailty were statistically significant (P > 0.09).
Table 4.
Adjusteda Difference in FEV1 or FVC Associated With a 10-μg/m3 Increase in Recent PM10 Level, a 10-ppb Increase in Recent Ozone Level (During Ozone Season), or a 1-Month Increase in Typical PM10 Months or Typical Ozone Months (During Ozone Season), by Sex, Cardiovascular Health Study, 1989–1997
| Pollutant and Summary | Difference in FEV1, mL |
Difference in FVC, mL |
||||||
|---|---|---|---|---|---|---|---|---|
| Men |
Women |
Men |
Women |
|||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| PM10 | ||||||||
| Current-month mean | −1.7 | −13.9, 10.4 | −8.4 | −16.1, −0.7 | −10.3 | −26.9, 6.3 | −16.9 | −28.3, −5.6 |
| Prior-month mean | 3.1 | −9.0, 15.1 | −3.3 | −10.8, 4.3 | −7.3 | −23.8, 9.2 | −7.7 | −18.7, 3.4 |
| 5-month mean | −6.7 | −26.9, 13.4 | −20.2 | −32.6, −7.7 | −29.8 | −56.9, −2.7 | −31.8 | −49.9, −13.6 |
| Typical months | −0.9 | −1.4, −0.4 | −0.5 | −0.8, −0.2 | −4.3 | −5.0, −3.7 | −2.6 | −3.0, −2.2 |
| Ozone | ||||||||
| Current-month mean | −5.3 | −23.2, 12.6 | 3.6 | −7.3, 14.5 | −4.9 | −28.5, 18.7 | −0.3 | −16.5, 16.0 |
| Prior-month mean | 7.5 | −25.7, 40.8 | 8.0 | −12.2, 28.3 | −18.5 | −63.2, 26.2 | −3.3 | −34.2, 27.6 |
| Typical months | −2.4 | −3.3, −1.5 | −1.2 | −1.7, −0.7 | −8.7 | −9.8, −7.6 | −5.3 | −6.0, −4.6 |
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PM10, particulate matter with an aerodynamic diameter less than 10 µm.
a Adjusted for anthropometric, demographic, and behavioral covariates and current frailty status.
Figure 2.
Difference in forced expiratory volume in 1 second (FEV1) (top) or forced vital capacity (FVC) (bottom) associated with a 10-μg/m3 increase in recent particulate matter with an aerodynamic diameter less than 10 µm (PM10) or a 10-ppb increase in recent ozone (O3) during ozone season, according to current frailty status, for men (left) and women (right), after adjustment for anthropometric, demographic, and behavioral covariates, Cardiovascular Health Study, 1989–1997.
Long-term pollution associations
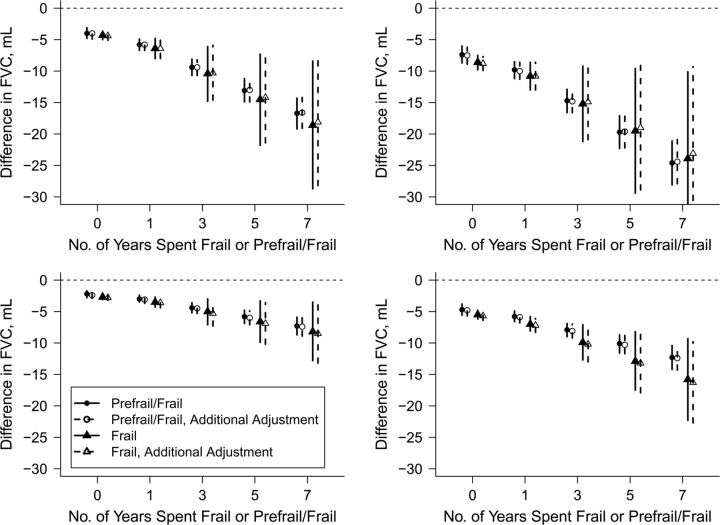
Increased typical PM10 and ozone months were associated with decreased lung function after adjustment for anthropometric, demographic, and behavioral covariates (Table 4). Participants who spent more years frail or prefrail/frail had significantly larger declines in FVC (frail: P < 0.030; prefrail/frail: P < 0.001) but not FEV1 (frail and prefrail/frail: P > 0.17) (see Figure 3 and Web Figure 1, where plotted values are 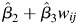 from equation 1). For example, the estimated decrease in FVC associated with exposure to an additional typical month (30 µg/m3 monthly mean) of ambient PM10 was 2.2 mL (95% CI: 1.7, 2.8) for a female participant who was robust over a 7-year interval as compared with a decrease of 4.4 mL (95% CI: 3.6, 5.2) if the same participant had instead spent 3 of those 7 years prefrail/frail.
from equation 1). For example, the estimated decrease in FVC associated with exposure to an additional typical month (30 µg/m3 monthly mean) of ambient PM10 was 2.2 mL (95% CI: 1.7, 2.8) for a female participant who was robust over a 7-year interval as compared with a decrease of 4.4 mL (95% CI: 3.6, 5.2) if the same participant had instead spent 3 of those 7 years prefrail/frail.
Figure 3.
Difference in forced vital capacity (FVC) associated with a 1-month increase in typical pollution months (left, particulate matter with an aerodynamic diameter less than 10 µm; right, ozone) for men (top) and women (bottom), according to the number of years spent frail or prefrail/frail, after adjustment for anthropometric, demographic, and behavioral covariates (solid lines) and additional adjustment for cardiovascular and respiratory disease covariates (dashed lines), Cardiovascular Health Study, 1989–1997.
Sensitivity analyses
Results were qualitatively similar (unless specified, results not shown) when we 1) additionally adjusted for cardiovascular and respiratory disease covariates (long-term associations: Figure 3), 2) excluded low-quality PFT (Web Table 1 and Web Figures 2–4), 3) included an additional quadratic term for age, 4) multiply imputed missing adjustment covariates, 5) adjusted for baseline frailty status instead of current frailty status, and 6) excluded current-year frailty status from the cumulative frailty metric. When we considered modification of recent pollution associations by baseline frailty instead of current frailty, again only 5-month mean PM10 level showed a trend (not significant). In models including multiple summaries for the same pollutant on different time scales, the estimated associations of current-month and 5-month mean pollution were attenuated, while associations of cumulative pollution were similar. In multipollutant models, associations of 5-month mean PM10 were attenuated and statistically significant only for FVC in women (Web Table 2). When we stratified results by community, Sacramento County had the largest-magnitude (negative) interaction regression coefficients for both cumulative pollutant exposures.
DISCUSSION
In a large community-dwelling cohort of older adults, we found strong evidence that cumulative ozone or PM10 exposure was associated with decreased lung function. A history of frailty amplified the adverse associations of cumulative exposure with FVC but not FEV1. Five-month average PM10 level was negatively associated with lung function, but there were no significant differences by frailty status.
Previous studies have found associations of particulate matter and ozone with lung function in older adults. For 57 older adults in Seattle, Washington, a 10-μg/m3 increase in prior-day particulate matter with an aerodynamic diameter less than 2.5 µm (PM2.5) was associated with a 40.4-mL decrease (95% CI: 9.6, 71.1) in FEV1 (11). For 1,100 older men in the Normative Aging Study, a 15-ppb increase in prior-48-hour ozone was associated with decreases in FEV1 and FVC of 1.25% (95% CI: 0.54, 1.96) and 1.29% (95% CI: 0.63, 1.95), respectively (13). Midterm (6-month) ozone associations with FEV1 and FVC have been found in children (6), though we are not aware of similar studies in older adults. In a study by Abbey et al. (41), summaries of 20-year PM10 exposure (and, to a lesser extent, ozone) were negatively associated with lung function in 1,391 adult nonsmokers. Cross-sectional surveys of adults in England related lung function to 2-year average ambient pollutant exposures and found negative associations of PM10 with FEV1 that were stronger for men and older adults, but no evidence of associations of ozone with FEV1 (14).
A previously developed conceptual framework describes the most “frail” segment of the population as being at greater risk for air-pollution-related mortality (42, 43). To our knowledge, no studies have considered gerontologic measures of health status as susceptibility factors. In a study of Chinese older adults, Sun and Gu (44) investigated the associations of air pollution with Activities of Daily Living, Instrumental Activities of Daily Living, Mini-Mental State Examination score, and self-rated health but did not consider these as modifying factors.
Strengths of this study include the large sample size, repeated measurements, the long follow-up period, individualized ambient pollutant exposures, and annual frailty assessment in a population where frailty has been well-studied. Frequent frailty assessment and summaries of frailty history are important because frailty is a dynamic process, with individuals transitioning in both directions along the frailty gradient (45).
In contrast to other studies of long-term exposure associations that use study-period average exposures (5), we used a cumulative exposure summary to relate only prior air pollution levels to each lung function measurement. Advantages of the typical pollution month's exposure metric include its 1) temporal ordering of exposure and outcome assessment, 2) accounting for exposure as a function not only of concentration but also of duration, 3) interpretability due to its similarity to the pack-years metric for smoking history, and 4) mathematical motivation stemming from models for change. However, implied assumptions of no safe level of exposure and no recovery from high past exposures may be questionable in studies of air pollution associations with health. Alternative cumulative summaries could use thresholds or weighting, but then the pack-years analogy and mathematical motivation would no longer hold.
Limitations of this study include the inability to determine whether the strengthening of the midterm association of PM10 over a longer time scale (5-month mean vs. 1-month mean) may have a biologic explanation or may be deattenuation from reduced measurement error. We were unable to investigate the associations with different particulate matter size fractions, because PM2.5 data were not available. We controlled for community, season, and their interaction, but residual confounding may still have existed. PM10 concentrations are affected by differences in location-specific seasonal patterns and sources of PM2.5 and the coarse fraction (PM2.5–10). In the East (Forsyth County and, to a lesser degree, Pittsburgh), PM2.5 concentrations peak in the summer, largely because of the transport of primary emissions and the formation of secondary aerosols resulting from photochemical processes. In Sacramento County, winter air inversions trap primary fine particles (including PM2.5), and in the fall, higher PM10 levels reflect windblown coarse fraction particles.
Interpolation of ambient air pollutant levels to participants' residences may reduce spatial exposure misclassification, which can attenuate associations (46). However, we still lacked data on indoor/outdoor activity patterns and personal exposure. Ambient exposures differ from personal exposure but are still of interest. Previous studies have found associations of ambient exposures with lung function, and the National Ambient Air Quality Standards regulate ambient levels of air pollutants. Frail participants might spend more time indoors and have less exposure to ambient pollution, potentially attenuating associations. No data exist for exposures incurred prior to baseline in the CHS, but we may have partially accounted for previous exposure effects by adjusting for respiratory and cardiovascular disease, age, community, and individual-level random intercepts.
Participants dropped out of the study or had intermittent missing spirometry data for many reasons, including morbidity and death. Under the assumption that the missingness mechanism for PFT is related only to observed data (i.e., that data are missing at random) (47), a linear mixed-effects model that is “correct” for the mean and covariance structure is an appropriate analytic method, and there is no need to multiply impute the missing response values, as has been done previously (35). Dropout due to morbidity or death prior to frailty diagnosis has been hypothesized as an explanation for lower-than-expected frailty incidence rates in many studies. Differential dropout of the frail would probably attenuate the modification by frailty status of pollution associations with lung function.
The high correlation between FVC and FEV1 (r = 0.90) suggests that we would have seen similar strong interactions between cumulative exposure and frailty history had the interaction been spuriously induced by uncontrolled confounding, yet we observed the interaction for FVC and not FEV1. FEV1 measures large airway flow, with declines being indicative of obstructive disease. FVC measures total capacity, including large and small airways, and it is reduced (along with FEV1) in restrictive disease. Since cumulative exposure was more strongly associated with decreases in FVC, particularly in participants with histories of frailty, chronic pollution exposure in older adults may more adversely affect smaller airways and may contribute to restrictive disease.
Increased susceptibility in older adults with frailty histories may arise from: 1) decreased physiologic (especially cardiopulmonary) reserve for offsetting the pathways by which air pollution affects pulmonary function; 2) potentially increased air-pollution-related inflammation in the frail as compared with the nonfrail; and 3) repercussions of frailty-related sarcopenia. In future work, researchers might investigate whether a chain reaction exists wherein reductions in lung function (potentially from air pollution) causally contribute to frailty, which could further increase susceptibility to air pollution.
In conclusion, this study provides novel evidence that frailty history modifies cumulative associations between air pollution and lung function in older adults. This offers insight into older adult susceptibility to air pollution and may have clinical implications for identifying which older adults are at increased risk.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Biostatistics, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Thomas A. Louis); Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Paulo H. M. Chaves); Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Linda P. Fried); Department of Internal Medicine, School of Medicine, University of California, Davis, Sacramento, California (Helene G. Margolis); UCD Center for Healthcare Policy and Research, University of California, Davis, Sacramento, California (Helene G. Margolis); and Division of Biostatistics, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California (Sandrah P. Eckel).
This work was supported by grants from the National Institutes of Health (grant T32 AG00247 from the National Institute on Aging and grant R01 DK061662 from the National Institute of Diabetes and Digestive and Kidney Diseases). The Cardiovascular Health Study (CHS) was supported by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133 and grant HL080295 from the National Heart, Lung, and Blood Institute, with an additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through grants AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
The authors thank Fred Lurmann (Sonoma Technology, Inc., Petaluma, California) for development of participant air pollution exposure estimates in the CHS, Dr. Paul L. Enright for his contributions to lung function testing in the CHS, and Dr. John Robbins for providing insights into the CHS cohort and data.
Dr. Sandrah P. Eckel won the 2010 American Statistical Association Statistics in Epidemiology Section's Young Investigator Award for this paper.
Conflict of interest: none declared.
REFERENCES
- 1.Götschi T, Heinrich J, Sunyer J, et al. Long-term effects of ambient air pollution on lung function: a review. Epidemiology. 2008;19(5):690–701. doi: 10.1097/EDE.0b013e318181650f. [DOI] [PubMed] [Google Scholar]
- 2.Avol EL, Gauderman WJ, Tan SM, et al. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001;164(11):2067–2072. doi: 10.1164/ajrccm.164.11.2102005. [DOI] [PubMed] [Google Scholar]
- 3.Horak F, Jr, Studnicka M, Gartner C, et al. Particulate matter and lung function growth in children: a 3-yr follow-up study in Austrian schoolchildren. Eur Respir J. 2002;19(5):838–845. doi: 10.1183/09031936.02.00512001. [DOI] [PubMed] [Google Scholar]
- 4.Neuberger M, Moshammer H, Kundi M. Declining ambient air pollution and lung function improvement in Austrian children. Atmos Environ. 2002;36(11):1733–1736. [Google Scholar]
- 5.Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 6.Ihorst G, Frischer T, Horak F, et al. Long- and medium-term ozone effects on lung growth including a broad spectrum of exposure. Eur Respir J. 2004;23(2):292–299. doi: 10.1183/09031936.04.00021704. [DOI] [PubMed] [Google Scholar]
- 7.Gauderman WJ, Vora H, McConnell R, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369(9561):571–577. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 8.Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, et al. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med. 2007;176(4):377–384. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- 9.Downs SH, Schindler C, Liu LJ, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. SAPALDIA Team. N Engl J Med. 2007;357(23):2338–2347. doi: 10.1056/NEJMoa073625. [DOI] [PubMed] [Google Scholar]
- 10.Sekine K, Shima M, Nitta Y, et al. Long term effects of exposure to automobile exhaust on the pulmonary function of female adults in Tokyo, Japan. Occup Environ Med. 2004;61(4):350–357. doi: 10.1136/oem.2002.005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trenga CA, Sullivan JH, Schildcrout JS, et al. Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest. 2006;129(6):1614–1622. doi: 10.1378/chest.129.6.1614. [DOI] [PubMed] [Google Scholar]
- 12.Lee JT, Son JY, Cho YS. The adverse effects of fine particle air pollution on respiratory function in the elderly. Sci Total Environ. 2007;385(1–3):28–36. doi: 10.1016/j.scitotenv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Alexeeff SE, Litonjua AA, Wright RO, et al. Ozone exposure, antioxidant genes, and lung function in an elderly cohort: VA Normative Aging Study. Occup Environ Med. 2008;65(11):736–742. doi: 10.1136/oem.2007.035253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes LJ, Kapetanakis V, Rudnicka AR, et al. Chronic exposure to outdoor air pollution and lung function in adults. Thorax. 2009;64(8):657–663. doi: 10.1136/thx.2008.109389. [DOI] [PubMed] [Google Scholar]
- 15.Höppe P, Peters A, Rabe G, et al. Environmental ozone effects in different population subgroups. Int J Hyg Environ Health. 2003;206(6):505–516. doi: 10.1078/1438-4639-00250. [DOI] [PubMed] [Google Scholar]
- 16.Schikowski T, Ranft U, Sugiri D, et al. Decline in air pollution and change in prevalence in respiratory symptoms and chronic obstructive pulmonary disease in elderly women. Respir Res. 2010;11(1):113. doi: 10.1186/1465-9921-11-113. doi:10.1186/1465-9921-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Research Council, National Academy of Sciences. Research Priorities for Airborne Particulate Matter. Washington, DC: National Academy Press; 1998. [Google Scholar]
- 18.Sandström T, Frew AJ, Svartengren M, et al. The need for a focus on air pollution research in the elderly. Eur Respir J Suppl. 2003;40:92s–95s. doi: 10.1183/09031936.03.00403503. [DOI] [PubMed] [Google Scholar]
- 19.Annesi-Maesano I, Agabiti N, Pistelli R, et al. Subpopulations at increased risk of adverse health outcomes from air pollution. Eur Respir J Suppl. 2003;40:57s–63s. doi: 10.1183/09031936.03.00402103. [DOI] [PubMed] [Google Scholar]
- 20.Peters A. Susceptible subgroups: the challenge of studying interactions. Epidemiology. 2004;15(2):131–132. doi: 10.1097/01.ede.0000112218.51486.bc. [DOI] [PubMed] [Google Scholar]
- 21.Dychtwald K. Healthy Aging: Challenges and Solutions. Sudbury, MA: Jones & Bartlett Publishers; 1999. [Google Scholar]
- 22.Fries JF. Compression of morbidity in the elderly. Vaccine. 2000;18(16):1584–1589. doi: 10.1016/s0264-410x(99)00490-9. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed N, Mandel R, Fain MJ. Frailty: an emerging geriatric syndrome. Am J Med. 2007;120(9):748–753. doi: 10.1016/j.amjmed.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Abellan van Kan G, Rolland Y, Houles M, et al. The assessment of frailty in older adults. Clin Geriatr Med. 2010;26(2):275–286. doi: 10.1016/j.cger.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. Cardiovascular Health Study Collaborative Research Group. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 26.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the Women's Health and Aging Studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 27.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 28.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(4):358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 29.Enright PL, Kronmal RA, Higgins M, et al. Spirometry reference values for women and men 65 to 85 years of age. Cardiovascular Health Study. Am Rev Respir Dis. 1993;147(1):125–133. doi: 10.1164/ajrccm/147.1.125. [DOI] [PubMed] [Google Scholar]
- 30.Enright PL, Kronmal RA, Higgins MW, et al. Prevalence and correlates of respiratory symptoms and disease in the elderly. Cardiovascular Health Study. Chest. 1994;106(3):827–834. doi: 10.1378/chest.106.3.827. [DOI] [PubMed] [Google Scholar]
- 31.Griffith KA, Sherrill DL, Siegel EM, et al. Predictors of loss of lung function in the elderly: the Cardiovascular Health Study. Am J Respir Crit Care Med. 2001;163(1):61–68. doi: 10.1164/ajrccm.163.1.9906089. [DOI] [PubMed] [Google Scholar]
- 32.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 33.Harms CA. Does gender affect pulmonary function and exercise capacity? Respir Physiol Neurobiol. 2006;151(2-3):124–131. doi: 10.1016/j.resp.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Tager IB, Balmes J, Lurmann F, et al. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology. 2005;16(6):751–759. doi: 10.1097/01.ede.0000183166.68809.b0. [DOI] [PubMed] [Google Scholar]
- 35.Jiang R, Burke GL, Enright PL, et al. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol. 2008;168(6):602–610. doi: 10.1093/aje/kwn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hastie T, Tibshirani R. Generalized Additive Models. New York, NY: Chapman & Hall, Inc; 1990. [Google Scholar]
- 37.Jones R. Longitudinal Data With Serial Correlation: A State-Space Approach. New York, NY: Chapman & Hall, Inc; 1993. [Google Scholar]
- 38.Pinheiro J, Bates D. Mixed-Effects Models in S and S-PLUS. New York, NY: Springer Publishing Company; 2000. [Google Scholar]
- 39.Pinheiro J, Bates D, DebRoy S, et al. nlme: Linear and Nonlinear Mixed Effects Models. Vienna, Austria: R Foundation for Statistical Computing; 2008. (R package, version 3.1-89) [Google Scholar]
- 40.Thomas D. Statistical Methods in Environmental Epidemiology. New York, NY: Oxford University Press; 2009. [Google Scholar]
- 41.Abbey DE, Burchette RJ, Knutsen SF, et al. Long-term particulate and other air pollutants and lung function in nonsmokers. Am J Respir Crit Care Med. 1998;158(1):289–298. doi: 10.1164/ajrccm.158.1.9710101. [DOI] [PubMed] [Google Scholar]
- 42.Zeger SL, Dominici F, Samet J. Harvesting-resistant estimates of air pollution effects on mortality. Epidemiology. 1999;10(2):171–175. [PubMed] [Google Scholar]
- 43.Künzli N, Medina S, Kaiser R, et al. Assessment of deaths attributable to air pollution: should we use risk estimates based on time series or on cohort studies? Am J Epidemiol. 2001;153(11):1050–1055. doi: 10.1093/aje/153.11.1050. [DOI] [PubMed] [Google Scholar]
- 44.Sun R, Gu D. Air pollution, economic development of communities, and health status among the elderly in urban China. Am J Epidemiol. 2008;168(11):1311–1318. doi: 10.1093/aje/kwn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166(4):418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 46.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, Inc; 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.